Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Bladder Cancer Overview
3. mTOR Cell Signaling Pathway
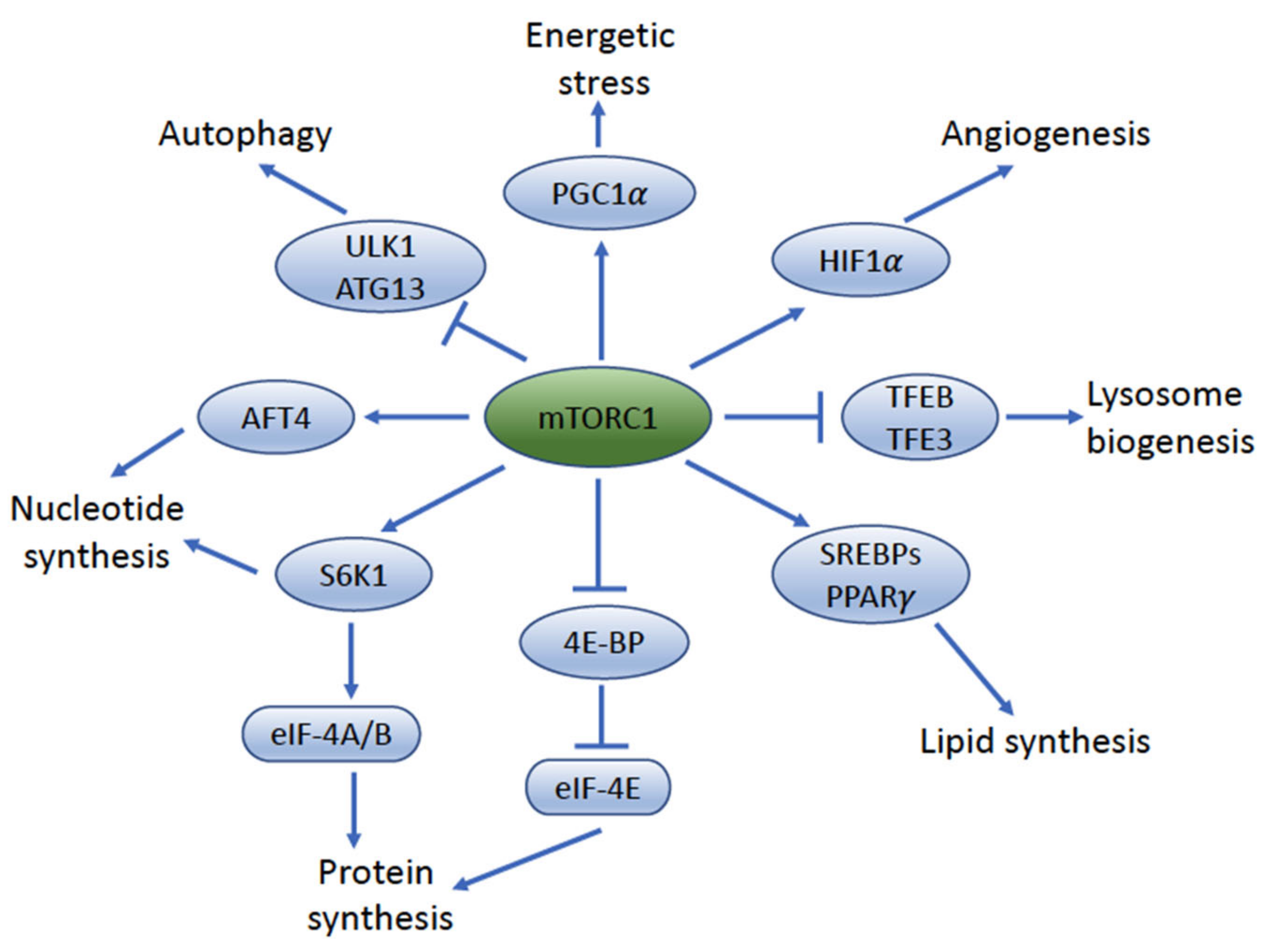
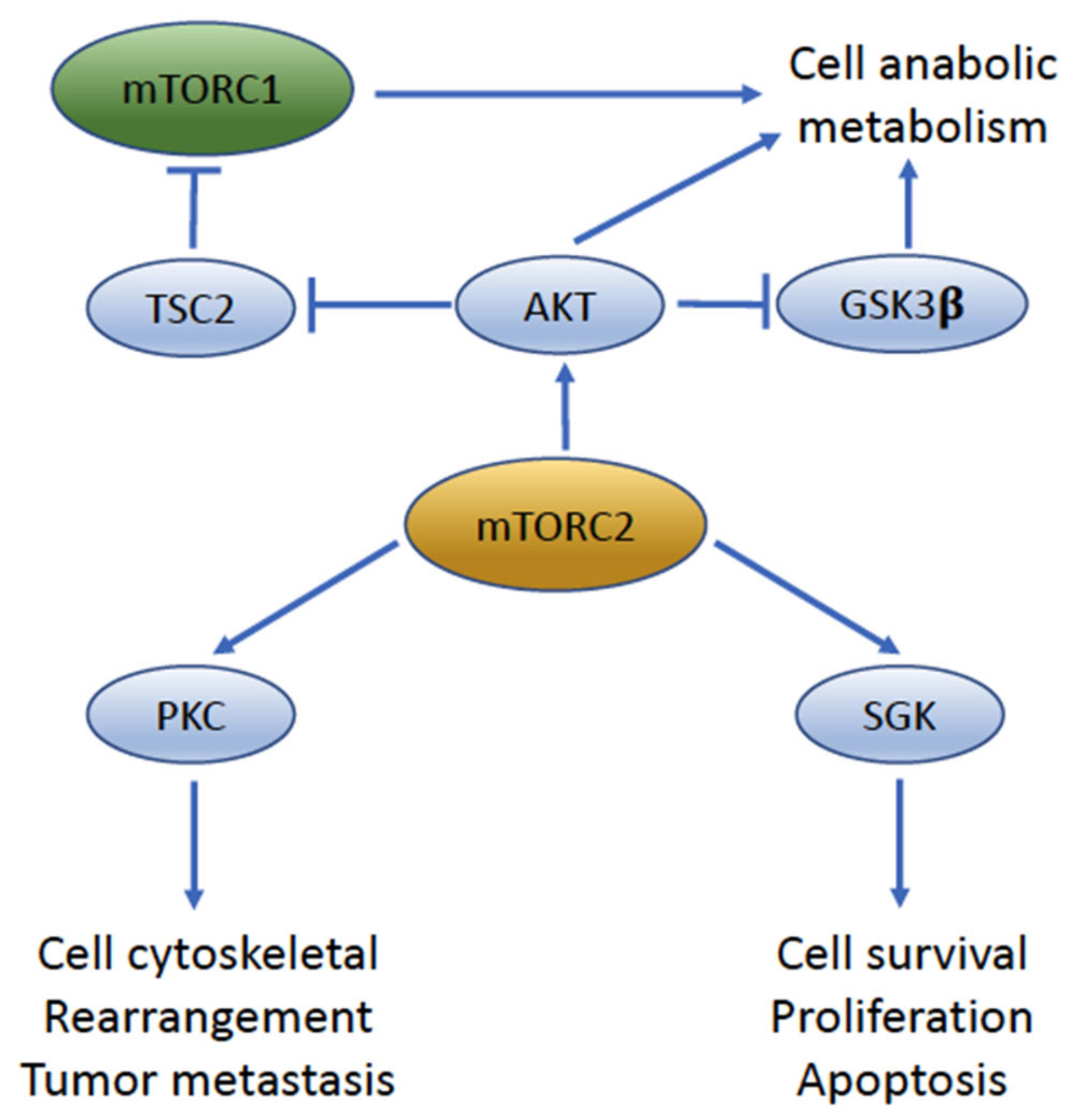
4. mTOR Complexes: mTORC1 and mTORC2
5. mTORC1 Signaling Impacts UC Cell Size and Proliferation
6. mTORC2 Signaling Is a Key Driver of UC Migration and Invasion
7. Genetic Alterations and Dysregulation of mTOR Signaling in Urothelial Carcinoma
8. Potential Targets of the PI3K/AKT/mTOR Pathway in Urothelial Cancer
8.1. PI3K Inhibitors
8.2. AKT Inhibitors
| Inhibitor | Target | Disease Status | Study/Trial | Reference |
|---|---|---|---|---|
| Copanlisib | Pan-PI3K | Advanced UC | Phase I | [157] |
| Alpelisib | PI3Kα | Advanced UC | Phase Ib | [158] |
| Buparlisib | Pan-PI3K | Metastatic UC | Phase II | [145] |
| Pilaralisib | Pan-PI3K | Advanced UC | Phase I | [144] |
| MK-2206 | Allosteric pan-AKT | UC cell lines | Preclincial | [148] |
| AZ7328 | Allosteric pan-AKT | UC cell lines | Preclincial | [149] |
| Borussertib | Allosteric pan-AKT | UC cell lines | Preclincial | [151] |
| Capivasertib | ATP-competitive pan-AKT | UC cell lines | Preclincial | [152] |
| Ipatasertib | ATP-competitive pan-AKT | Advanced/metastatic UC | Phase Ib | [156] |
| Everolimus | Allosteric mTOR | Advanced/metastatic UC | Phase II | [159,160,161,162] |
| Temsirolimus | Allosteric mTOR | Metastatic UC | Phase II | [163,164] |
| ABI-009 | Allosteric mTOR | BCG refractory NIMBC | Phase I/II | [165] |
| OSI-027 | mTORC1, mTORC2 | UC cell lines | Preclinical | [166] |
| Dactolisib | Dual PI3K/mTOR | UC cell lines | Preclinical | [167] |
| Sapanisertib | mTORC1, mTORC2 | Metastatic UC | Phase II | [168] |
| Vistusertib | mTORC1, mTORC2 | UC cell lines Advanced UC | Preclinical Phase Ib | [152] [169] |
| Erdafitinib | FGFR1–4 | Metastatic UC | Phase II | [170] |
| AZD4547 | FGFR1–3 | Advanced UC | Phase Ib | [169] |
| Infigratinib | FGFR1–3 | Advanced UC | Phase I | [171] |
| Pemigatinib | FGFR1–3 | Metastatic UC | Phase II | [172] |
| Rogaratinib | FGFR1–4 | Metastatic UC | Phase II/III | [173] |
8.3. mTOR Inhibitors
8.4. FGFR Inhibitors
9. Future of Targeting mTOR in Bladder Cancer Therapy
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mock, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2016; Volume 8. [Google Scholar]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, S.; Svatek, R.; Singh, P.; Woldu, S. Bladder Neoplasms: Muscle Invasive Bladder Cancer. Available online: https://university.auanet.org/core/oncology-adult/bladder-neoplasms-muscle-invasive-bladder-cancer/index.cfm (accessed on 8 December 2021).
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Houede, N.; Pourquier, P. Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use in the treatment of bladder cancers. Pharmacol. Ther. 2015, 145, 1–18. [Google Scholar] [CrossRef]
- Sathe, A.; Nawroth, R. Targeting the PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol. Biol. 2018, 1655, 335–350. [Google Scholar] [CrossRef]
- Creighton, C.J. The clinical applications of The Cancer Genome Atlas project for bladder cancer. Expert Rev. Anticancer Ther. 2018, 18, 973–980. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Kogevinas, M. Bladder Cancer. In Occupational Cancers; Anttila, S., Boffetta, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 487–506. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. IARC Monogr. Eval. Carcinog Risks Hum. 2014, 106, 1–512. [Google Scholar]
- Sui, X.; Lei, L.; Chen, L.; Xie, T.; Li, X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget. 2017, 8, 93279–93294. [Google Scholar] [CrossRef]
- Tamas, E.F.; Stephenson, A.J.; Campbell, S.C.; Montague, D.K.; Trusty, D.C.; Hansel, D.E. Histopathologic features and clinical outcomes in 71 cases of bladder diverticula. Arch. Pathol. Lab. Med. 2009, 133, 791–796. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, B.; Tian, Y.; Chen, Y.; Luo, D.; Lin, Y.; Li, H.; Wang, K.J. Total fluid consumption and risk of bladder cancer: A meta-analysis with updated data. Oncotarget 2017, 8, 55467–55477. [Google Scholar] [CrossRef]
- Ahmad, I.; Sansom, O.J.; Leung, H.Y. Exploring molecular genetics of bladder cancer: Lessons learned from mouse models. Dis. Model. Mech. 2012, 5, 323–332. [Google Scholar] [CrossRef]
- Hansel, D.E.; Miller, J.S.; Cookson, M.S.; Chang, S.S. Challenges in the pathology of non-muscle-invasive bladder cancer: A dialogue between the urologic surgeon and the pathologist. Urology 2013, 81, 1123–1130. [Google Scholar] [CrossRef]
- Heney, N.M.; Ahmed, S.; Flanagan, M.J.; Frable, W.; Corder, M.P.; Hafermann, M.D.; Hawkins, I.R. Superficial Bladder Cancer: Progression and Recurrence. J. Urol. 1983, 130, 1083–1086. [Google Scholar] [CrossRef]
- Kashif Khan, M.; Ahmed, I.; Raza, S.J. Factors effecting recurrence and progression of high grade non invasive bladder cancer treated by intravesical BCG. Pak. J. Med. Sci. 2014, 30, 326–330. [Google Scholar] [CrossRef]
- Cordon-Cardo, C. Molecular alterations associated with bladder cancer initiation and progression. Scand. J. Urol. Nephrol. Suppl. 2008, 42, 154–165. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Lin Gellert, L.; Maschino, A.; Gotto, G.T.; Kim, P.H.; Al-Ahmadie, H.; Bochner, B.H. The role of PTEN tumor suppressor pathway staining in carcinoma in situ of the bladder. Urol. Oncol. 2014, 32, 657–662. [Google Scholar] [CrossRef][Green Version]
- Puzio-Kuter, A.M.; Castillo-Martin, M.; Kinkade, C.W.; Wang, X.; Shen, T.H.; Matos, T.; Shen, M.M.; Cordon-Cardo, C.; Abate-Shen, C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009, 23, 675–680. [Google Scholar] [CrossRef]
- Kachrilas, S.; Dellis, A.; Papatsoris, A.; Avgeris, S.; Anastasiou, D.; Gavriil, A.; Horti, M.; Tseleni Balafouta, S.; Livadas, K.; Stravopodis, D.J.; et al. PI3K/AKT pathway genetic alterations and dysregulation of expression in bladder cancer. J. BUON 2019, 24, 329–337. [Google Scholar]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e525. [Google Scholar] [CrossRef]
- Hurst, C.D.; Alder, O.; Platt, F.M.; Droop, A.; Stead, L.F.; Burns, J.E.; Burghel, G.J.; Jain, S.; Klimczak, L.J.; Lindsay, H.; et al. Genomic Subtypes of Non-invasive Bladder Cancer with Distinct Metabolic Profile and Female Gender Bias in KDM6A Mutation Frequency. Cancer Cell. 2017, 32, 701–715.e707. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Currie, R.A.; Walker, K.S.; Gray, A.; Deak, M.; Casamayor, A.; Downes, C.P.; Cohen, P.; Alessi, D.R.; Lucocq, J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 1999, 337 Pt 3, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Wang, J.; Su, B.; Wu, D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 2011, 286, 10998–11002. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Hodakoski, C.; Barrows, D.; Mense, S.M.; Parsons, R.E. PTEN function: The long and the short of it. Trends Biochem Sci. 2014, 39, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cordes, I.; Kluth, M.; Zygis, D.; Rink, M.; Chun, F.; Eichelberg, C.; Dahlem, R.; Fisch, M.; Hoppner, W.; Wagner, W.; et al. PTEN deletions are related to disease progression and unfavourable prognosis in early bladder cancer. Histopathology 2013, 63, 670–677. [Google Scholar] [CrossRef]
- Aveyard, J.S.; Skilleter, A.; Habuchi, T.; Knowles, M.A. Somatic mutation of PTEN in bladder carcinoma. Br. J. Cancer 1999, 80, 904–908. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Samarghandian, S.; Najafi, M. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur. J. Pharmacol. 2020, 881, 173226. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef]
- Ramlaul, K.; Fu, W.; Li, H.; de Martin Garrido, N.; He, L.; Trivedi, M.; Cui, W.; Aylett, C.H.S.; Wu, G. Architecture of the Tuberous Sclerosis Protein Complex. J. Mol. Biol. 2021, 433, 166743. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Amin, A.G.; Cooper, J.B.; Das, K.; Schmidt, M.H.; Murali, R. Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects. Adv. Biol. Regul. 2017, 64, 39–48. [Google Scholar] [CrossRef]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef]
- Sabatini, D.M.; Erdjument-Bromage, H.; Lui, M.; Tempst, P.; Snyder, S.H. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 1994, 78, 35–43. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef]
- Oshiro, N.; Takahashi, R.; Yoshino, K.; Tanimura, K.; Nakashima, A.; Eguchi, S.; Miyamoto, T.; Hara, K.; Takehana, K.; Avruch, J.; et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J. Biol. Chem. 2007, 282, 20329–20339. [Google Scholar] [CrossRef]
- Vander Haar, E.; Lee, S.I.; Bandhakavi, S.; Griffin, T.J.; Kim, D.H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 2007, 9, 316–323. [Google Scholar] [CrossRef]
- Kovacina, K.S.; Park, G.Y.; Bae, S.S.; Guzzetta, A.W.; Schaefer, E.; Birnbaum, M.J.; Roth, R.A. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 2003, 278, 10189–10194. [Google Scholar] [CrossRef]
- Sancak, Y.; Thoreen, C.C.; Peterson, T.R.; Lindquist, R.A.; Kang, S.A.; Spooner, E.; Carr, S.A.; Sabatini, D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell. 2007, 25, 903–915. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Tian, Y.; Li, J.; Qi, Y.; Zhao, D.; Wu, Z.; Huang, M.; Wong, C.C.L.; Wang, H.W.; et al. Cryo-EM structure of human mTOR complex 2. Cell Res. 2018, 28, 518–528. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Liu, P.; Gan, W.; Chin, Y.R.; Ogura, K.; Guo, J.; Zhang, J.; Wang, B.; Blenis, J.; Cantley, L.C.; Toker, A.; et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015, 5, 1194–1209. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Frank, A.R.; Jewell, J.L. Amino acid and small GTPase regulation of mTORC1. Cell Logist. 2017, 7, e1378794. [Google Scholar] [CrossRef]
- Shen, K.; Huang, R.K.; Brignole, E.J.; Condon, K.J.; Valenstein, M.L.; Chantranupong, L.; Bomaliyamu, A.; Choe, A.; Hong, C.; Yu, Z.; et al. Architecture of the human GATOR1 and GATOR1-Rag GTPases complexes. Nature 2018, 556, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.; Borden, K.L. The oncogene eIF4E: Using biochemical insights to target cancer. J. Interferon Cytokine Res. 2013, 33, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.; Gingras, A.C.; Pause, A.; Sonenberg, N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene 1996, 13, 2415–2420. [Google Scholar]
- Kim, S.J.; Kim, J.H.; Jung, H.S.; Lee, T.J.; Lee, K.M.; Chang, I.H. Phosphorylated p70S6K in noninvasive low-grade urothelial carcinoma of the bladder: Correlation with tumor recurrence. Asian J. Androl. 2014, 16, 611–617. [Google Scholar] [CrossRef]
- Jefferies, H.B.; Fumagalli, S.; Dennis, P.B.; Reinhard, C.; Pearson, R.B.; Thomas, G. Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997, 16, 3693–3704. [Google Scholar] [CrossRef]
- Caron, A.; Richard, D.; Laplante, M. The Roles of mTOR Complexes in Lipid Metabolism. Annu. Rev. Nutr. 2015, 35, 321–348. [Google Scholar] [CrossRef]
- Cam, H.; Easton, J.B.; High, A.; Houghton, P.J. mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1alpha. Mol. Cell. 2010, 40, 509–520. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 2009, 20, 1981–1991. [Google Scholar] [CrossRef]
- Yuan, H.X.; Russell, R.C.; Guan, K.L. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy 2013, 9, 1983–1995. [Google Scholar] [CrossRef]
- Ling, N.X.Y.; Kaczmarek, A.; Hoque, A.; Davie, E.; Ngoei, K.R.W.; Morrison, K.R.; Smiles, W.J.; Forte, G.M.; Wang, T.; Lie, S.; et al. mTORC1 directly inhibits AMPK to promote cell proliferation under nutrient stress. Nat. Metab. 2020, 2, 41–49. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Tan, C.Y.; Hagen, T. mTORC1 dependent regulation of REDD1 protein stability. PLoS ONE 2013, 8, e63970. [Google Scholar] [CrossRef]
- Lin, A.; Yao, J.; Zhuang, L.; Wang, D.; Han, J.; Lam, E.W.; Network, T.R.; Gan, B. The FoxO-BNIP3 axis exerts a unique regulation of mTORC1 and cell survival under energy stress. Oncogene 2014, 33, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.; Guernah, I.; Jin, D.; Grisendi, S.; Alimonti, A.; Teruya-Feldstein, J.; Cordon-Cardo, C.; Simon, M.C.; Rafii, S.; Pandolfi, P.P. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature 2006, 442, 779–785. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; de Stanchina, E.; Teresky, A.K.; Jin, S.; Lowe, S.; Levine, A.J. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: Stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007, 67, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hau, A.M.; Beach, J.R.; Harwalker, J.; Mantuano, E.; Gonias, S.L.; Egelhoff, T.T.; Hansel, D.E. Mammalian target of rapamycin complex 2 (mTORC2) is a critical determinant of bladder cancer invasion. PLoS ONE 2013, 8, e81081. [Google Scholar] [CrossRef] [PubMed]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef]
- Dowling, R.J.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakis, E.; Wang, X.; Larsson, O.; Selvaraj, A.; Liu, Y.; et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef]
- Kwon, J.K.; Kim, S.-J.; Hoon Kim, J.; Mee Lee, K.; Ho Chang, I. Dual inhibition by S6K1 and Elf4E is essential for controlling cellular growth and invasion in bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 51.e27–51.e35. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, T.J.; Chang, I.H. Role of the mTOR Pathway in the Progression and Recurrence of Bladder Cancer: An Immunohistochemical Tissue Microarray Study. Korean J. Urol. 2011, 52, 466–473. [Google Scholar] [CrossRef]
- Xie, X.; Hu, H.; Tong, X.; Li, L.; Liu, X.; Chen, M.; Yuan, H.; Xie, X.; Li, Q.; Zhang, Y.; et al. The mTOR-S6K pathway links growth signalling to DNA damage response by targeting RNF168. Nat. Cell Biol. 2018, 20, 320–331. [Google Scholar] [CrossRef]
- Liu, H.-J.; Lam, H.C.; Baglini, C.V.; Nijmeh, J.; Cottrill, A.A.; Chan, S.Y.; Henske, E.P. Rapamycin-upregulated miR-29b promotes mTORC1-hyperactive cell growth in TSC2-deficient cells by downregulating tumor suppressor retinoic acid receptor β (RARβ). Oncogene 2019, 38, 7367–7383. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Agarwal, N.; Rinaldetti, S.; Cheikh, B.B.; Zhou, Q.; Hass, E.P.; Jones, R.T.; Joshi, M.; LaBarbera, D.V.; Knott, S.R.V.; Cech, T.R.; et al. TRIM28 is a transcriptional activator of the mutant TERT promoter in human bladder cancer. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Ikenoue, T.; Inoki, K.; Yang, Q.; Zhou, X.; Guan, K.L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008, 27, 1919–1931. [Google Scholar] [CrossRef]
- Hresko, R.C.; Mueckler, M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol Chem. 2005, 280, 40406–40416. [Google Scholar] [CrossRef]
- Oh, W.J.; Wu, C.C.; Kim, S.J.; Facchinetti, V.; Julien, L.A.; Finlan, M.; Roux, P.P.; Su, B.; Jacinto, E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010, 29, 3939–3951. [Google Scholar] [CrossRef]
- Li, X.; Gao, T. mTORC2 phosphorylates protein kinase Czeta to regulate its stability and activity. EMBO Rep. 2014, 15, 191–198. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Betz, C.; Hall, M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013, 203, 563–574. [Google Scholar] [CrossRef]
- Ebner, M.; Sinkovics, B.; Szczygiel, M.; Ribeiro, D.W.; Yudushkin, I. Localization of mTORC2 activity inside cells. J. Cell Biol. 2017, 216, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.R.; Fritzen, A.M.; James, D.E.; Jensen, T.E.; Kleinert, M.; Richter, E.A. Growth Factor-Dependent and -Independent Activation of mTORC2. Trends Endocrinol. Metab. 2020, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kovalski, J.R.; Bhaduri, A.; Zehnder, A.M.; Neela, P.H.; Che, Y.; Wozniak, G.G.; Khavari, P.A. The Functional Proximal Proteome of Oncogenic Ras Includes mTORC2. Mol. Cell. 2019, 73, 830–844 e812. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, W.; Li, G.; Cui, W. Weighing In on mTOR Complex 2 Signaling: The Expanding Role in Cell Metabolism. Oxid. Med. Cell Longev. 2018, 2018, 7838647. [Google Scholar] [CrossRef] [PubMed]
- Zinzalla, V.; Stracka, D.; Oppliger, W.; Hall, M.N. Activation of mTORC2 by association with the ribosome. Cell 2011, 144, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Saci, A.; Cantley, L.C.; Carpenter, C.L. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol. Cell. 2011, 42, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Lotfi, P.; Chavan, A.J.; Montano, N.M.; Bolourani, P.; Weeks, G.; Shen, Z.; Briggs, S.P.; Pots, H.; Van Haastert, P.J.; et al. The small GTPases Ras and Rap1 bind to and control TORC2 activity. Sci. Rep. 2016, 6, 25823. [Google Scholar] [CrossRef] [PubMed]
- Senoo, H.; Kamimura, Y.; Kimura, R.; Nakajima, A.; Sawai, S.; Sesaki, H.; Iijima, M. Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration. Nat. Cell Biol. 2019, 21, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Gulhati, P.; Bowen, K.A.; Liu, J.; Stevens, P.D.; Rychahou, P.G.; Chen, M.; Lee, E.Y.; Weiss, H.L.; O’Connor, K.L.; Gao, T.; et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011, 71, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Ruegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Lamouille, S.; Connolly, E.; Smyth, J.W.; Akhurst, R.J.; Derynck, R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 2012, 125, 1259–1273. [Google Scholar] [CrossRef]
- Morrison Joly, M.; Williams, M.M.; Hicks, D.J.; Jones, B.; Sanchez, V.; Young, C.D.; Sarbassov, D.D.; Muller, W.J.; Brantley-Sieders, D.; Cook, R.S. Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Res. 2017, 19, 74. [Google Scholar] [CrossRef]
- Sahu, D.; Huan, J.; Wang, H.; Sahoo, D.; Casteel, D.E.; Klemke, R.L.; Boss, G.R.; Hansel, D.E. Bladder Cancer Invasion Is Mediated by Mammalian Target of Rapamycin Complex 2–Driven Regulation of Nitric Oxide and Invadopodia Formation. Am. J. Pathol. 2021, 191, 2203–2218. [Google Scholar] [CrossRef]
- Hau, A.M.; Gupta, S.; Leivo, M.Z.; Nakashima, K.; Macias, J.; Zhou, W.; Hodge, A.; Wulfkuhle, J.; Conkright, B.; Bhuvaneshwar, K.; et al. Dynamic Regulation of Caveolin-1 Phosphorylation and Caveolae Formation by Mammalian Target of Rapamycin Complex 2 in Bladder Cancer Cells. Am. J. Pathol. 2019, 189, 1846–1862. [Google Scholar] [CrossRef]
- Hau, A.M.; Leivo, M.Z.; Gilder, A.S.; Hu, J.J.; Gonias, S.L.; Hansel, D.E. mTORC2 activation is regulated by the urokinase receptor (uPAR) in bladder cancer. Cell Signal. 2017, 29, 96–106. [Google Scholar] [CrossRef]
- Dohn, L.H.; Pappot, H.; Iversen, B.R.; Illemann, M.; Hoyer-Hansen, G.; Christensen, I.J.; Thind, P.; Salling, L.; von der Maase, H.; Laerum, O.D. uPAR Expression Pattern in Patients with Urothelial Carcinoma of the Bladder--Possible Clinical Implications. PLoS ONE 2015, 10, e0135824. [Google Scholar] [CrossRef][Green Version]
- Iwata, T.; Kimura, S.; Abufaraj, M.; Janisch, F.; Parizi, M.K.; Haitel, A.; Rink, M.; Roupret, M.; Fajkovic, H.; Seebacher, V.; et al. Prognostic role of the urokinase plasminogen activator (uPA) system in patients with nonmuscle invasive bladder cancer. Urol. Oncol. 2019, 37, 774–783. [Google Scholar] [CrossRef]
- Gupta, S.; Hau, A.M.; Al-Ahmadie, H.A.; Harwalkar, J.; Shoskes, A.C.; Elson, P.; Beach, J.R.; Hussey, G.S.; Schiemann, W.P.; Egelhoff, T.T.; et al. Transforming Growth Factor-beta Is an Upstream Regulator of Mammalian Target of Rapamycin Complex 2-Dependent Bladder Cancer Cell Migration and Invasion. Am. J. Pathol. 2016, 186, 1351–1360. [Google Scholar] [CrossRef]
- Kazyken, D.; Magnuson, B.; Bodur, C.; Acosta-Jaquez, H.A.; Zhang, D.; Tong, X.; Barnes, T.M.; Steinl, G.K.; Patterson, N.E.; Altheim, C.H.; et al. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Platt, F.M.; Hurst, C.D.; Taylor, C.F.; Gregory, W.M.; Harnden, P.; Knowles, M.A. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin. Cancer Res. 2009, 15, 6008–6017. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Arantes-Rodrigues, R.; Sousa-Diniz, C.; Colaco, A.; Lourenco, L.; De La Cruz, L.F.; Da Silva, V.M.; Afonso, J.; Lopes, C.; Santos, L. The effects of sirolimus on urothelial lesions chemically induced in ICR mice by BBN. Anticancer Res. 2009, 29, 3221–3226. [Google Scholar] [PubMed]
- Solomon, J.P.; Hansel, D.E. The Emerging Molecular Landscape of Urothelial Carcinoma. Surg Pathol. Clin. 2016, 9, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Sjodahl, G.; Lauss, M.; Gudjonsson, S.; Liedberg, F.; Hallden, C.; Chebil, G.; Mansson, W.; Hoglund, M.; Lindgren, D. A systematic study of gene mutations in urothelial carcinoma; inactivating mutations in TSC2 and PIK3R1. PLoS ONE 2011, 6, e18583. [Google Scholar] [CrossRef] [PubMed]
- Gildea, J.J.; Herlevsen, M.; Harding, M.A.; Gulding, K.M.; Moskaluk, C.A.; Frierson, H.F.; Theodorescu, D. PTEN can inhibit in vitro organotypic and in vivo orthotopic invasion of human bladder cancer cells even in the absence of its lipid phosphatase activity. Oncogene 2004, 23, 6788–6797. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Al-Ahmadie, H.; Schultz, N.; Hanrahan, A.J.; Ostrovnaya, I.; Balar, A.V.; Kim, P.H.; Lin, O.; Weinhold, N.; Sander, C.; et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J. Clin. Oncol. 2013, 31, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, H.; Kishimoto, H.; Sasaki, T.; Horie, Y.; Natsui, M.; Shibata, Y.; Hamada, K.; Yajima, N.; Kawahara, K.; Sasaki, M.; et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res. 2006, 66, 8389–8396. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Habuchi, T.; Kennedy, W.; Cuthbert-Heavens, D. Mutation spectrum of the 9q34 tuberous sclerosis gene TSC1 in transitional cell carcinoma of the bladder. Cancer Res. 2003, 63, 7652–7656. [Google Scholar]
- Billerey, C.; Chopin, D.; Aubriot-Lorton, M.H.; Ricol, D.; Gil Diez de Medina, S.; Van Rhijn, B.; Bralet, M.P.; Lefrere-Belda, M.A.; Lahaye, J.B.; Abbou, C.C.; et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 2001, 158, 1955–1959. [Google Scholar] [CrossRef]
- Nassar, A.H.; Umeton, R.; Kim, J.; Lundgren, K.; Harshman, L.; Van Allen, E.M.; Preston, M.; Dong, F.; Bellmunt, J.; Mouw, K.W.; et al. Mutational Analysis of 472 Urothelial Carcinoma Across Grades and Anatomic Sites. Clin. Cancer Res. 2019, 25, 2458–2470. [Google Scholar] [CrossRef]
- van Rhijn, B.W.G.; Mertens, L.S.; Mayr, R.; Bostrom, P.J.; Real, F.X.; Zwarthoff, E.C.; Boormans, J.L.; Abas, C.; van Leenders, G.; Gotz, S.; et al. FGFR3 Mutation Status and FGFR3 Expression in a Large Bladder Cancer Cohort Treated by Radical Cystectomy: Implications for Anti-FGFR3 Treatment?(dagger). Eur. Urol. 2020, 78, 682–687. [Google Scholar] [CrossRef]
- Chaux, A.; Cohen, J.S.; Schultz, L.; Albadine, R.; Jadallah, S.; Murphy, K.M.; Sharma, R.; Schoenberg, M.P.; Netto, G.J. High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: A study using formalin-fixed, paraffin-embedded archival tissues. Hum. Pathol. 2012, 43, 1590–1595. [Google Scholar] [CrossRef]
- Rotterud, R.; Nesland, J.M.; Berner, A.; Fossa, S.D. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 2005, 95, 1344–1350. [Google Scholar] [CrossRef]
- Chow, N.H.; Chan, S.H.; Tzai, T.S.; Ho, C.L.; Liu, H.S. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin. Cancer Res. 2001, 7, 1957–1962. [Google Scholar]
- Hansel, D.E.; Swain, E.; Dreicer, R.; Tubbs, R.R. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am. J. Clin. Pathol. 2008, 130, 274–281. [Google Scholar] [CrossRef]
- Gonzalez-Roibon, N.; Kim, J.J.; Faraj, S.F.; Chaux, A.; Bezerra, S.M.; Munari, E.; Ellis, C.; Sharma, R.; Keizman, D.; Bivalacqua, T.J.; et al. Insulin-like growth factor-1 receptor overexpression is associated with outcome in invasive urothelial carcinoma of urinary bladder: A retrospective study of patients treated using radical cystectomy. Urology 2014, 83, 1444.E1–1444.E6. [Google Scholar] [CrossRef]
- Grabiner, B.C.; Nardi, V.; Birsoy, K.; Possemato, R.; Shen, K.; Sinha, S.; Jordan, A.; Beck, A.H.; Sabatini, D.M. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014, 4, 554–563. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef]
- Murugan, A.K.; Alzahrani, A.; Xing, M. Mutations in critical domains confer the human mTOR gene strong tumorigenicity. J. Biol Chem. 2013, 288, 6511–6521. [Google Scholar] [CrossRef]
- Bellmunt, J.; Werner, L.; Leow, J.J.; Mullane, S.A.; Fay, A.P.; Riester, M.; Van Hummelen, P.; Taplin, M.E.; Choueiri, T.K.; Van Allen, E.; et al. Somatic Copy Number Abnormalities and Mutations in PI3K/AKT/mTOR Pathway Have Prognostic Significance for Overall Survival in Platinum Treated Locally Advanced or Metastatic Urothelial Tumors. PLoS ONE 2015, 10, e0124711. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.; Hui, G.; Mathis, C.; Chamie, K.; Pantuck, A.J.; Drakaki, A. The Current Status and Future Role of the Phosphoinositide 3 Kinase/AKT Signaling Pathway in Urothelial Cancer: An Old Pathway in the New Immunotherapy Era. Clin. Genitourin Cancer 2018, 16, e269–e276. [Google Scholar] [CrossRef]
- Winters, B.R.; Vakar-Lopez, F.; Brown, L.; Montgomery, B.; Seiler, R.; Black, P.C.; Boormans, J.L.; Dall Era, M.; Davincioni, E.; Douglas, J.; et al. Mechanistic target of rapamycin (MTOR) protein expression in the tumor and its microenvironment correlates with more aggressive pathology at cystectomy. Urol. Oncol. 2018, 36, 342.e7–342.e14. [Google Scholar] [CrossRef]
- Hau, A.M.; Nakasaki, M.; Nakashima, K.; Krish, G.; Hansel, D.E. Differential mTOR pathway profiles in bladder cancer cell line subtypes to predict sensitivity to mTOR inhibition. Urol Oncol. 2017, 35, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Rowley, B.R.; Bull, C.O.; Schneider, C.; Haegebarth, A.; Schatz, C.A.; Fracasso, P.R.; Wilkie, D.P.; Hentemann, M.; Wilhelm, S.M.; et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 2013, 12, 2319–2330. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H.; et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kalpha-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef]
- Ross, R.L.; McPherson, H.R.; Kettlewell, L.; Shnyder, S.D.; Hurst, C.D.; Alder, O.; Knowles, M.A. PIK3CA dependence and sensitivity to therapeutic targeting in urothelial carcinoma. BMC Cancer 2016, 16, 553. [Google Scholar] [CrossRef]
- Borcoman, E.; De La Rochere, P.; Richer, W.; Vacher, S.; Chemlali, W.; Krucker, C.; Sirab, N.; Radvanyi, F.; Allory, Y.; Pignot, G.; et al. Inhibition of PI3K pathway increases immune infiltrate in muscle-invasive bladder cancer. Oncoimmunology 2019, 8, e1581556. [Google Scholar] [CrossRef]
- Edelman, G.; Rodon, J.; Lager, J.; Castell, C.; Jiang, J.; Van Allen, E.M.; Wagle, N.; Lindeman, N.I.; Sholl, L.M.; Shapiro, G.I. Phase I Trial of a Tablet Formulation of Pilaralisib, a Pan-Class I PI3K Inhibitor, in Patients with Advanced Solid Tumors. Oncologist 2018, 23, e401–e438. [Google Scholar] [CrossRef]
- McPherson, V.; Reardon, B.; Bhayankara, A.; Scott, S.N.; Boyd, M.E.; Garcia-Grossman, I.R.; Regazzi, A.M.; McCoy, A.S.; Kim, P.H.; Al-Ahmadie, H.; et al. A phase 2 trial of buparlisib in patients with platinum-resistant metastatic urothelial carcinoma. Cancer 2020. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Sathe, A.; Chalaud, G.; Oppolzer, I.; Wong, K.Y.; von Busch, M.; Schmid, S.C.; Tong, Z.; Retz, M.; Gschwend, J.E.; Schulz, W.A.; et al. Parallel PI3K, AKT and mTOR inhibition is required to control feedback loops that limit tumor therapy. PLoS ONE 2018, 13, e0190854. [Google Scholar] [CrossRef]
- Sathe, A.; Guerth, F.; Cronauer, M.V.; Heck, M.M.; Thalgott, M.; Gschwend, J.E.; Retz, M.; Nawroth, R. Mutant PIK3CA controls DUSP1-dependent ERK 1/2 activity to confer response to AKT target therapy. Br. J. Cancer 2014, 111, 2103–2113. [Google Scholar] [CrossRef][Green Version]
- Dickstein, R.J.; Nitti, G.; Dinney, C.P.; Davies, B.R.; Kamat, A.M.; McConkey, D.J. Autophagy limits the cytotoxic effects of the AKT inhibitor AZ7328 in human bladder cancer cells. Cancer Biol. Ther. 2012, 13, 1325–1338. [Google Scholar] [CrossRef]
- Peng, M.; Deng, J.; Zhou, S.; Xiao, D.; Long, J.; Zhang, N.; He, C.; Mo, M.; Yang, X. Dual Inhibition of Pirarubicin-Induced AKT and ERK Activations by Phenformin Sensitively Suppresses Bladder Cancer Growth. Front. Pharmacol. 2019, 10, 1159. [Google Scholar] [CrossRef]
- Weisner, J.; Landel, I.; Reintjes, C.; Uhlenbrock, N.; Trajkovic-Arsic, M.; Dienstbier, N.; Hardick, J.; Ladigan, S.; Lindemann, M.; Smith, S.; et al. Preclinical Efficacy of Covalent-Allosteric AKT Inhibitor Borussertib in Combination with Trametinib in KRAS-Mutant Pancreatic and Colorectal Cancer. Cancer Res. 2019, 79, 2367–2378. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.J.; Lee, I.K.; Min, S.C.; Sung, H.H.; Jeong, B.C.; Lee, J.; Park, S.H. Synergistic Effects of Combination Therapy with AKT and mTOR Inhibitors on Bladder Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2825. [Google Scholar] [CrossRef]
- Xing, Y.; Lin, N.U.; Maurer, M.A.; Chen, H.; Mahvash, A.; Sahin, A.; Akcakanat, A.; Li, Y.; Abramson, V.; Litton, J.; et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019, 21, 78. [Google Scholar] [CrossRef]
- Molife, L.R.; Yan, L.; Vitfell-Rasmussen, J.; Zernhelt, A.M.; Sullivan, D.M.; Cassier, P.A.; Chen, E.; Biondo, A.; Tetteh, E.; Siu, L.L.; et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J. Hematol. Oncol. 2014, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, L.G.; Edwards, M.; O’Donnell, S.; Doherty, P.; MacDougall, T.; Tith, K.; Kazakin, J.; Schwartz, B. Clinical report: One year of treatment of Proteus syndrome with miransertib (ARQ 092). Cold Spring Harb. Mol. Case Stud. 2020, 6, a004549. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, S.J.; Tabernero, J.; Molife, L.R.; Soria, J.C.; Cervantes, A.; Vogelzang, N.J.; Patel, M.R.; Hussain, M.; Baron, A.; Argiles, G.; et al. Antitumor activity of ipatasertib combined with chemotherapy: Results from a phase Ib study in solid tumors. Ann. Oncol. 2020, 31, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Alberts, S.R.; Pena, C.; Genvresse, I.; Ajavon-Hartmann, A.; Xia, C.; Kelly, A.; Grilley-Olson, J.E. Phase I dose-escalation study of copanlisib in combination with gemcitabine or cisplatin plus gemcitabine in patients with advanced cancer. Br. J. Cancer 2018, 118, 462–470. [Google Scholar] [CrossRef]
- Hyman, D.M.; Tran, B.; Paz-Ares, L.; Machiels, J.-P.; Schellens, J.H.; Bedard, P.L.; Campone, M.; Cassier, P.A.; Sarantopoulos, J.; Vaishampayan, U.; et al. Combined PIK3CA and FGFR Inhibition With Alpelisib and Infigratinib in Patients With PIK3CA-Mutant Solid Tumors, With or Without FGFR Alterations. JCO Precision Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Iyer, G.; Regazzi, A.M.; Al-Ahmadie, H.; Gerst, S.R.; Ostrovnaya, I.; Gellert, L.L.; Kaplan, R.; Garcia-Grossman, I.R.; Pendse, D.; et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 2013, 112, 462–470. [Google Scholar] [CrossRef]
- Niegisch, G.; Retz, M.; Thalgott, M.; Balabanov, S.; Honecker, F.; Ohlmann, C.H.; Stockle, M.; Bogemann, M.; Vom Dorp, F.; Gschwend, J.; et al. Second-Line Treatment of Advanced Urothelial Cancer with Paclitaxel and Everolimus in a German Phase II Trial (AUO Trial AB 35/09). Oncology 2015, 89, 70–78. [Google Scholar] [CrossRef]
- Seront, E.; Rottey, S.; Sautois, B.; Kerger, J.; D’Hondt, L.A.; Verschaeve, V.; Canon, J.L.; Dopchie, C.; Vandenbulcke, J.M.; Whenham, N.; et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: Clinical activity, molecular response, and biomarkers. Ann. Oncol. 2012, 23, 2663–2670. [Google Scholar] [CrossRef]
- Adib, E.; Klonowska, K.; Giannikou, K.; Do, K.T.; Pruitt-Thompson, S.; Bhushan, K.; Milstein, M.I.; Hedglin, J.; Kargus, K.E.; Sholl, L.M.; et al. Phase II Clinical Trial of Everolimus in a Pan-Cancer Cohort of Patients with mTOR Pathway Alterations. Clin. Cancer Res. 2021, 27, 3845–3853. [Google Scholar] [CrossRef]
- Gerullis, H.; Eimer, C.; Ecke, T.H.; Georgas, E.; Freitas, C.; Kastenholz, S.; Arndt, C.; Heusch, C.; Otto, T. A phase II trial of temsirolimus in second-line metastatic urothelial cancer. Med. Oncol. 2012, 29, 2870–2876. [Google Scholar] [CrossRef]
- Pulido, M.; Roubaud, G.; Cazeau, A.L.; Mahammedi, H.; Vedrine, L.; Joly, F.; Mourey, L.; Pfister, C.; Goberna, A.; Lortal, B.; et al. Safety and efficacy of temsirolimus as second line treatment for patients with recurrent bladder cancer. BMC Cancer 2018, 18, 194. [Google Scholar] [CrossRef]
- McKiernan, J.M.; Lascano, D.; Ahn, J.; Ghandour, R.; Pak, J.S.; RoyChoudhury, A.; Chang, S.; DeCastro, G.J.; Desai, N. A phase I/II multi-center study of intravesical nanoparticle albumin-bound rapamycin (ABI-009) in the treatment of BCG refractory non-muscle invasive bladder cancer. J. Clin. Oncol. 2015, 33, TPS4576. [Google Scholar] [CrossRef]
- Becker, M.N.; Wu, K.J.; Marlow, L.A.; Kreinest, P.A.; Vonroemeling, C.A.; Copland, J.A.; Williams, C.R. The combination of an mTORc1/TORc2 inhibitor with lapatinib is syn.nergistic in bladder cancer in vitro. Urol. Oncol. 2014, 32, 317–326. [Google Scholar] [CrossRef]
- Moon, d.G.; Lee, S.E.; Oh, M.M.; Lee, S.C.; Jeong, S.J.; Hong, S.K.; Yoon, C.Y.; Byun, S.S.; Park, H.S.; Cheon, J. NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cells. Int. J. Oncol. 2014, 45, 1027–1035. [Google Scholar] [CrossRef]
- Kim, J.W.; Milowsky, M.I.; Hahn, N.M.; Kwiatkowski, D.J.; Morgans, A.K.; Davis, N.B.; Appleman, L.J.; Gupta, S.; Lara, P.L.N.; Hoffman-Censits, J.H.; et al. Sapanisertib, a dual mTORC1/2 inhibitor, for TSC1- or TSC2-mutated metastatic urothelial carcinoma (mUC). J. Clin. Oncol. 2021, 39, 431. [Google Scholar] [CrossRef]
- Powles, T.; Carroll, D.; Chowdhury, S.; Gravis, G.; Joly, F.; Carles, J.; Fléchon, A.; Maroto, P.; Petrylak, D.; Rolland, F.; et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat. Med. 2021, 27, 793–801. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Pal, S.K.; Rosenberg, J.E.; Hoffman-Censits, J.H.; Berger, R.; Quinn, D.I.; Galsky, M.D.; Wolf, J.; Dittrich, C.; Keam, B.; Delord, J.P.; et al. Efficacy of BGJ398, a Fibroblast Growth Factor Receptor 1–3 Inhibitor, in Patients with Previously Treated Advanced Urothelial Carcinoma with FGFR3 Alterations. Cancer Discov. 2018, 8, 812–821. [Google Scholar] [CrossRef]
- Necchi, A.; Pouessel, D.; Leibowitz-Amit, R.; Flechon, A.; Gupta, S.; Barthelemy, P.; Maio, M.; Zhu, X.; Asatiani, E.; Serbest, G.; et al. 900PInterim results of fight-201, a phase II, open-label, multicenter study of INCB054828 in patients (pts) with metastatic or surgically unresectable urothelial carcinoma (UC) harboring fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations (GA). Ann. Oncol. 2018, 29, viii319–viii320. [Google Scholar] [CrossRef]
- Quinn, D.I.; Petrylak, D.P.; Bellmunt, J.; Necchi, A.; Gurney, H.; Lee, J.-L.; Van Der Heijden, M.S.; Rosenbaum, E.; Penel, N.; Pang, S.-T.; et al. FORT-1: Phase II/III study of rogaratinib versus chemotherapy (CT) in patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) selected based on FGFR1/3 mRNA expression. J. Clin. Oncol. 2020, 38, 489. [Google Scholar] [CrossRef]
- Saunders, R.N.; Metcalfe, M.S.; Nicholson, M.L. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001, 59, 3–16. [Google Scholar] [CrossRef]
- Ehninger, D.; Neff, F.; Xie, K. Longevity, aging and rapamycin. Cell Mol. Life Sci. 2014, 71, 4325–4346. [Google Scholar] [CrossRef] [PubMed]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, N.; Yoshino, K.; Hidayat, S.; Tokunaga, C.; Hara, K.; Eguchi, S.; Avruch, J.; Yonezawa, K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 2004, 9, 359–366. [Google Scholar] [CrossRef]
- Mansure, J.J.; Nassim, R.; Chevalier, S.; Rocha, J.; Scarlata, E.; Kassouf, W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol. Ther. 2009, 8, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Bachir, B.G.; Souhami, L.; Mansure, J.J.; Cury, F.; Vanhuyse, M.; Brimo, F.; Aprikian, A.G.; Tanguay, S.; Sturgeon, J.; Kassouf, W. Phase I Clinical Trial of Everolimus Combined with Trimodality Therapy in Patients with Muscle-Invasive Bladder Cancer. Bladder Cancer 2017, 3, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Milowsky, M.I.; Ostrovnaya, I.; Gerst, S.R.; Rosenberg, J.E.; Voss, M.H.; Apolo, A.B.; Regazzi, A.M.; McCoy, A.S.; Boyd, M.E.; et al. Phase I Study of Everolimus in Combination with Gemcitabine and Split-Dose Cisplatin in Advanced Urothelial Carcinoma. Bladder Cancer 2016, 2, 111–117. [Google Scholar] [CrossRef]
- Dalbagni, G.; Benfante, N.; Sjoberg, D.D.; Bochner, B.H.; Machele Donat, S.; Herr, H.W.; Mc Coy, A.S.; Fahrner, A.J.; Retinger, C.; Rosenberg, J.E.; et al. Single Arm Phase I/II Study of Everolimus and Intravesical Gemcitabine in Patients with Primary or Secondary Carcinoma In Situ of the Bladder who failed Bacillus Calmette Guerin (NCT01259063). Bladder Cancer 2017, 3, 113–119. [Google Scholar] [CrossRef]
- Iyer, G.; Hanrahan, A.J.; Milowsky, M.I.; Al-Ahmadie, H.; Scott, S.N.; Janakiraman, M.; Pirun, M.; Sander, C.; Socci, N.D.; Ostrovnaya, I.; et al. Genome Sequencing Identifies a Basis for Everolimus Sensitivity. Science 2012, 338, 221. [Google Scholar] [CrossRef]
- Rodriguez-Pascual, J.; Cheng, E.; Maroto, P.; Duran, I. Emergent toxicities associated with the use of mTOR inhibitors in patients with advanced renal carcinoma. Anticancer Drugs 2010, 21, 478–486. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Valentino, J.D.; Gulhati, P.; Evers, B.M. mTOR inhibitors in cancer therapy. Cancer Lett. 2012, 319, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X. Research progress of mTOR inhibitors. Eur. J. Med. Chem. 2020, 208, 112820. [Google Scholar] [CrossRef]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chene, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Chi, B.H.; Kim, S.J.; Seo, H.K.; Seo, H.H.; Lee, S.J.; Kwon, J.K.; Lee, T.J.; Chang, I.H. P70S6K and Elf4E dual inhibition is essential to control bladder tumor growth and progression in orthotopic mouse non-muscle invasive bladder tumor model. J. Korean Med. Sci. 2015, 30, 308–316. [Google Scholar] [CrossRef]
- Casadei, C.; Dizman, N.; Schepisi, G.; Cursano, M.C.; Basso, U.; Santini, D.; Pal, S.K.; De Giorgi, U. Targeted therapies for advanced bladder cancer: New strategies with FGFR inhibitors. Ther. Adv. Med. Oncol. 2019, 11, 1758835919890285. [Google Scholar] [CrossRef]
- Roubal, K.; Myint, Z.W.; Kolesar, J.M. Erdafitinib: A novel therapy for FGFR-mutated urothelial cancer. Am. J. Health Syst. Pharm. 2020, 77, 346–351. [Google Scholar] [CrossRef]
- Porebska, N.; Latko, M.; Kucinska, M.; Zakrzewska, M.; Otlewski, J.; Opalinski, L. Targeting Cellular Trafficking of Fibroblast Growth Factor Receptors as a Strategy for Selective Cancer Treatment. J. Clin. Med. 2018, 8, 7. [Google Scholar] [CrossRef]
- Katoh, M.; Nakagama, H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Necchi, A.; Park, S.H.; García-Donas, J.; Huddart, R.A.; Burgess, E.F.; Fleming, M.T.; Rezazadeh Kalebasty, A.; Mellado, B.; Varlamov, S.; et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: Long-term follow-up of a phase 2 study. Lancet Oncol. 2022, 23, 248–258. [Google Scholar] [CrossRef]
- Herrera-Abreu, M.T.; Pearson, A.; Campbell, J.; Shnyder, S.D.; Knowles, M.A.; Ashworth, A.; Turner, N.C. Parallel RNA interference screens identify EGFR activation as an escape mechanism in FGFR3-mutant cancer. Cancer Discov. 2013, 3, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sustic, T.; Leite de Oliveira, R.; Lieftink, C.; Halonen, P.; van de Ven, M.; Beijersbergen, R.L.; van den Heuvel, M.M.; Bernards, R.; van der Heijden, M.S. A Functional Genetic Screen Identifies the Phosphoinositide 3-kinase Pathway as a Determinant of Resistance to Fibroblast Growth Factor Receptor Inhibitors in FGFR Mutant Urothelial Cell Carcinoma. Eur. Urol. 2017, 71, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Song, B.; Ai, H. Combined inhibition of FGFR and mTOR pathways is effective in suppressing ovarian cancer. Am. J. Transl Res. 2019, 11, 1616–1625. [Google Scholar] [PubMed]
- Wheler, J.J.; Atkins, J.T.; Janku, F.; Moulder, S.L.; Stephens, P.J.; Yelensky, R.; Valero, V.; Miller, V.; Kurzrock, R.; Meric-Bernstam, F. Presence of both alterations in FGFR/FGF and PI3K/AKT/mTOR confer improved outcomes for patients with metastatic breast cancer treated with PI3K/AKT/mTOR inhibitors. Oncoscience 2016, 3, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Fujishita, T.; Kojima, Y.; Kajino-Sakamoto, R.; Taketo, M.M.; Aoki, M. Tumor microenvironment confers mTOR inhibitor resistance in invasive intestinal adenocarcinoma. Oncogene 2017, 36, 6480–6489. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.X.; Tan, G.H.; Hui Low, S.H.; Mohd Omar, M.F.; Han, M.J.; Iacopetta, B.; Soo, R.; Beloueche-Babari, M.; Bhattacharya, B.; Soong, R. Acquired resistance to PI3K/mTOR inhibition is associated with mitochondrial DNA mutation and glycolysis. Oncotarget 2017, 8, 110133–110144. [Google Scholar] [CrossRef]
- Juengel, E.; Natsheh, I.; Najafi, R.; Rutz, J.; Tsaur, I.; Haferkamp, A.; Chun, F.K.; Blaheta, R.A. Mechanisms behind Temsirolimus Resistance Causing Reactivated Growth and Invasive Behavior of Bladder Cancer Cells In Vitro. Cancers 2019, 11, 777. [Google Scholar] [CrossRef]
- He, J.; McLaughlin, R.P.; van der Noord, V.; Foekens, J.A.; Martens, J.W.M.; van Westen, G.; Zhang, Y.; van de Water, B. Multi-targeted kinase inhibition alleviates mTOR inhibitor resistance in triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 178, 263–274. [Google Scholar] [CrossRef]
- Taglieri, L.; De Iuliis, F.; Giuffrida, A.; Giantulli, S.; Silvestri, I.; Scarpa, S. Resistance to the mTOR inhibitor everolimus is reversed by the downregulation of survivin in breast cancer cells. Oncol. Lett. 2017, 14, 3832–3838. [Google Scholar] [CrossRef]
- Haines, R.J.; Pendleton, L.C.; Eichler, D.C. Argininosuccinate synthase: At the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011, 2, 8–23. [Google Scholar]
- Delage, B.; Luong, P.; Maharaj, L.; O’Riain, C.; Syed, N.; Crook, T.; Hatzimichael, E.; Papoudou-Bai, A.; Mitchell, T.J.; Whittaker, S.J.; et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012, 3, e342. [Google Scholar] [CrossRef]
- Ohshima, K.; Nojima, S.; Tahara, S.; Kurashige, M.; Hori, Y.; Hagiwara, K.; Okuzaki, D.; Oki, S.; Wada, N.; Ikeda, J.I.; et al. Argininosuccinate Synthase 1-Deficiency Enhances the Cell Sensitivity to Arginine through Decreased DEPTOR Expression in Endometrial Cancer. Sci. Rep. 2017, 7, 45504. [Google Scholar] [CrossRef]
- Sahu, D.; Gupta, S.; Hau, A.M.; Nakashima, K.; Leivo, M.Z.; Searles, S.C.; Elson, P.; Bomalaski, J.S.; Casteel, D.E.; Boss, G.R.; et al. Argininosuccinate Synthetase 1 Loss in Invasive Bladder Cancer Regulates Survival through General Control Nonderepressible 2 Kinase-Mediated Eukaryotic Initiation Factor 2alpha Activity and Is Targetable by Pegylated Arginine Deiminase. Am. J. Pathol. 2017, 187, 200–213. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huan, J.; Grivas, P.; Birch, J.; Hansel, D.E. Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy. Cancers 2022, 14, 1555. https://doi.org/10.3390/cancers14061555
Huan J, Grivas P, Birch J, Hansel DE. Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy. Cancers. 2022; 14(6):1555. https://doi.org/10.3390/cancers14061555
Chicago/Turabian StyleHuan, Jianya, Petros Grivas, Jasmine Birch, and Donna E. Hansel. 2022. "Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy" Cancers 14, no. 6: 1555. https://doi.org/10.3390/cancers14061555
APA StyleHuan, J., Grivas, P., Birch, J., & Hansel, D. E. (2022). Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy. Cancers, 14(6), 1555. https://doi.org/10.3390/cancers14061555






