Characterization of Cellular and Acellular Analytes from Pre-Cystectomy Liquid Biopsies in Patients Newly Diagnosed with Primary Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
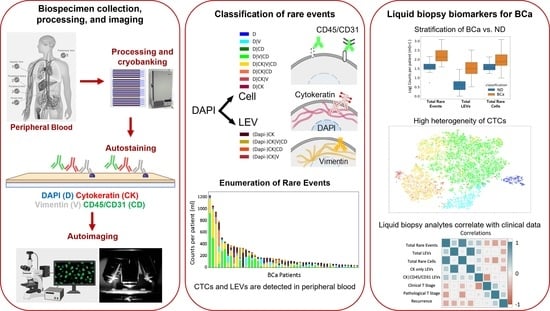
2.1. Study Design
2.2. Blood Sample Processing
2.3. Blood Sample Staining and Imaging
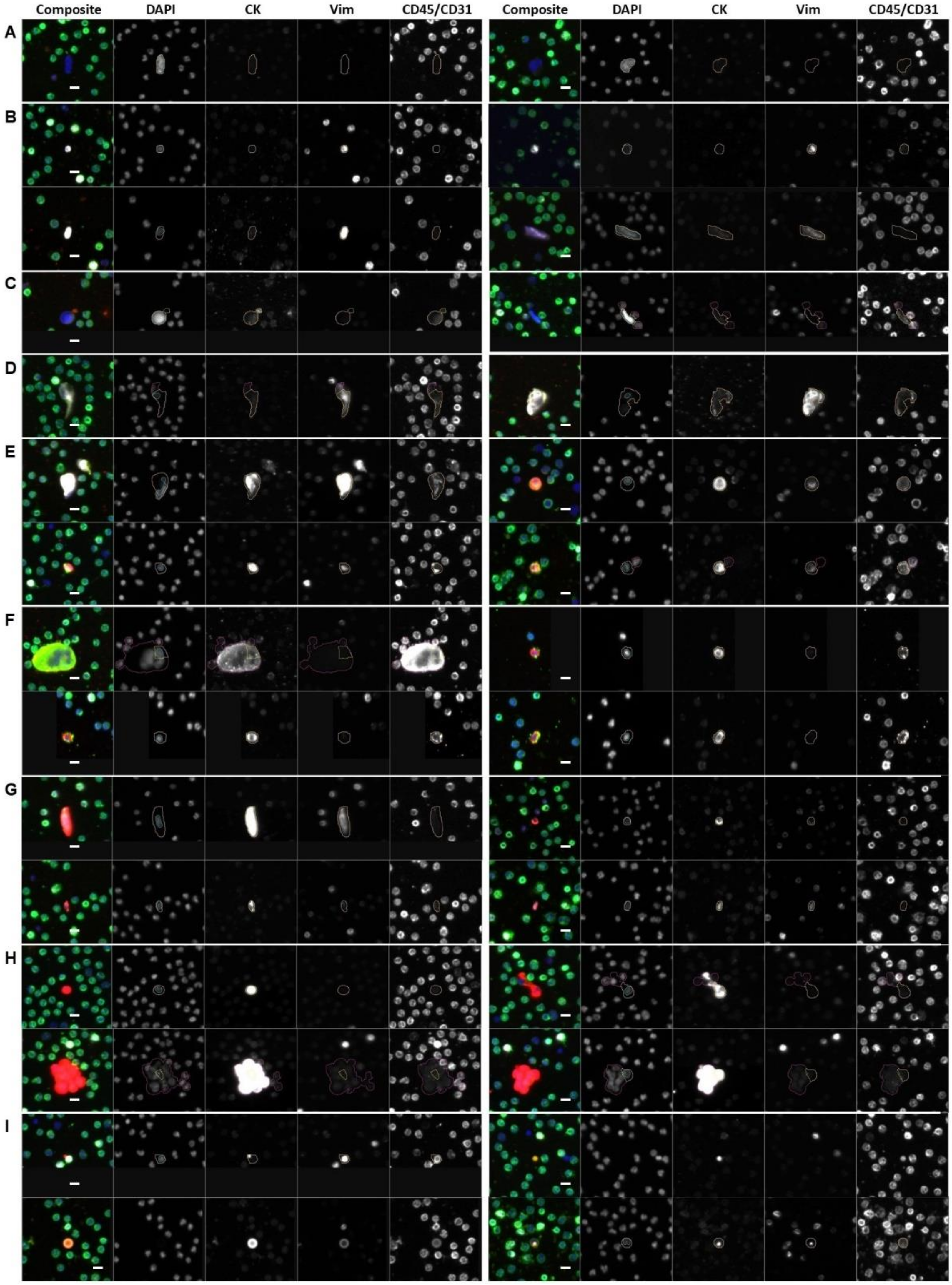
2.4. Rare Event Detection and Classification
2.5. Statistical Analysis
2.6. Patient Level Classification Modeling
3. Results
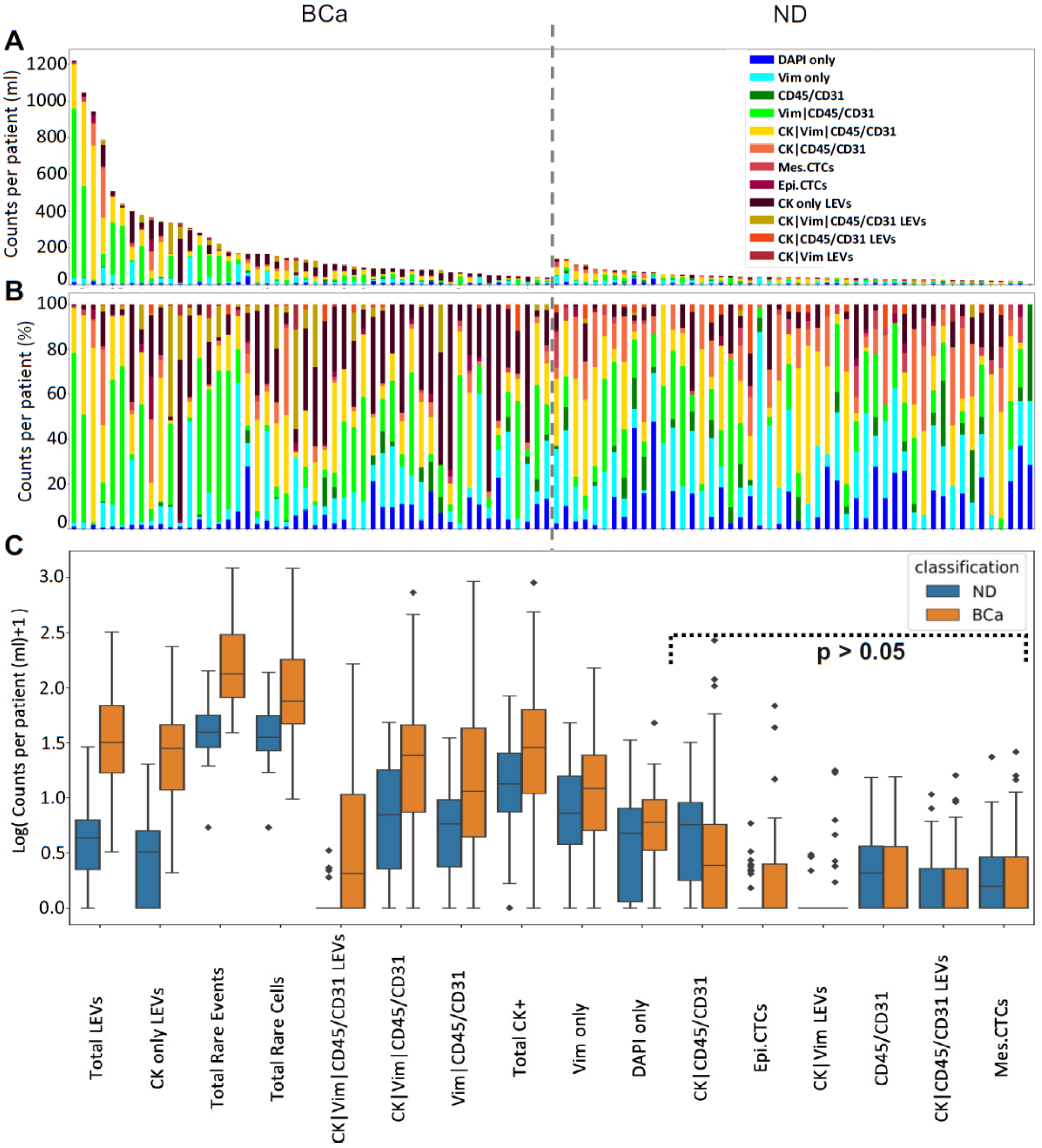
3.1. Liquid Biopsy Analysis Prior to Cystectomy
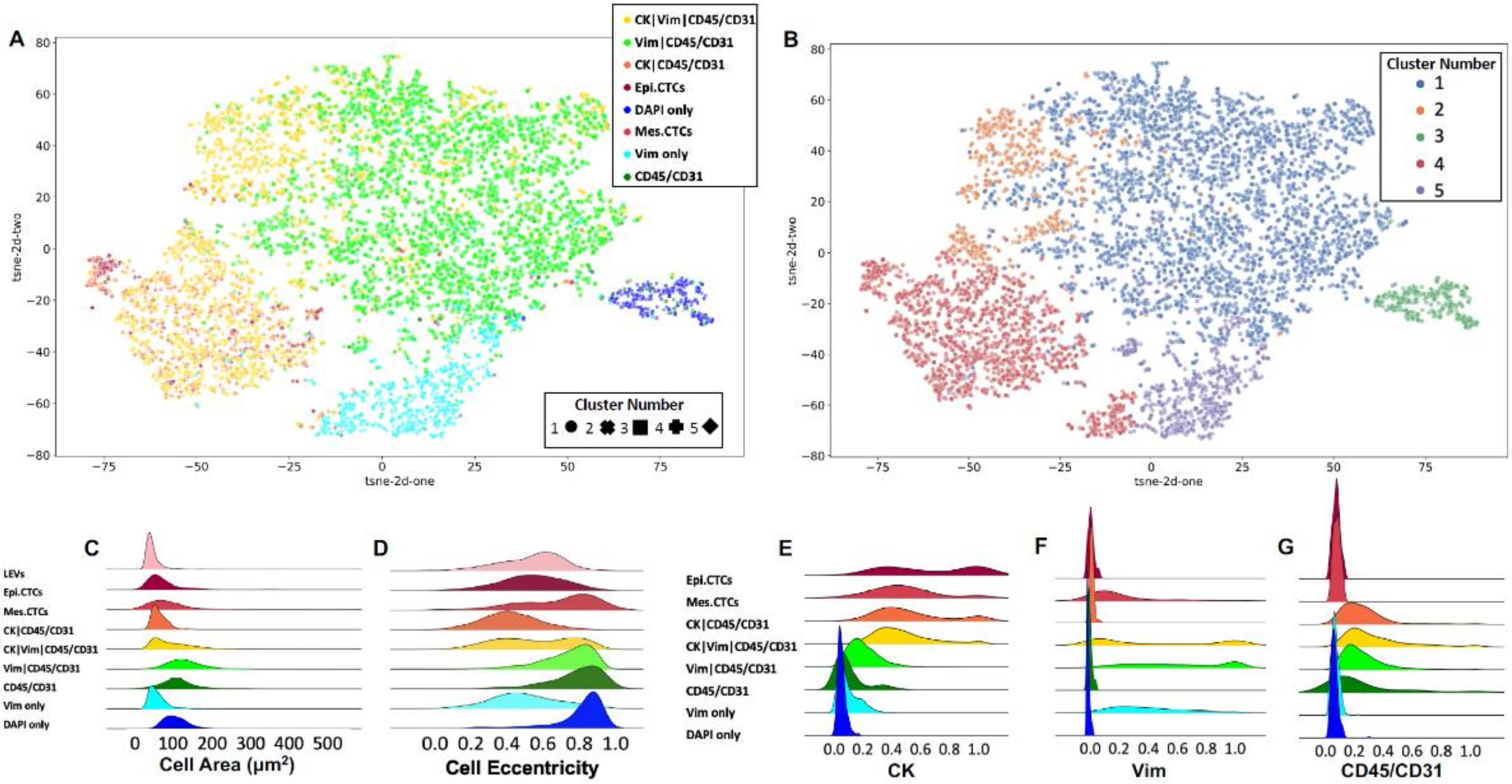
3.2. Rare Cell Characterization
3.3. LEV Detection
3.4. Keck Cohort with Clinical Data
3.5. Patient Level Classification Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel, D.E.; Amin, M.B.; Comperat, E.; Cote, R.J.; Knuchel, R.; Montironi, R.; Reuter, V.E.; Soloway, M.S.; Umar, S.A.; Van der Kwast, T.H. A contemporary update on pathology standards for bladder cancer: Transurethral resection and radical cystectomy specimens. Eur. Urol. 2013, 63, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, Z.; Mason, J.; Gill, K.; Miranda, G.; Gill, I.S.; Kuhn, P.; Newton, P.K. Machine learning models for predicting post-cystectomy recurrence and survival in bladder cancer patients. PLoS ONE 2019, 14, e0210976. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Hasnain, Z.; Miranda, G.; Gill, K.; Djaladat, H.; Desai, M.; Newton, P.K.; Gill, I.S.; Kuhn, P. Prediction of metastatic patterns in bladder cancer: Spatiotemporal progression and development of a novel, web-based platform for clinical utility. Eur. Urol. Open Sci. 2021, 32, 8–18. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Svatek, R.S.; Shariat, S.F.; Novara, G.; Skinner, E.C.; Fradet, Y.; Bastian, P.J.; Kamat, A.M.; Kassouf, W.; Karakiewicz, P.I.; Fritsche, H.M.; et al. Discrepancy between clinical and pathological stage: External validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011, 107, 898–904. [Google Scholar] [CrossRef]
- Blick, C.G.; Nazir, S.A.; Mallett, S.; Turney, B.W.; Onwu, N.N.; Roberts, I.S.; Crew, J.P.; Cowan, N.C. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: Results for 778 patients from a hospital haematuria clinic. BJU Int. 2012, 110, 84–94. [Google Scholar] [CrossRef]
- Lodewijk, I.; Dueñas, M.; Rubio, C.; Munera-Maravilla, E.; Segovia, C.; Bernardini, A.; Teijeira, A.; Paramio, J.M.; Suárez-Cabrera, C. Liquid biopsy biomarkers in bladder cancer: A current need for patient diagnosis and monitoring. Int. J. Mol. Sci. 2018, 19, 2514. [Google Scholar] [CrossRef] [Green Version]
- Busetto, G.M.; Ferro, M.; Del Giudice, F.; Antonini, G.; Chung, B.I.; Sperduti, I.; Giannarelli, D.; Lucarelli, G.; Borghesi, M.; Musi, G.; et al. The prognostic role of circulating tumor cells (CTC) in High-risk non-muscle-invasive bladder cancer. Clin. Genitourin. Cancer 2017, 15, e661–e666. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, W.; Deng, Q.; Tang, S.; Wang, P.; Xu, P.; Wang, J.; Yu, M. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta-analysis of 30 published studies. Oncotarget 2017, 8, 59527–59538. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef]
- Chai, S.; Matsumoto, N.; Storgard, R.; Peng, C.-C.; Aparicio, A.; Ormseth, B.; Rappard, K.; Cunningham, K.; Kolatkar, A.; Nevarez, R.; et al. Platelet-coated circulating tumor cells are a predictive biomarker in patients with metastatic castrate resistant prostate cancer. Mol. Cancer Res. 2021, 19, 2036–2045. [Google Scholar] [CrossRef]
- Malihi, P.D.; Graf, R.P.; Rodriguez, A.; Ramesh, N.; Lee, J.; Sutton, R.; Jiles, R.; Ruiz Velasco, C.; Sei, E.; Kolatkar, A.; et al. Single-cell circulating tumor cell analysis reveals genomic instability as a distinctive feature of aggressive prostate cancer. Clin. Cancer Res. 2020, 26, 4143–4153. [Google Scholar] [CrossRef]
- Malihi, P.D.; Morikado, M.; Welter, L.; Liu, S.T.; Miller, E.T.; Cadaneanu, R.M.; Knudsen, B.S.; Lewis, M.S.; Carlsson, A.; Velasco, C.R.; et al. Clonal diversity revealed by morphoproteomic and copy number profiles of single prostate cancer cells at diagnosis. Converg. Sci. Phys. Oncol. 2017, 4, 015003. [Google Scholar] [CrossRef]
- Kuhn, P.; Keating, S.M.; Baxter, G.T.; Thomas, K.; Kolatkar, A.; Sigman, C.C. Lessons learned: Transfer of the high-definition circulating tumor cell assay platform to development as a commercialized clinical assay platform. Clin. Pharmacol. Ther. 2017, 102, 777–785. [Google Scholar] [CrossRef]
- Rodríguez-Lee, M.; Kolatkar, A.; McCormick, M.; Dago, A.D.; Kendall, J.; Carlsson, N.A.; Bethel, K.; Greenspan, E.J.; Hwang, S.E.; Waitman, K.R.; et al. Effect of blood collection tube type and time to processing on the enumeration and high-content characterization of circulating tumor cells using the high-definition single-cell assay. Arch. Pathol. Lab. Med. 2018, 142, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Shishido, S.N.; Welter, L.; Rodriguez-Lee, M.; Kolatkar, A.; Xu, L.; Ruiz, C.; Gerdtsson, A.S.; Restrepo-Vassalli, S.; Carlsson, A.; Larsen, J.; et al. Preanalytical variables for the genomic assessment of the cellular and acellular fractions of the liquid biopsy in a cohort of breast cancer patients. J. Mol. Diagn. 2020, 22, 319–337. [Google Scholar] [CrossRef]
- Marrinucci, D.; Bethel, K.; Kolatkar, A.; Luttgen, M.S.; Malchiodi, M.; Baehring, F.; Voigt, K.; Lazar, D.; Nieva, J.; Bazhenova, L.; et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys. Biol. 2012, 9, 016003. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.; Kuhn, P.; Luttgen, M.S.; Dizon, K.K.; Troncoso, P.; Corn, P.G.; Kolatkar, A.; Hicks, J.B.; Logothetis, C.J.; Zurita, A.J. Paired high-content analysis of prostate cancer cells in bone marrow and blood characterizes increased androgen receptor expression in tumor cell clusters. Clin. Cancer Res. 2017, 23, 1722–1732. [Google Scholar] [CrossRef] [Green Version]
- Gerdtsson, A.S.; Setayesh, S.M.; Malihi, P.D.; Ruiz, C.; Carlsson, A.; Nevarez, R.; Matsumoto, N.; Gerdtsson, E.; Zurita, A.; Logothetis, C.; et al. Large extracellular vesicle characterization and association with circulating tumor cells in metastatic castrate resistant prostate cancer. Cancers 2021, 13, 1056. [Google Scholar] [CrossRef]
- Ruiz, C.; Li, J.; Luttgen, M.S.; Kolatkar, A.; Kendall, J.T.; Flores, E.; Topp, Z.; Samlowski, W.E.; McClay, E.; Bethel, K.; et al. Limited genomic heterogeneity of circulating melanoma cells in advanced stage patients. Phys. Biol. 2015, 12, 016008. [Google Scholar] [CrossRef] [Green Version]
- Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 1987, 100, 441–471. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons of grouped data by ranking methods. J. Econ. Entomol. 1946, 39, 269. [Google Scholar] [CrossRef]
- McIntosh, A.M.; Sharpe, M.; Lawrie, S.M. 9—Research Methods, Statistics And Evidence-Based Practice. In Companion to Psychiatric Studies, 8th ed.; Johnstone, E.C., Owens, D.C., Lawrie, S.M., McIntosh, A.M., Sharpe, M., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2010; pp. 157–198. [Google Scholar]
- Maaten, L.V.D.; Hinton, G.E. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Louppe, G.; Prettenhofer, P.; Weiss, R.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Ting, H.N.; Ng, K.H.; Ong, T.A. A review on the accuracy of bladder cancer detection methods. J. Cancer 2019, 10, 4038–4044. [Google Scholar] [CrossRef] [Green Version]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. 2015, 33, 66.e25–66.e31. [Google Scholar] [CrossRef]
- Gazzaniga, P.; Gradilone, A.; de Berardinis, E.; Busetto, G.M.; Raimondi, C.; Gandini, O.; Nicolazzo, C.; Petracca, A.; Vincenzi, B.; Farcomeni, A.; et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: A CellSearch analysis. Ann. Oncol. 2012, 23, 2352–2356. [Google Scholar] [CrossRef]
- Bethel, K.; Luttgen, M.S.; Damani, S.; Kolatkar, A.; Lamy, R.; Sabouri-Ghomi, M.; Topol, S.; Topol, E.J.; Kuhn, P. Fluid phase biopsy for detection and characterization of circulating endothelial cells in myocardial infarction. Phys. Biol. 2014, 11, 016002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.G.; Kong, M.Q.; Zhou, S.; Sheng, Y.F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, A.J.; Halabi, S.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Healy, P.; Anand, M.; Rothwell, C.J.; et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: The PROPHECY Study. J. Clin. Oncol. 2019, 37, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Dago, A.E.; Stepansky, A.; Carlsson, A.; Luttgen, M.; Kendall, J.; Baslan, T.; Kolatkar, A.; Wigler, M.; Bethel, K.; Gross, M.E.; et al. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS ONE 2014, 9, e101777. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; Winquist, E.; McLaughlin, B.; Lu, D.; Orr, S.; Fleisher, M.; Lowes, L.; Anderson, A.K.L.; et al. Validation of nuclear-localized AR-V7 on circulating tumor cells (CTC) as a treatment-selection biomarker for managing metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2018, 36, 273. [Google Scholar] [CrossRef]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016, 2, 1441–1449. [Google Scholar] [CrossRef]
- Thiele, J.A.; Bethel, K.; Kralickova, M.; Kuhn, P. Circulating tumor cells: Fluid surrogates of solid tumors. Annu. Rev. Pathol. 2017, 12, 419–447. [Google Scholar] [CrossRef]
- Birkenkamp-Demtroder, K.; Christensen, E.; Nordentoft, I.; Knudsen, M.; Taber, A.; Hoyer, S.; Lamy, P.; Agerbaek, M.; Jensen, J.B.; Dyrskjot, L. Monitoring treatment response and metastatic relapse in advanced bladder cancer by liquid biopsy analysis. Eur. Urol. 2018, 73, 535–540. [Google Scholar] [CrossRef]
- Birkenkamp-Demtroder, K.; Nordentoft, I.; Christensen, E.; Hoyer, S.; Reinert, T.; Vang, S.; Borre, M.; Agerbaek, M.; Jensen, J.B.; Orntoft, T.F.; et al. Genomic alterations in liquid biopsies from patients with bladder cancer. Eur. Urol. 2016, 70, 75–82. [Google Scholar] [CrossRef]
- Christensen, E.; Birkenkamp-Demtroder, K.; Nordentoft, I.; Hoyer, S.; van der Keur, K.; van Kessel, K.; Zwarthoff, E.; Agerbaek, M.; Orntoft, T.F.; Jensen, J.B.; et al. Liquid biopsy analysis of FGFR3 and PIK3CA hotspot mutations for disease surveillance in bladder cancer. Eur. Urol. 2017, 71, 961–969. [Google Scholar] [CrossRef]
- Patel, K.M.; van der Vos, K.E.; Smith, C.G.; Mouliere, F.; Tsui, D.; Morris, J.; Chandrananda, D.; Marass, F.; van den Broek, D.; Neal, D.E.; et al. Association of plasma and urinary mutant dna with clinical outcomes in muscle invasive bladder cancer. Sci. Rep. 2017, 7, 5554. [Google Scholar] [CrossRef] [Green Version]
- Chalfin, H.J.; Glavaris, S.A.; Gorin, M.A.; Kates, M.R.; Fong, M.H.; Dong, L.; Matoso, A.; Bivalacqua, T.J.; Johnson, M.H.; Pienta, K.J.; et al. Circulating tumor cell and circulating tumor dna assays reveal complementary information for patients with metastatic urothelial cancer. Eur. Urol. Oncol. 2021, 4, 310–314. [Google Scholar] [CrossRef] [Green Version]

| Variable | Category | Value |
|---|---|---|
| Age | 71.4 (53.4–86.1) | |
| BMI | 24.9 (21.2–36.9) | |
| Hgb | 11.1 (5.1–15.0) | |
| HCT | 34.2 (18.3–46.3) | |
| WBC | 7.6 (4.8–20.4) | |
| Platelets | 201.5 (57–387) | |
| BUN | 22.5 (13–70) | |
| Creatinine | 1.2 (0.5–3.1) | |
| Race | Caucasian | 20 |
| Asian | 2 | |
| Gender | Male | 18 |
| Female | 4 | |
| Smoker | Previous | 14 |
| Current | 4 | |
| Never | 4 | |
| Neoadjuvant Chemo | Yes | 10 |
| No | 12 | |
| Surgical Procedure | Anterior Exenteration | 1 |
| Radical Cystectomy | 4 | |
| Robotic Radical Cystectimy | 17 | |
| Urinary Diversion | Studer | 9 |
| Ileal Conduit | 11 | |
| Indiana Pouch | 2 | |
| Pure Urothelial (CS/PS) | 7/4 | |
| Predominant Histology (CS/PS) | No Tumor | 2/9 |
| Urothelial | 17/11 | |
| Other | 3/1 | |
| Plasmacytoid | 0/1 | |
| Squamous (CS/PS) | Absent | 16/12 |
| Present | 2/1 | |
| NA | 4/9 | |
| Glandular (CS/PS) | Absent | 16/12 |
| Present | 2/1 | |
| NA | 4/9 | |
| Neuro (CS/PS) | Absent | 18/12 |
| Present | 1/1 | |
| NA | 3/9 | |
| Subgroup (CS/PS) | OC | 16/15 |
| EV | 4/3 | |
| N+ | 2/4 | |
| T Stage (CS/PS) | T0 | 2/9 |
| Ta | 2/0 | |
| Tis | 1/4 | |
| T1 | 1/2 | |
| T2a | 11/0 | |
| T2b | 0/1 | |
| T3a | 0/3 | |
| T3b | 2/1 | |
| T4a | 3/2 | |
| N Stage (CS/PS) | NX | 2/0 |
| N0 | 19/18 | |
| N2 | 1/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishido, S.N.; Sayeed, S.; Courcoubetis, G.; Djaladat, H.; Miranda, G.; Pienta, K.J.; Nieva, J.; Hansel, D.E.; Desai, M.; Gill, I.S.; et al. Characterization of Cellular and Acellular Analytes from Pre-Cystectomy Liquid Biopsies in Patients Newly Diagnosed with Primary Bladder Cancer. Cancers 2022, 14, 758. https://doi.org/10.3390/cancers14030758
Shishido SN, Sayeed S, Courcoubetis G, Djaladat H, Miranda G, Pienta KJ, Nieva J, Hansel DE, Desai M, Gill IS, et al. Characterization of Cellular and Acellular Analytes from Pre-Cystectomy Liquid Biopsies in Patients Newly Diagnosed with Primary Bladder Cancer. Cancers. 2022; 14(3):758. https://doi.org/10.3390/cancers14030758
Chicago/Turabian StyleShishido, Stephanie N., Salmaan Sayeed, George Courcoubetis, Hooman Djaladat, Gus Miranda, Kenneth J. Pienta, Jorge Nieva, Donna E. Hansel, Mihir Desai, Inderbir S. Gill, and et al. 2022. "Characterization of Cellular and Acellular Analytes from Pre-Cystectomy Liquid Biopsies in Patients Newly Diagnosed with Primary Bladder Cancer" Cancers 14, no. 3: 758. https://doi.org/10.3390/cancers14030758
APA StyleShishido, S. N., Sayeed, S., Courcoubetis, G., Djaladat, H., Miranda, G., Pienta, K. J., Nieva, J., Hansel, D. E., Desai, M., Gill, I. S., Kuhn, P., & Mason, J. (2022). Characterization of Cellular and Acellular Analytes from Pre-Cystectomy Liquid Biopsies in Patients Newly Diagnosed with Primary Bladder Cancer. Cancers, 14(3), 758. https://doi.org/10.3390/cancers14030758