Genome-Wide Meta-Analysis Identifies Variants in DSCAM and PDLIM3 That Correlate with Efficacy Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
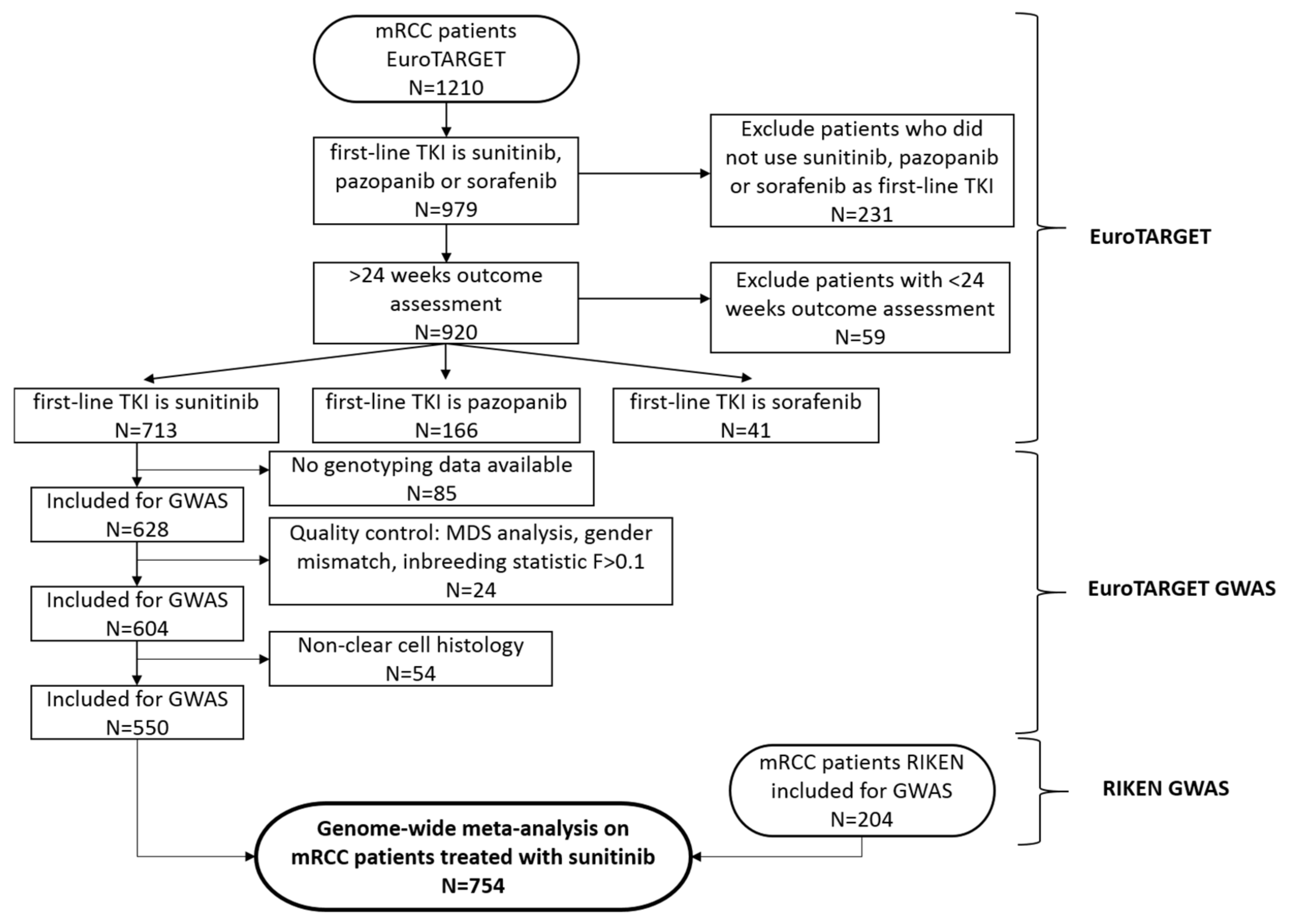
2.1. Patient Cohorts
2.2. Patient Selection
2.3. Clinical Data Collection
2.4. Genotyping and Quality Control (QC)
2.5. Genetic Association Analysis
3. Results
3.1. Patients and Genetic Data
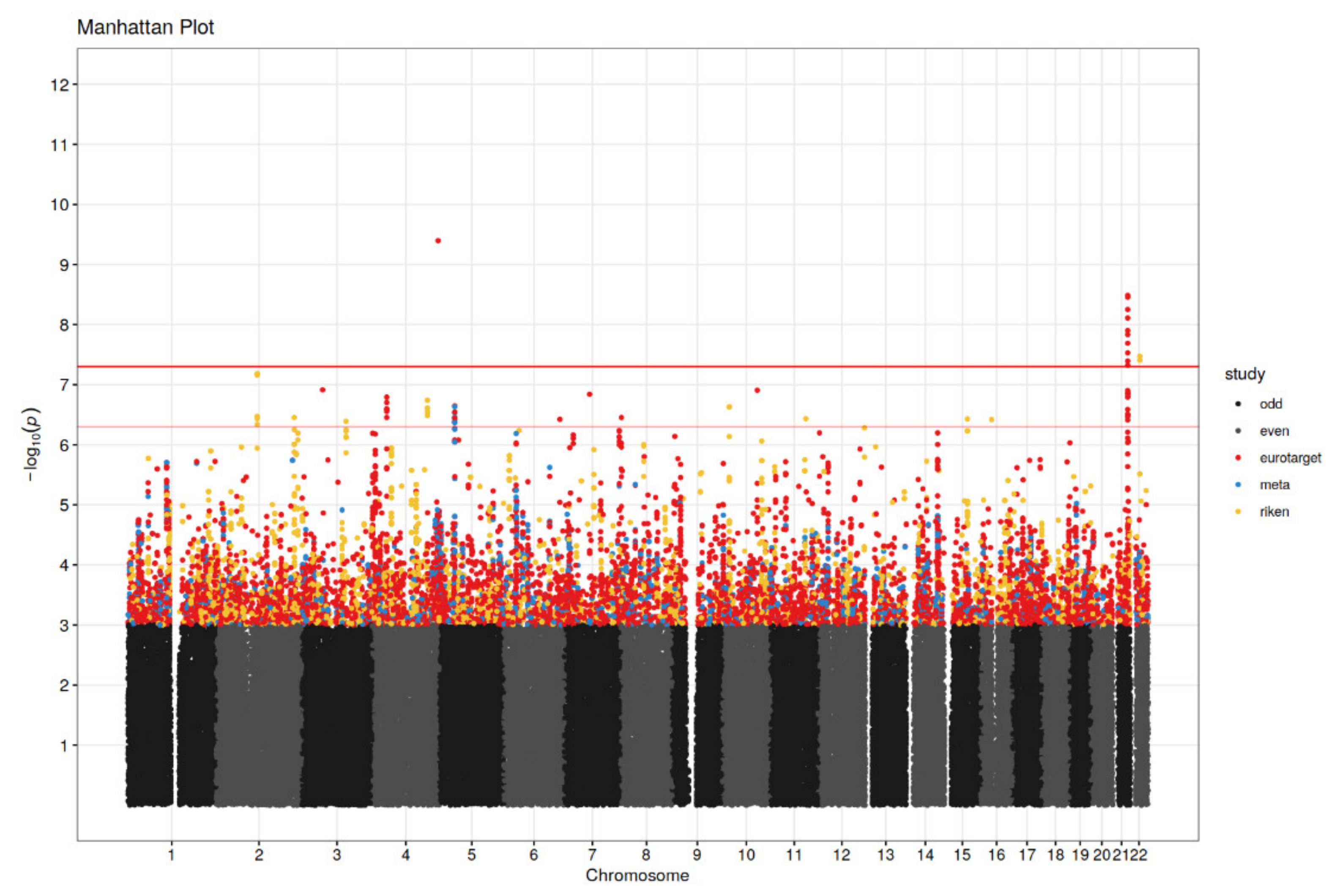
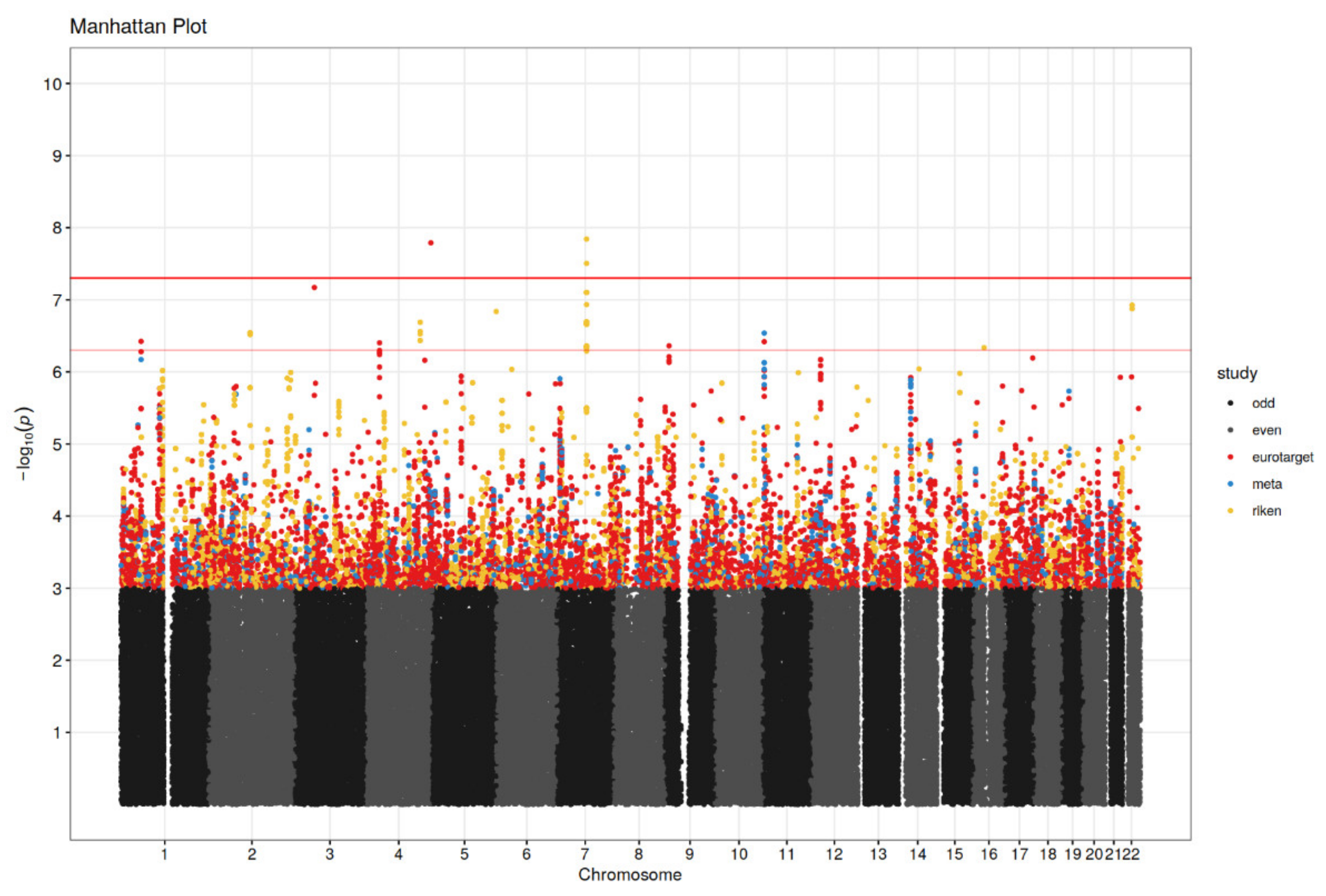
3.2. Genetic Association Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Erp, N.P.; Eechoute, K.; van der Veldt, A.A.; Haanen, J.B.; Reyners, A.K.L.; Mathijssen, A.H.J.; Boven, E.; van der Straaten, T.; Baak-Pablo, R.F.; Wessels, J.A.M.; et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J. Clin. Oncol. 2009, 27, 4406–4412. [Google Scholar] [CrossRef]
- Garcia-Donas, J.; Esteban, E.; Leandro-García, L.J.; Castellano, D.E.; González del Alba, A.; Climent, M.A.; Arranz, J.A.; Gallardo, E.; Puente, J.; Bellmunt, J.; et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: A multicentre, observational, prospective study. Lancet Oncol. 2011, 12, 1143–1150. [Google Scholar] [CrossRef]
- Alessandro Rizzo, A.; Mollica, V.; Santoni, M.; Ricci, A.D.; Rosellini, M.; Marchetti, A.; Montironi, R.; Ardizzoni, A.; Massari, F. Impact of Clinicopathological Features on Survival in Patients Treated with First-line Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Renal Cell Carcinoma: A Meta-analysis of Randomized Clinical Trials. Eur. Urol. Focus. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of renal cell carcinoma: Findings and clinical implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Houk, B.E.; Bello, C.L.; Kang, D.; Amantea, M. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin. Cancer Res. 2009, 15, 2497–2506. [Google Scholar] [CrossRef]
- Diekstra, M.H.M.; Swen, J.J.; Gelderblom, H.; Guchelaar, H.J. A decade of pharmacogenomics research on tyrosine kinase inhibitors in metastatic renal cell cancer: A systematic review. Exp. Rev. Mol. Diagn. 2016, 16, 605–618. [Google Scholar] [CrossRef]
- Rini, B.I.; Cohen, D.P.; Lu, D.R.; Chen, I.; Hariharan, S.; Gore, M.E.; Figlin, R.A.; Baum, M.S.; Motzer, R.J. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 2011, 103, 763–773. [Google Scholar] [CrossRef]
- Van der Veldt, A.A.; Eechoute, K.; Gelderblom, H.; Gietema, J.; Guchelaar, H.J.; van Erp, N.P.; van den Eertwegh, A.J.M.; Haanen, J.B.; Mathijssen, R.H.J.; Wessels, J.A.M. Genetic polymorphisms associated with a prolonged progression-free survival in patients with metastatic renal cell cancer treated with sunitinib. Clin. Cancer Res. 2011, 17, 620–629. [Google Scholar] [CrossRef]
- Kim, J.J.; Vaziri, S.A.J.; Rini, B.I.; Elson, P.; Garcia, J.A.; Wirka, R.; Dreicer, R.; Ganapathi, M.K.; Ganapathi, R. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer 2012, 118, 1946–1954. [Google Scholar] [CrossRef]
- Eechoute, K.; van der Veldt, A.A.M.; Oosting, S.; Kappers, M.H.W.; Wessels, J.A.M.; Gelderblom, H.; Guchelaar, H.J.; Reyners, A.K.L.; van Herpen, C.M.L.; Haanen, J.B.; et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin. Pharmacol. Ther. 2012, 92, 503–510. [Google Scholar] [CrossRef]
- Beuselinck, B.; Karadimou, A.; Lambrechts, D.; Claes, B.; Wolter, P.; Couchy, G.; Berkers, J.; Paridaens, R.; Schöffski, P.; Méjean, A.; et al. Single-nucleotide polymorphisms associated with outcome in metastatic renal cell carcinoma treated with sunitinib. Br. J. Cancer 2013, 108, 887–900. [Google Scholar] [CrossRef][Green Version]
- Beuselinck, B.; Lambrechts, D.; Van Brussel, T.; Wolter, P.; Cardinaels, N.; Joniau, S.; Lerut, E.; Karadimou, A.; Couchy, G.; Sebe, P.; et al. Efflux pump ABCB1 single nucleotide polymorphisms and dose reductions in patients with metastatic renal cell carcinoma treated with sunitinib. Acta Oncol. 2014, 53, 1413–1422. [Google Scholar] [CrossRef]
- Beuselinck, B.; Karadimou, A.; Lambrechts, D.; Claes, B.; Wolter, P.; Couchy, G.; Berkers, J.; van Poppel, H.; Paridaens, R.; Schöffski, P.; et al. VEGFR1 single nucleotide polymorphisms associated with outcome in patients with metastatic renal cell carcinoma treated with sunitinib—A multicentric retrospective analysis. Acta Oncol. 2014, 53, 103–112. [Google Scholar] [CrossRef]
- Diekstra, M.H.M.; Klümpen, H.J.; Lolkema, M.P.J.K.; Yu, H.; Kloth, J.S.L.; Gelderblom, H.; van Schaik, R.H.N.; Gurney, H.; Swen, J.J.; Huitema, A.D.R.; et al. Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU12662. Clin. Pharmacol. Ther. 2014, 96, 81–89. [Google Scholar] [CrossRef]
- Motzer, R.J.; Johnson, T.; Choueiri, T.K.; Deen, K.C.; Xue, Z.; Pandite, L.N.; Carpenter, C.; Xu, C.F. Hyperbilirubinemia in pazopanib- or sunitinib-treated patients in COMPARZ is associated with UGT1A1 polymorphisms. Ann. Oncol. 2013, 24, 2927–2928. [Google Scholar] [CrossRef]
- Xu, C.F.; Johnson, T.; Garcia-Donas, J.; Choueiri, T.K.; Sternberg, C.N.; Davis, I.D.; Bing, N.; Deen, K.C.; Xue, Z.; McCann, L.; et al. IL8 polymorphisms and overall survival in pazopanib- or sunitinib-treated patients with renal cell carcinoma. Br. J. Cancer 2015, 112, 1190–1198. [Google Scholar] [CrossRef][Green Version]
- Diekstra, M.H.M.; Swen, J.J.; Boven, E.; Castellano, D.; Gelderblom, H.; Mathijssen, R.H.J.; Rodríguez-Antona, C.; García-Donas, J.; Rini, B.I.; Guchelaar, H.J. CYP3A5 and ABCB1 Polymorphisms as Predictors for Sunitinib Outcome in Metastatic Renal Cell Carcinoma. Eur. Urol. 2015, 68, 621–629. [Google Scholar] [CrossRef]
- Low, S.K.; Takahashi, A.; Mushiroda, T.; Kubo, M. Genome-Wide Association Study: A useful tool to identify common genetic variants associated with drug toxicity and efficacy in cancer pharmacogenomics. Clin. Cancer Res. 2014, 20, 2541–2552. [Google Scholar] [CrossRef]
- Johnson, T.; Xu, C.F.; Choueiri, T.K.; Figlin, R.A.; Sternberg, C.N.; King, K.S.; Xue, Z.; Stinnett, S.; Deen, K.C.; Carpenter, C.; et al. Genome-wide association study (GWAS) of efficacy and safety endpoints in pazopanib- or sunitinib-treated patients with renal cell carcinoma (RCC), ASCO annual meeting abstract. J. Clin. Oncol. 2014, 32, 5s. Available online: https://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.4503 (accessed on 4 April 2022). [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef]
- Van der Zanden, L.F.; Vermeulen, S.H.; Oskarsdottir, A.; Maurits, J.S.F.; Diekstra, M.H.M.; Ambert, V.; Cambon-Thomsen, A.; Castellano, D.; Fritsch, A.; Garcia Donas, J.; et al. Description of the EuroTARGET cohort: A European collaborative project on Targeted therapy in Renal cell cancer: Genetic and Tumour related biomarkers for response and toxicity. Urol. Oncol. 2017, 35, e9–e16. [Google Scholar] [CrossRef]
- De Groot, S.; Sleijfer, S.; Redekop, W.K.; Oosterwijk, E.; Haanen, J.B.A.G.; Kiemeney, L.A.L.M.; Uyl-de Groot, C.A. Variation in use of targeted therapies for metastatic renal cell carcinoma: Results from a Dutch population-based registry. BMC Cancer 2016, 16, 364. [Google Scholar] [CrossRef]
- Low, S.-K.; Fukunaga, K.; Takahashi, A.; Matsuda, K.; Hongo, F.; Nakanishi, H.; Kitamura, H.; Inoue, T.; Kato, Y.; Tomita, Y.; et al. Association Study of a Functional Variant on ABCG2 Gene With Sunitinib-Induced Severe Adverse Drug Reaction. PLoS ONE 2016, 11, e0148177. [Google Scholar] [CrossRef]
- Illumina Human OmniExpress BeadChips. Available online: https://support.illumina.com/content/illumina-support/us/en/downloads.html (accessed on 4 April 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 4 April 2022).
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Marchini, J.; Howie, B.; Myers, S.; McVean, G.; Donnelly, P. A new multipoint method for genome-wide association studies via imputation of genotypes. Nat. Genet. 2007, 39, 906–913. [Google Scholar] [CrossRef]
- Delaneau, O.; Marchini, J.; Zagury, J.F. A linear complexity phasing method for thousands of genomes. Nat. Methods 2011, 9, 179–181. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium; Abecasis, G.R.; Auton, A.; Brooks, L.D.; DePristo, M.A.; Durbin, R.M.; Handsaker, R.E.; Kang, H.M.; Marth, G.T.; McVean, G.A. An integrated map of genetic variation from 1092 human genomes. Nature 2012, 491, 56–65. Available online: http://www.1000genomes.org/home. [CrossRef]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef]
- Wang, D.; Fang, J.; Lv, J.; Pan, Z.; Yin, X.; Cheng, H.; Guo, X. Novel polymorphisms in PDLIM3 and PDLIM5 gene encoding Z-line proteins increase risk of idiopathic dilated cardiomyopathy. J. Cell Mol. Med. 2019, 23, 7054–7062. [Google Scholar] [CrossRef]
- Ono, R.; Kaisho, T.; Tanaka, T. PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm. Sci. Rep. 2015, 5, 18327. [Google Scholar] [CrossRef] [PubMed]
- Krcmery, J.; Camarata, T.; Kulisz, A.; Simon, H.G. Nucleocytoplasmic functions of the PDZ-LIM protein family: New insights into organ development. Bioessays 2010, 32, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Núñez, R.; Barraza, N.; Gonzalez-Jamett, A.; Cárdenas, A.M.; Barnier, J.V.; Caviedes, P. Overexpressed Down Syndrome Cell Adhesion Molecule (DSCAM) Deregulates P21-Activated Kinase (PAK) Activity in an In Vitro Neuronal Model of Down Syndrome: Consequences on Cell Process Formation and Extension. Neurotox. Res. 2016, 30, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.L.; Jing, L.J.; Li, J.Y.; Lu, J.J.; Han, R.; Wang, S.Y.; Peng, T.; Jia, Y.J. Expression and significance of DSCAM in the cerebral cortex of APP transgenic mice. Neurosci. Lett. 2011, 491, 153–157. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Xu, L.; An, H.; Liu, W.; Liu, Y.; Lin, Z.; Xu, J. p21-activated kinase 1 determines stem-like phenotype and sunitinib resistance via NF-κB/IL-6 activation in renal cell carcinoma. Cell Death Dis. 2015, 6, e1637. [Google Scholar] [CrossRef]
- Nie, C.; Qin, X.; Li, X.; Tian, B.; Zhao, Y.; Jin, Y.; Li, Y.; Wang, Q.; Zeng, D.; Hong, A.; et al. CACNA2D3 Enhances the Chemosensitivity of Esophageal Squamous Cell Carcinoma to Cisplatin via Inducing Ca 2+-Mediated Apoptosis and Suppressing PI3K/Akt Pathways. Front. Oncol. 2019, 9, 185. [Google Scholar] [CrossRef]
- Shao, J.; Lu, J.; Zhu, W.; Yu, H.; Jing, X.; Wang, Y.L.; Wang, X.; Wang, X.J. Derepression of LOXL4 inhibits liver cancer growth by reactivating compromised p53. Cell Death Differ. 2019, 26, 2237–2252. [Google Scholar] [CrossRef]
- De Jong, L.M.; Jiskoot, W.; Swen, J.J.; Manson, M.L. Distinct effects of inflammation on cytochrome P450 regulation and drug metabolism: Lessons from experimental models and a potential role for pharmacogenetics. Genes 2020, 11, 1509. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
| EuroTARGET | RIKEN | |||
|---|---|---|---|---|
| Characteristic | Value (Range) | % | Value (Range) | % |
| Gender | ||||
| ■ male | 405 | 74 | 151 | 74 |
| ■ female | 145 | 26 | 53 | 26 |
| Median age at start sunitinib (years) | 63 (33–87) | |||
| Country | ||||
| ■ Netherlands | 281 | 51 | ||
| ■ Spain (SOGUG) | 168 | 30.5 | ||
| ■ Germany (CESAR) | 39 | 7 | ||
| ■ Iceland (Landspitali University Hospital) | 27 | 5 | ||
| ■ United Kingdom (Addenbrooke’s Cambridge) | 20 | 3.5 | ||
| ■ Romania (University of Medicine and Farmacy Carol Davila) | 15 | 3 | ||
| ■ Japan | 204 | 100 | ||
| Prior nephrectomy before start sunitinib | ||||
| ■ yes | 201 | 36.5 | 166 | 81 |
| ■ no | 349 | 63.5 | 32 | 16 |
| ■ unknown | 6 | 3 | ||
| WHO performance status (EuroTARGET), ECOG performance status (RIKEN) | ||||
| ■ 0 | 206 | 37.5 | 146 | 72 |
| ■ 1 | 206 | 37.5 | 44 | 22 |
| ■ 2 | 25 | 4.5 | 10 | 5 |
| ■ 3 | 2 | 0.3 | 2 | 1 |
| ■ 4 | 1 | 0.2 | 0 | 0 |
| ■ unknown | 110 | 20 | 2 | 1 |
| Treatment line sunitinib | ||||
| ■ 1 | 512 | 93 | 98 | 48 |
| ■ 2 | 32 | 6 | 41 | 20 |
| ■ 3 | 5 | 0.9 | 39 | 19 |
| ■ 4 | 1 | 0.1 | 15 | 7 |
| ■ 5 | 4 | 2 | ||
| ■ Unknown | 7 | 3 | ||
| Heng prognostic risk group * | ||||
| ■ Good (0 risk factors) | 80 | 14.5 | ||
| ■ Intermediate (1–2 risk factors) | 298 | 54.2 | ||
| ■ Poor (3–6 risk factors) | 172 | 31.3 | ||
| MSKCC prognostic risk group ** | ||||
| ■ Favourable (0 risk factors) | 38 | 19 | ||
| ■ Intermediate (1–2 risk factors) | 124 | 61 | ||
| ■ Poor (3–6 risk factors) | 32 | 16 | ||
| ■ Unknown | 10 | 5 | ||
| Prior drugs (excluding TKI or TKI-like antitumor treatment) | ||||
| ■ Yes | 38 | 7 | 102 | 50 |
| ■ No | 512 | 93 | 95 | 47 |
| ■ Unknown | 7 | 3 | ||
| Duration of prior drug treatment (months) | 1 to 23 | 1 to 123 months | ||
| Sunitinib starting dose (daily dose in mg) | ||||
| ■ 50 | 482 | 87.6 | 125 | 61 |
| ■ 37.5 | 46 | 8.4 | 72 | 35 |
| ■ 25 | 20 | 3.6 | 7 | 3 |
| ■ 12.5 | 2 | 0.4 | 0 | 0 |
| Median treatment duration of sunitinib | 10 months (2 days–113 months) | 5 months (2 days–53 months) | ||
| Dose reduction | ||||
| ■ yes | 223 | 41 | 0 | 0 |
| ■ no | 310 | 56 | 201 | 99 |
| ■ unknown | 17 | 3 | 3 | 1 |
| Dose reduction within 12 weeks (equal to 2 cycles) of treatment | ||||
| ■ yes | 111 | 20 | 0 | 0 |
| ■ no | 416 | 76 | 201 | 99 |
| ■ unknown | 23 | 4 | 3 | 1 |
| Dose reduction within 24 weeks (equal to 4 cycles) of treatment | ||||
| ■ yes | 145 | 26 | 0 | 0 |
| ■ no | 382 | 70 | 201 | 99 |
| ■ unknown | 23 | 4 | 3 | 1 |
| SNP | Chromosome | Position | Gene | MAF (%) | Allele * | p-Value | Hazard Ratio (HR) | Beta | Se | SOE | Association |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs28520013 | 4 | 186442067 | PDLIM3 | 2.30 | G/T | 4.02 × 10−10 | 7.26 | 1.98 | 0.32 | E | GWAS significant |
| rs2205096 | 21 | 41683405 | DSCAM | 6.30 | T/A | 5.60 × 10−9 | 2.50 | 0.92 | 0.16 | E | |
| rs111356738 | 21 | 41677845 | DSCAM | 5.30 | G/A | 4.77 × 10−8 | 2.51 | 0.92 | 0.17 | E | |
| rs76403021 | 3 | 58195886 | DNASE1L3 | G/C | 1.22 × 10−7 | 4.23 | 1.44 | 0.27 | E | GWAS suggestive | |
| rs595883 | 10 | 98483774 | Position close to LOXL4, PYROXD2, HPS1, and HPSE2 | 2.00 | C/T | 1.24 × 10−7 | 1.63 | 0.49 | 0.09 | E | |
| rs118150161 | 7 | 71421614 | CALN1 | 2.00 | T/C | 1.45 × 10−7 | 3.83 | 1.34 | 0.26 | E | |
| rs113168647 | 21 | 41679847 | DSCAM | 2.00 | C/T | 1.59 × 10−7 | 2.46 | 0.90 | 0.17 | E | |
| rs7282179 | 21 | 41670052 | DSCAM | 5.00 | T/C | 1.61 × 10−7 | 2.26 | 0.81 | 0.16 | E | |
| rs79433348 | 4 | 41381011 | LIMCH1 | 6.40 | A/C | 1.61 × 10−7 | 2.70 | 0.99 | 0.19 | E | |
| rs79160607 | 4 | 41395370 | LIMCH1 | 3.60 | A/G | 1.98 × 10−7 | 2.57 | 0.94 | 0.18 | E |
| SNP | Chromosome | Position | Gene | MAF (%) | Allele * | p-Value | Hazard Ratio (HR) | Beta | Se | SOE | Association |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs28520013 | 4 | 186442067 | PDLIM3 | 2.30 | G | 1.62 × 10−8 | 5.96 | 1.79 | 0.32 | E | GWAS significant |
| rs62256189 | 3 | 55094589 | CACNA2D3 | 2.50 | G/T | 6.75 × 10−8 | 3.20 | 1.16 | 0.22 | E | GWAS suggestive |
| rs2271583 | 11 | 3685655 | ART1 | 14.70 | G/A | 2.90 × 10−7 | 1.72 | 0.54 | 0.11 | E | |
| rs80071112 | 1 | 57650640 | DAB1 | 2.90 | C/T | 3.78 × 10−7 | 3.09 | 1.13 | 0.22 | E | |
| rs79160607 | 4 | 41395370 | LIMCH1 | 4.00 | A/G | 3.95 × 10−7 | 2.49 | 0.91 | 0.18 | E | |
| rs10959526 | 9 | 11107610 | Position close to PTPRD | 4.20 | T/C | 4.36 × 10−7 | 2.52 | 0.93 | 0.18 | E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diekstra, M.H.M.; Swen, J.J.; van der Zanden, L.F.M.; Vermeulen, S.H.; Boven, E.; Mathijssen, R.H.J.; Fukunaga, K.; Mushiroda, T.; Hongo, F.; Oosterwijk, E.; et al. Genome-Wide Meta-Analysis Identifies Variants in DSCAM and PDLIM3 That Correlate with Efficacy Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib. Cancers 2022, 14, 2838. https://doi.org/10.3390/cancers14122838
Diekstra MHM, Swen JJ, van der Zanden LFM, Vermeulen SH, Boven E, Mathijssen RHJ, Fukunaga K, Mushiroda T, Hongo F, Oosterwijk E, et al. Genome-Wide Meta-Analysis Identifies Variants in DSCAM and PDLIM3 That Correlate with Efficacy Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib. Cancers. 2022; 14(12):2838. https://doi.org/10.3390/cancers14122838
Chicago/Turabian StyleDiekstra, Meta H. M., Jesse J. Swen, Loes F. M. van der Zanden, Sita H. Vermeulen, Epie Boven, Ron H. J. Mathijssen, Koya Fukunaga, Taisei Mushiroda, Fumiya Hongo, Egbert Oosterwijk, and et al. 2022. "Genome-Wide Meta-Analysis Identifies Variants in DSCAM and PDLIM3 That Correlate with Efficacy Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib" Cancers 14, no. 12: 2838. https://doi.org/10.3390/cancers14122838
APA StyleDiekstra, M. H. M., Swen, J. J., van der Zanden, L. F. M., Vermeulen, S. H., Boven, E., Mathijssen, R. H. J., Fukunaga, K., Mushiroda, T., Hongo, F., Oosterwijk, E., Cambon-Thomsen, A., Castellano, D., Fritsch, A., Donas, J. G., Rodriguez-Antona, C., Ruijtenbeek, R., Radu, M. T., Eisen, T., Junker, K., ... Guchelaar, H.-J. (2022). Genome-Wide Meta-Analysis Identifies Variants in DSCAM and PDLIM3 That Correlate with Efficacy Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib. Cancers, 14(12), 2838. https://doi.org/10.3390/cancers14122838









