Late Cardiological Sequelae and Long-Term Monitoring in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Identification
2.2. Eligibility Criteria
2.3. Risk of Bias and Quality of Evidence Assessment
2.4. Study Selection and Data Extraction
2.5. Data Synthesis
3. Results
3.1. Incidence of CVD in cHL and DLBCL Survivors
3.2. Risk of Long-Term CVD with the Use of Less Cardiotoxic Therapies: Reduced-Field/Dose of Radiotherapy and Liposomal Doxorubicin
3.3. Preferable Cardiovascular Monitoring for cHL and DLBCL Long-Term Survivors
3.3.1. Detection, Monitoring, and Risk Factors for LV Dysfunction
Two-Dimensional Echocardiography and Echocardiographic Parameters Other Than LVEF to Early Detect LV Dysfunctions
Nonechocardiographic Imaging and Markers for the Early Detection of LV Dysfunctions: Magnetic Resonance Imaging (MRI), Cardiac Troponins, and Natriuretic Peptide Levels
3.3.2. Detection and Monitoring for CHD
3.3.3. Detection and Monitoring for Valvular Disorders
3.3.4. Detection and Monitoring for Pericardial Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armitage, J.O. Early-Stage Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 363, 653–662. [Google Scholar] [CrossRef]
- Van Leeuwen, F.E.; Ng, A.K. Late sequelae in Hodgkin lymphoma survivors. Hematol. Oncol. 2017, 35, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Minoia, C.; Bari, A.; Nassi, L.; Banzi, R.; Gerardi, C.; Lenti, V.; Calabrese, M.; Spina, M.; Guarini, A. Management of lymphoma survivor patients in Italy: An evaluation by Fondazione Italiana Linfomi. Tumori J. 2021, 107, 91–94. [Google Scholar] [CrossRef]
- Hu, C.-S.; Wu, Q.-H.; Hu, D.-Y. Cardiovascular, diabetes, and cancer strips: Evidences, mechanisms, and classifications. J. Thorac. Dis. 2014, 6, 1319–1328. [Google Scholar] [CrossRef]
- Ciavarella, S.; Minoia, C.; Quinto, A.M.; Oliva, S.; Carbonara, S.; Cormio, C.; Cox, M.C.; Bravo, E.; Santoro, F.; Napolitano, M.; et al. Improving Provision of Care for Long-term Survivors of Lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Minoia, C.; Gerardi, C.; Allocati, E.; Daniele, A.; De Sanctis, V.; Bari, A.; Guarini, A. The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2021, 13, 3135. [Google Scholar] [CrossRef] [PubMed]
- Galper, S.L.; Yu, J.; Mauch, P.M.; Strasser, J.F.; Silver, B.; LaCasce, A.; Marcus, K.J.; Stevenson, M.A.; Chen, M.H.; Ng, A.K. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011, 117, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, S.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; Van Leeuwen, F.E. Cardiovascular Disease After Hodgkin Lymphoma Treatment. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Moser, E.C.; Noordijk, E.M.; Van Leeuwen, F.E.; Le Cessie, S.; Baars, J.W.; Thomas, J.; Carde, P.; Meerwaldt, J.H.; Van Glabbeke, M.; Kluin-Nelemans, H.C. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 2006, 107, 2912–2919. [Google Scholar] [CrossRef]
- Hequet, O.; Le, Q.; Moullet, I.; Pauli, E.; Salles, G.; Espinouse, D.; Dumontet, C.; Thieblemont, C.; Arnaud, P.; Antal, D.; et al. Subclinical Late Cardiomyopathy After Doxorubicin Therapy for Lymphoma in Adults. J. Clin. Oncol. 2004, 22, 1864–1871. [Google Scholar] [CrossRef]
- Minoia, C.; De Fazio, V.; Scognamillo, G.; Scattone, A.; Maggialetti, N.; Ferrari, C.; Guarini, A. Long-Lasting Remission in De Novo Breast Myeloid Sarcoma Treated with Decitabine and Radiotherapy. Diagnostics 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, F.; Greco, G.; Cataldi, S.; Minoia, C.; Loseto, G.; Guarini, A. Effects of Physical Exercise Intervention on Psychological and Physical Fitness in Lymphoma Patients. Medicina 2019, 55, 379. [Google Scholar] [CrossRef] [Green Version]
- Majhail, N.S. Long-term complications after hematopoietic cell transplantation. Hematol. Stem Cell Ther. 2017, 10, 220–227. [Google Scholar] [CrossRef]
- Armenian, S.H.; Sun, C.-L.; Francisco, L.; Baker, K.S.; Weisdorf, D.J.; Forman, S.J.; Bhatia, S. Health behaviors and cancer screening practices in long-term survivors of hematopoietic cell transplantation (HCT): A report from the BMT Survivor Study. Bone Marrow Transplant. 2011, 47, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf (accessed on 5 June 2021).
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, B.K.A.; Osman, M.; Haykal, T.; Chahine, A.; Ahmed, S.; Osman, K.; Hassan, M.; Bachuwa, G.; Deepak, L.B. Meta-Analysis of Carvedilol for the Prevention of An-thracycline-Induced Cardiotoxicity. Am. J. Cardiol. 2018, 122, 1959–1964. [Google Scholar]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalam, K.; Marwick, T.H. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: A systematic review and meta-analysis. Eur. J. Cancer 2013, 49, 2900–2909. [Google Scholar] [CrossRef]
- Luminari, S.; Viel, E.; Ferreri, A.J.M.; Zaja, F.; Chimienti, E.; Musuraca, G.; Tucci, A.; Balzarotti, M.; Tani, M.; Salvi, F.; et al. Nonpegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi. Hematol. Oncol. 2018, 36, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Casadei, B.; Pellegrini, C.; Tonialini, L.; Argnani, L.; Zinzani, P.L. Interesting activity of pegylated liposomal doxorubicin in primary refractory and multirelapsed Hodgkin lymphoma patients: Bridge to transplant. Hematol. Oncol. 2018, 36, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Cuomo, A.; Della Pepa, R.; Ciervo, D.; Cella, L.; Pirozzi, F.; Parrella, P.; Campi, G.; Franco, R.; Varricchi, G.; et al. What Is the Cardiac Impact of Chemotherapy and Subsequent Radiotherapy in Lymphoma Patients? Antioxid. Redox Signal. 2019, 31, 1166–1174. [Google Scholar] [CrossRef]
- Ratosa, I.; IvaneticPantar, M. Cardiotoxicity of mediastinal radiotherapy. Rep. Pract. Oncol. Radiother. 2019, 24, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Specht, L.; Yahalom, J.; Illidge, T.; Berthelsen, A.K.; Constine, L.S.; Eich, H.T.; Girinsky, T.; Hoppe, R.T.; Mauch, P.; Mikhaeel, N.G.; et al. Modern Radiation Therapy for Hodgkin Lymphoma: Field and Dose Guidelines From the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. 2014, 89, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Negishi, T.; Coté, M.-A.; Penicka, M.; Massey, R.; Cho, G.-Y.; Hristova, K.; Vinereanu, D.; Popescu, B.A.; Izumo, M.; et al. Single Versus Standard Multiview Assessment of Global Longitudinal Strain for the Diagnosis of Cardiotoxicity During Cancer Therapy. JACC Cardiovasc. Imaging 2018, 11, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognosticvalue of troponin I in cardiacriskstratification of cancerpatientsundergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, D.; Ciceri, F.; Latini, R.; Franzosi, M.G.; Sandri, M.T.; Civelli, M.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Cavina, R.; et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International Cardio Oncology Society-one trial. Eur. J. Cancer 2018, 94, 126–137. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’Connel, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 July 2021).
- Stone, C.R.; Mickle, A.T.; Boyne, D.J.; Mohamed, A.; Rabi, D.M.; Brenner, D.R.; Friedenreich, C.M. Treatment for lymphoma and late cardiovascular disease risk: A systematic review and meta-analysis. Heal. Sci. Rep. 2019, 2, e135. [Google Scholar] [CrossRef]
- Holtzman, A.L.; Stahl, J.M.; Zhu, S.; Morris, C.G.; Hoppe, B.S.; Kirwan, J.E.; Mendenhall, N.P. Does the Incidence of Treatment-Related Toxicity Plateau After Radiation Therapy: The Long-Term Impact of Integral Dose in Hodgkin’s Lymphoma Survivors. Adv. Radiat. Oncol. 2019, 4, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Maraldo, M.V.; Giusti, F.; Vogelius, I.R.; Lundemann, M.; van der Kaaij, M.A.E.; Ramadan, S.; Meulemans, B.; Henry-Amar, M.; Aleman, B.M.P.; Raemaekers, J.; et al. Cardiovascular disease after treatment for Hodgkin’s lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015, 2, e492–e502. [Google Scholar] [CrossRef]
- Clavert, A.; Peric, Z.; Brissot, E.; Malard, F.; Guillaume, T.; Delaunay, J.; Dubruille, V.; Le Gouill, S.; Mahe, B.; Gastinne, T.; et al. Late Complications and Quality of Life after Reduced-Intensity Conditioning Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, F.A.; Ntentas, G.; Darby, S.C.; Schaapveld, M.; Hauptmann, M.; Lugtenburg, P.J.; Janus, C.P.M.; Daniels, L.; Van Leeuwen, F.E.; Cutter, D.J.; et al. Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood 2017, 129, 2257–2265. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Schaapveld, M.; Cutter, D.J.; Janus, C.P.; Krol, A.D.; Hauptmann, M.; Kooijman, K.; Roesink, J.; van der Maazen, R.; Darby, S.C.; et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2016, 34, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Sun, C.-L.; Shannon, T.; Mills, G.; Francisco, L.; Venkataraman, K.; Wong, F.L.; Forman, S.J.; Bhatia, S. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011, 118, 6023–6029. [Google Scholar] [CrossRef] [Green Version]
- Matasar, M.J.; Ford, J.S.; Riedel, E.R.; Salz, T.; Oeffinger, K.C.; Straus, D.J. Late Morbidity and Mortality in Patients with Hodgkin’s Lymphoma Treated During Adulthood. J. Natl. Cancer Inst. 2015, 107, djv018. [Google Scholar] [CrossRef] [PubMed]
- Cutter, D.J.; Schaapveld, M.; Darby, S.C.; Hauptmann, M.; Van Nimwegen, F.A.; Krol, S.; Janus, C.P.M.; Van Leeuwen, F.E.; Aleman, B.M.P. Risk for Valvular Heart Disease After Treatment for Hodgkin Lymphoma. J. Natl. Cancer Inst. 2015, 107, djv008. [Google Scholar] [CrossRef] [PubMed]
- Aleman, B.M.P.; van den Belt-Dusebout, A.W.; Bruin, M.L.d.; van’t Veer, M.B.; Baaijens, M.H.A.; de Boer, J.P.; Hart, A.A.M.; Klokman, W.J.; Kuenen, M.A.; Ouwens, G.M.; et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007, 109, 1878–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baech, J.; Hansen, S.M.; Lund, P.E.; Soegaard, P.; Brown, P.D.N.; Haaber, J.; Jørgensen, J.; Starklint, J.; Josefsson, P.; Poulsen, C.B.; et al. Cumulative anthracycline exposure and risk of cardiotoxicity; a Danish nationwide cohort study of 2440 lymphoma patients treated with or without anthracyclines. Br. J. Haematol. 2018, 183, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Patel, C.G.; Michaelson, E.; Chen, Y.-H.; Silver, B.; Marcus, K.J.; Stevenson, M.A.; Mauch, P.M.; Ng, A.K. Reduced Mortality Risk in the Recent Era in Early-Stage Hodgkin Lymphoma Patients Treated With Radiation Therapy With or Without Chemotherapy. Int. J. Radiat. Oncol. 2018, 100, 498–506. [Google Scholar] [CrossRef]
- Fu, J.; Upshaw, J.; Cohen, J.; Rodday, A.M.; Saunders, T.; Kelly, M.; Evens, A.M.; Parsons, S.K. Assessing the risk of cardiac toxicity after contemporary treatment for Hodgkin lymphoma: A systematic review. Leuk. Lymphoma 2017, 59, 1976–1980. [Google Scholar] [CrossRef]
- Kang, Y.; Xiao, F.; Chen, H.; Wang, W.; Shen, L.; Zhao, H.; Shen, X.; Chen, F.; He, B. Subclinical Anthracycline-Induced Cardiotoxicity in the Long-Term Follow-Up of Lymphoma Survivors: A Multi-Layer Speckle Tracking Analysis. Arq. Bras. Cardiol. 2018, 110, 219–228. [Google Scholar] [CrossRef]
- Machann, W.; Beer, M.; Breunig, M.; Störk, S.; Angermann, C.; Seufert, I.; Schwab, F.; Kölbl, O.; Flentje, M.; Vordermark, D. Cardiac Magnetic Resonance Imaging Findings in 20-year Survivors of Mediastinal Radiotherapy for Hodgkin’s Disease. Int. J. Radiat. Oncol. 2011, 79, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.T.; Russell, D.J.; Marwick, T.H. Long-term Risk of Heart Failure and Myocardial Dysfunction After Thoracic Radiotherapy: A Systematic Review. Can. J. Cardiol. 2015, 32, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-R.; Gjesdal, O.; Wethal, T.; Haugaa, K.; Fosså, A.; Fosså, S.D.; Edvardsen, T. Left Ventricular Function Assessed by Two-Dimensional Speckle Tracking Echocardiography in Long-Term Survivors of Hodgkin’s Lymphoma Treated by Mediastinal Radiotherapy with or Without Anthracycline Therapy. Am. J. Cardiol. 2011, 107, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Schnittger, I.; Strauss, H.W.; Vagelos, R.H.; Lee, B.K.; Mariscal, C.S.; Tate, D.J.; Horning, S.J.; Hoppe, R.T.; Hancock, S.L. Screening for Coronary Artery Disease After Mediastinal Irradiation for Hodgkin’s Disease. J. Clin. Oncol. 2007, 26, 43–49. [Google Scholar] [CrossRef]
- Murbraech, K.; Smeland, K.B.; Holte, H.; Loge, J.H.; Lund, M.B.; Wethal, T.; Holte, E.; Rösner, A.; Dalen, H.; Kvaløy, S.; et al. Heart Failure and Asymptomatic Left Ventricular Systolic Dysfunction in Lymphoma Survivors Treated With Autologous Stem-Cell Transplantation: A National Cross-Sectional Study. J. Clin. Oncol. 2015, 33, 2683–2691. [Google Scholar] [CrossRef]
- Armenian, S.H.; Mertens, L.; Slorach, C.; Venkataraman, K.; Mascarenhas, K.; Nathwani, N.; Wong, F.L.; Forman, S.J.; Bhatia, S. Prevalence of anthracycline-related cardiac dysfunction in long-term survivors of adult-onset lymphoma. Cancer 2017, 124, 850–857. [Google Scholar] [CrossRef]
- Andersen, R.; Wethal, T.; Günther, A.; Fosså, A.; Edvardsen, T.; Fosså, S.D.; Kjekshus, J. Relation of Coronary Artery Calcium Score to Premature Coronary Artery Disease in Survivors >15 Years of Hodgkin’s Lymphoma. Am. J. Cardiol. 2010, 105, 149–152. [Google Scholar] [CrossRef]
- Daniëls, L.A.; Krol, S.D.; De Graaf, M.A.; Scholte, A.J.; van Veer, M.B. Putter, H.; De Roos, A.; Schalij, M.J.; Van De Poll-Franse, L.V.; Creutzberg, C.L. Impact of Cardiovascular Counseling and Screening in Hodgkin Lymphoma Survivors. Int. J. Radiat. Oncol. 2014, 90, 164–171. [Google Scholar] [CrossRef]
- Wethal, T.; Lund, M.-B.; Edvardsen, T.; Fossa, S.D.; Pripp, A.H.; Holte, H.; Kjekshus, J.; Fossa, A. Valvular dysfunction and left ventricular changes in Hodgkin’s lymphoma survivors. A longitudinal study. Br. J. Cancer 2009, 101, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijl, J.M.; Roos, M.; van Leeuwen-Segarceanu, E.M.; Vos, J.M.; Bos, W.J.W.; Biesma, D.H.; Post, M.C. Assessment of Valvular Disorders in Survivors of Hodgkin’s Lymphoma Treated by Mediastinal Radiotherapy ± Chemotherapy. Am. J. Cardiol. 2016, 117, 691–696. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Lee, B.K.; Mariscal, C.S.; Schnittger, I. Asymptomatic cardiac disease following mediastinal irradiation. J. Am. Coll. Cardiol. 2003, 42, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Hershman, D.L.; McBride, R.B.; Eisenberger, A.; Tsai, W.Y.; Grann, V.R.; Jacobson, J.S. Doxorubicin, Cardiac Risk Factors, and Cardiac Toxicity in Elderly Patients with Diffuse B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2008, 26, 3159–3165. [Google Scholar] [CrossRef]
- Olivieri, J.; Perna, G.P.; Bocci, C.; Montevecchi, C.; Olivieri, A.; Leoni, P.; Gini, G. Modern Management of Anthracycline-Induced Cardiotoxicity in Lymphoma Patients: Low Occurrence of Cardiotoxicity with Comprehensive Assessment and Tailored Substitution by Nonpegylated Liposomal Doxorubicin. Oncology 2017, 22, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, B.A.; Hornby, C.; Cunninghame, J.; Burns, M.; MacManus, M.; Ryan, G.; Lau, E.; Seymour, J.F.; Wirth, A. Minimising critical organ irradiation in limited stage Hodgkin lymphoma: A dosimetric study of the benefit of involved node radiotherapy. Ann. Oncol. 2012, 23, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Bolzan, C.; D’Arienzo, M.; Bracci, S.; Fanelli, A.; Cox, M.C.; Valeriani, M.; Osti, M.F.; Minniti, G.; Chiacchiararelli, L.; et al. Intensity modulated radiotherapy in early stage Hodgkin lymphoma patients: Is it better than three dimensional conformal radiotherapy? Radiat. Oncol. 2012, 7, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiandra, C.; Filippi, A.R.; Catuzzo, P.; Botticella, A.; Ciammella, P.; Franco, P.; Borca, V.C.; Ragona, R.; Tofani, S.; Ricardi, U. Different IMRT solutions vs. 3D-Conformal Radiotherapy in early stage Hodgkin’s lymphoma: Dosimetric comparison and clinical considerations. Radiat. Oncol. 2012, 7, 186. [Google Scholar] [CrossRef] [Green Version]
- Kriz, J.; Spickermann, M.; Lehrich, P.; Schmidberger, H.; Reinartz, G.; Eich, H.; Haverkamp, U. Breath-hold technique in conventional APPA or intensity-modulated radiotherapy for Hodgkin’s lymphoma. Strahlenther. Onkol. 2015, 191, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.R.; on behalf of the Fondazione Italiana Linfomi (FIL) Radiotherapy Committee; Meregalli, S.; DI Russo, A.; Levis, M.; Ciammella, P.; Buglione, M.; Guerini, A.E.; De Marco, G.; De Sanctis, V.; et al. Fondazione Italiana Linfomi (FIL) expert consensus on the use of intensity-modulated and image-guided radiotherapy for Hodgkin’s lymphoma involving the mediastinum. Radiat. Oncol. 2020, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baues, C.; Marnitz, S.; Engert, A.; Baus, W.; Jablonska, K.; Fogliata, A.; Vásquez-Torres, A.; Scorsetti, M.; Cozzi, L. Proton versus photon deep inspiration breath hold technique in patients with hodgkin lymphoma and mediastinal radi-ation: A planning comparison of deep inspiration breath hold intensity modulation radiotherapy and intensity modulated proton therapy. Radiat Oncol. 2018, 13, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Moran, J.M.; Koelling, T.; Chughtai, A.; Chan, J.L.; Freedman, L.; Hayman, J.A.; Jagsi, R.; Jolly, S.; Larouere, J.; et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Duane, F.; Aznar, M.C.; Bartlett, F.; Cutter, D.J.; Darby, S.C.; Jagsi, R.; Lorenzen, E.L.; McArdle, O.; McGale, P.; Myerson, S.; et al. A cardiac contouring atlas for radiotherapy. Radiother. Oncol. 2017, 122, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Levis, M.; Filippi, A.R.; Fiandra, C.; De Luca, V.; Bartoncini, S.; Vella, D.; Ragona, R.; Ricardi, U. Inclusion of heart substructures in the optimization process of volumetric modulated arc therapy techniques may reduce the risk of heart disease in Hodgkin’s lymphoma patients. Radiother. Oncol. 2019, 138, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Villarraga, H.R.; Herrmann, J.; Nkomo, V.T. Cardio-Oncology: Role of Echocardiography. Prog. Cardiovasc. Dis. 2014, 57, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Wintersperger, B.J.; Flamm, S.D.; Marwick, T.H. Cardiac MRI in the Assessment of Cardiac Injury and Toxicity From Cancer Chemotherapy. Circ. Cardiovasc. Imaging 2013, 6, 1080–1091. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Sanft, T.; Moslehi, J.J.; Overholser, L.; Armenian, S.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; et al. NCCN Guidelines Insights: Survivorship, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Meijboom, W.B.; van Mieghem, C.A.; Mollet, N.R.; Pugliese, F.; Weustink, A.C.; van Pelt, N.; Cademartiri, F.; Nieman, K.; Boersma, E.; de Jaegere, P.; et al. 64-Slice Computed Tomography Coronary Angiography in Patients With High, Intermediate, or Low Pretest Probability of Significant Coronary Artery Disease. J. Am. Coll. Cardiol. 2007, 50, 1469–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen-Segarceanu, E.M.; Bos, W.-J.W.; Dorresteijn, L.D.; Rensing, B.J.; van der Heyden, J.A.; Vogels, O.J.; Biesma, D.H. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat. Rev. 2011, 37, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, C.; Mariani, J.; Wheeler, G.; Kaye, D.M. Cardiac Complications of Thoracic Irradiation. J. Am. Coll. Cardiol. 2013, 61, 2319–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
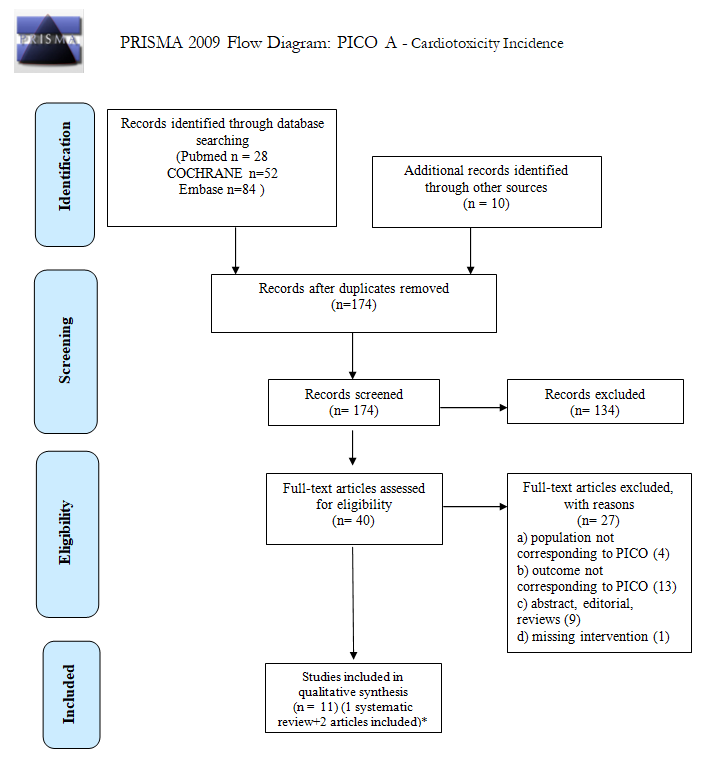
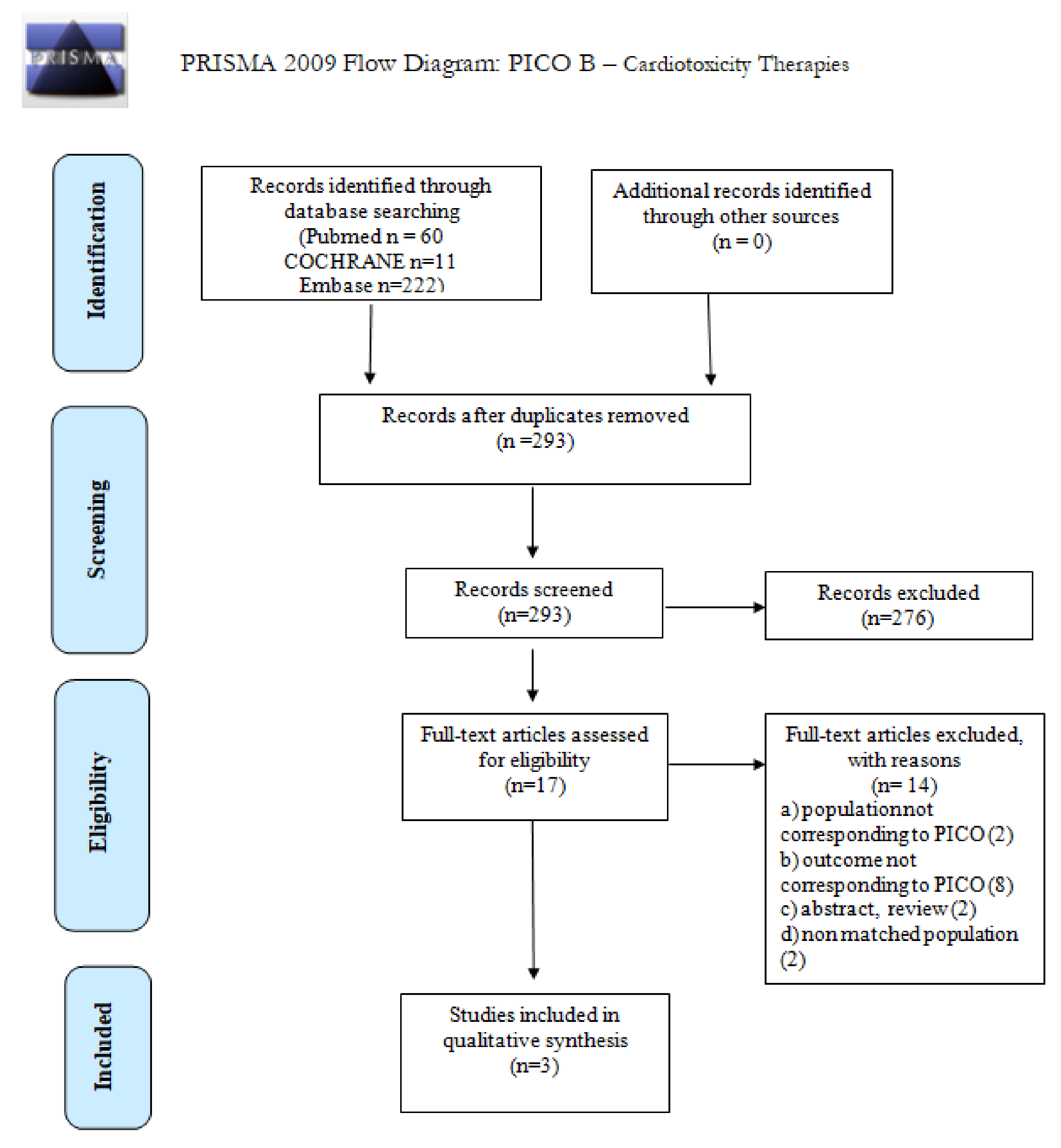
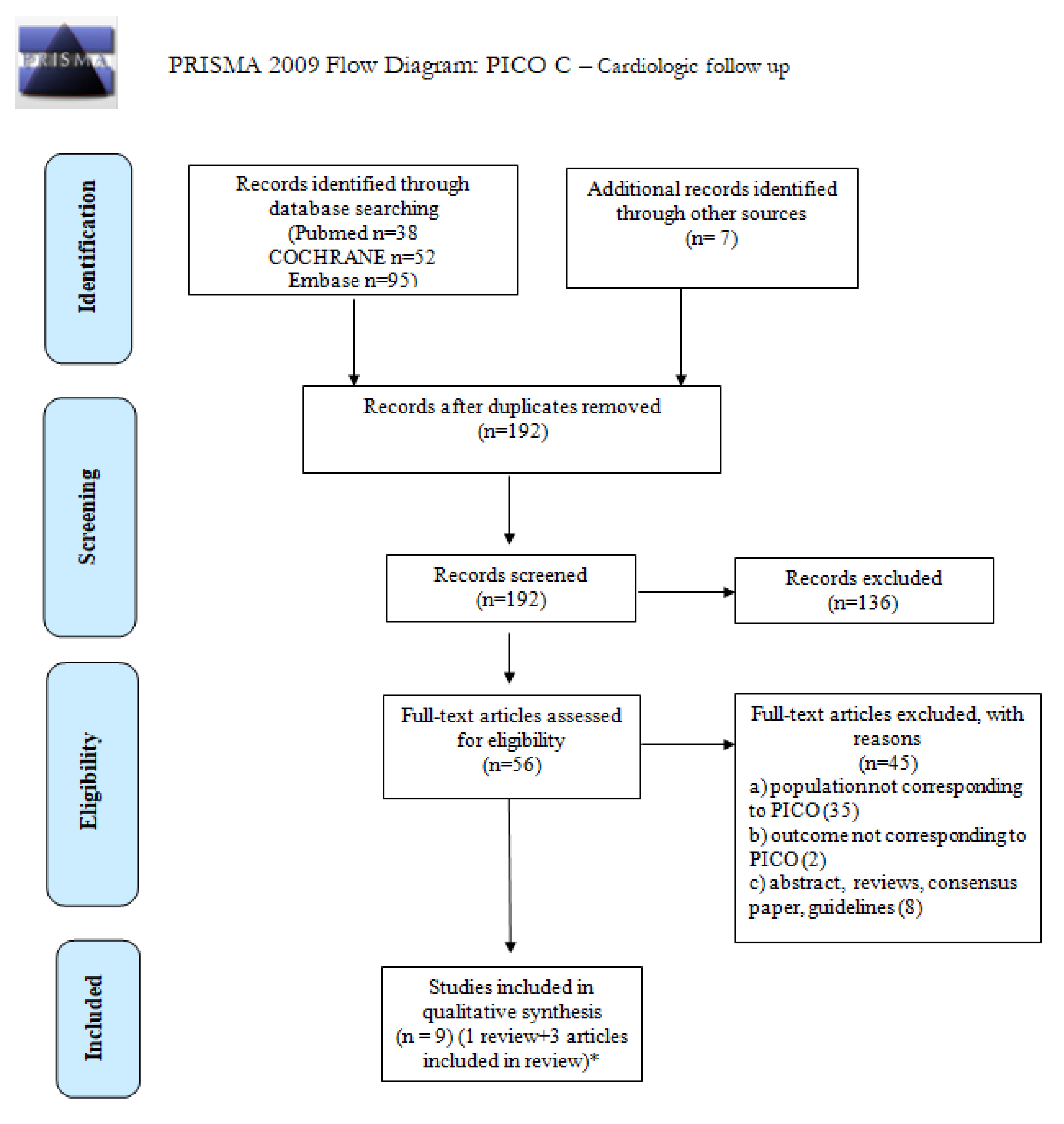
| What is the incidence of CVD in long-term survivors of cHL or DLBCL after first-line treatment? | P: patient population in long-term survivors of cHL or DLBCL (>5 years free of disease and treatment), with age >18 years at diagnosis; |
| I: chemotherapy or chemotherapy + standard dose radiotherapy; | |
| C1: homogeneous population for age and sex untreated (control group); | |
| C2: long-term survivors of cHL or DLBCL (>5 years free of disease and treatment) treated with different therapeutic regimens, without anthracyclines; | |
| O: diagnosis of cardiotoxicity of any degree; | |
| S: RCTs, retrospective registry studies, (controlled) cohort studies, and any reviews of such studies. | |
| What is the incidence of CVD in long-term survivors of cHL or DLBCL after second-line treatment (including ASCT)? | P: patient population in long-term survivors of cHL or DLBCL (>5 years free of disease and treatment), with age >18 years at diagnosis; |
| I: second-line chemotherapy and autologous transplantation, radiotherapy; | |
| C1: homogeneous population for age and sex untreated (control group); | |
| C2: long-term survivors of cHL or DLBCL (>5 years free of disease and treatment) treated with other chemotherapy/radiation treatment regimens; | |
| O: diagnosis of cardiotoxicity of any degree; | |
| S: RCTs, retrospective registry studies, (controlled) cohort studies, and any reviews of such studies. | |
| Has the incidence of cardiotoxicity in long-surviving patients with cHL or DLBCL changed with the introduction of modern radiotherapy and liposomal doxorubicin after first-line treatment? | P: patient population in long-term survivors of cHL or DLBCL (>5 years free of disease and treatment), with age >18 years at diagnosis; |
| I1: new chemotherapy approaches; | |
| I2: new radiotherapy approaches; | |
| C1: previous chemotherapy/radiotherapy regimens | |
| O: diagnosis of cardiotoxicity of any degree; | |
| S: RCTs, retrospective registry studies, (controlled) cohort studies, and any reviews of such studies. | |
| Has the incidence of cardiotoxicity in long-surviving patients with cHL or DLBCL changed with the introduction of modern radiotherapy after second-line treatment (including ASCT)? | P: patient population in long-term survivors of cHL or DLBCL (>5 years free of disease and treatment), with age >18 years at diagnosis; |
| I1: new radiotherapy approaches; | |
| C: previous radiotherapy regimens | |
| O: diagnosis of cardiotoxicity of any degree; | |
| S: RCTs, retrospective registry studies, (controlled) cohort studies, and any reviews of such studies. | |
| How to perform an optimal cardio-oncological follow-up in long-term survivors of cHL or DLBCL after first-line treatment? Which parameters to evaluate and how often? | P: patient population in long-term survivors of cHL or DLBCL (>5 years free of disease and treatment), with age >18 years at diagnosis; |
| I1: multiparametric assessment that includes specialist cardiology visit and calculation of LVEF with conventional echocardiography, strain-rate calculation, monitoring with biomarkers, monitoring with MRI, monitoring with MUGA-scan; | |
| I2: timing validation of the cardiological assessment during the follow-up; | |
| C: specialist cardiology visits and calculation of LVEF with conventional echocardiography; | |
| O: diagnosis of cardiotoxicity of any degree; | |
| S: RCTs, retrospective registry studies, (controlled) cohort studies, and any reviews of such studies. | |
| How to perform an optimal cardio-oncological follow-up in long-term survivors of cHL or DLBCL after second-line treatments (including ASCT)? Which parameters to evaluate and how often? | P: patient population in long-term survivors of cHL or DLBCL (>5 years free from disease and treatments), with age >18 years at diagnosis, already treated in the first line with chemotherapy/radiotherapy according to standard schedules and dosages; |
| I1: multiparametric evaluation that includes specialist cardiology visit and calculation of LVEF with conventional echocardiography, strain-rate calculation, monitoring with biomarkers, monitoring with MRI, monitoring with MUGA-scan; | |
| I2: validation of the timing of the cardiological assessment during the follow-up; | |
| C: specialist cardiology visits and calculation of LVEF with conventional echocardiography; | |
| O: diagnosis of cardiotoxicity of any degree; | |
| S: RCTs, retrospective registry studies, (controlled) cohort studies, and any reviews of such studies |
| Summary of Findings | ||||
|---|---|---|---|---|
| PICO 1 | ||||
| Study | Study Design and Sample Size | Intervention and Comparison | Primary Outcomes | Secondary Outcomes |
| Holtzman A.L. 2019 [34] | Retrospective cohort study (365 cHL patients) | Primary RT | 9% of CHD incidence at 10 years, 15% at 20 years, 26% at 30 years, 30% at 40 years of follow-up | - |
| Maraldo M.V. 2015 [35] | Retrospective cohort study (6030 cHL patients) | Primary CT with anthracycline and vinca-alkaloid; RT | 19% of CHD, 12% CHF, 16% arrhythmia, 11% VHD incidence | The mean heart radiation dose per 1 Gy increase and the dose of anthracylines were significant predictors of CHD |
| Clavert A. 2016 [36] | Retrospective cohort study (110 cHL e NHL) | Allotransplant | 47% of CVD incidence at 10 years (95% CI 35–59), and main presentations were heart failure (14%) and arterial hypertension (7%) | - |
| van Nimwegen F.A. 2015 [8] | Retrospective cohort study (2524 cHL patients) | RT only, or RT and CT (with or without anthracycline), or CT only (with or without anthracycline) | The 40-year cumulative incidence of CVD was 50% (95% CI, 47–52%) | - |
| van Nimwegen F.A. 2017 [37] | Case-control study (2617 cHL patients) | RT; anthracycline-containing CT; without anthracycline-containing CT | 25-year cumulative risks of heart failure following mean left ventricular doses of 0–15 Gy, 16–20 Gy, and ≥21 Gy were 4.4%, 6.2%, and 13.3%, respectively, in patients treated without anthracycline-containing CT; and 11.2%, 15.9%, and 32.9%, respectively, in patients treated with anthracyclines | - |
| van Nimwegen F.A. 2016 [38] | Nested case-control study (2617 cHL) | RT; anthracycline-containing CT; without anthracycline-containing CT | 2.5-fold risk of CHD for patients receiving a MHD of 20 Gy from mediastinal radiotherapy | Risk of CHD increased linearly with increasing MHD (excess relative risk [ERR]) per Gray, 7.4%; 95% CI, 3.3% to 14.8%) |
| Galper S.L. 2011 [7] | Retrospective cohort study (1279 patients) | Mediastinal RT or mediastinal RT and CT | 5-, 10-, 15-, and 20-year cumulative incidence rates of cardiac events were 2.2%, 4.5%, 9.6%, 16% | - |
| Armenian S.H. 2011 [39] | Retrospective cohort study (NHL 598, cHL 284 patients) Case-control study (88 patients) | Hematopoietic cell transplantation or conventional therapy | The incidence of CHF for HCT survivors was increased 4.5-fold compared with the controls | The cumulative incidence of CHF was 4,8% at 5 years and 9.1% at 15 years after HCT |
| Stone C.R. 2019 [33] | Systematic review and meta-analysis (22 studies, total of 32,438 patients) | Treatment for lymphoma | Relative to the general population, lymphoma survivors had statistically significant 2- to 3-fold increases in the risk for CVD | - |
| Matasar M.J. 2015 [40] | Retrospective cohort study (746 cHL patients) | First-line therapy for HL | Mortality: 30.4% of patients had died, 47.1% from HL, and 52.9% from other causes, including second primary malignancies (n2) and CVD (27) | Cardiovascular morbidity (54.6%) |
| Cutter D.J. 2015 [41] | Case-control study (1852 cHL patients) | RT | VHD incidence: 89 case patients with VHD were identified (66 severe or life-threatening) | For doses above 30 Gy the percentage growth in VHD rate per Gy increases progressively with increasing dose |
| Aleman B.M. 2007 [42] | Retrospective cohort study (1474 cHL patients) | RT only (27.5%), CT only (4.8%), RT + CT-anthracylines (29.5%), RT + CT no anthracylines (37.9%), unknown (8.2%) | The 25-year cumulative incidence of CHF after mediastinal RT and anthracyclines in competing risk analyses was 7.9% | - |
| Baech J. 2018 [43] | Retrospective Cohort study (2440 NHL patients) | Anthracycline-containing CT (R-CHOP or R-CHOEP); CT without anthracyclines | Patients treated with 3–5 cycles of R-CHOP/CHOEP had risks of CVD at 1, 5, and 8 years of 2.5%, 10.5%, and 17.2%, respectively | - |
| PICO 2 | ||||
| Study (Reference) | Study Design and Sample size | Intervention and Comparison | Primary Outcomes | Secondary Outcomes |
| Patel C.G. 2017 [44] | Retrospective cohort study (1541 cHL patients) | 1968–1982: RT field TNI 11.6%, mantle/para-aortic 73.4%; mantle 9.2%, inverted Y/pelvis 4.1%, IFRT 1.6%; 1983–1992: RT fields TNI 1,4 mantle/paraaortic 59.4%; mantle 33.1%, inverted Y/pelvis 3.2%, IFRT 3.0%; 1993–2007: RT fields TNI 0%, mantle/para-aortic 5.6%, mantle 40.9%, inverted Y/pelvis 3.4%, IFRT 42.2%, 0 | 15-years OS rates were 78%, 85%, and 88% (p < 0.01) according treatment periods | - |
| Fu J. 2017 [45] | Systematic review (articles from 1990–2016) | No studies fulfilled all review criteria (assessing the risk of cardiac toxicity after contemporary treatment for HL) | - | |
| Matasar M.J. 2015 [40] | Retrospective cohort study (746 cHL patients) | CT containing anthracycline; RT in doses from 20 to 36 Gy | Patients treated with less than 35 Gy did not have lower rates od CHD | - |
| PICO 3 | ||||
| Study | Study Design and Sample Size | Intervention and Comparison | Primary Outcomes | Secondary Outcomes |
| Kang Y. 2018 [46] | Cohort study (45 NHL patients) | Echocardiographic imaging; multilayer speckle tracking echocardiography | Compared with controls, patients had no different conventional parameters of systolic and diastolic function, but significantly lower GCS and GLS, significant reduction of circumferential strain (CS) of subendocardial layer, transmural CS gradient, and longitudinal strain of all three layers | In contrast, the two groups did not differ in transmural longitudinal strain gradient and radial strains |
| Machann W. 2011 [47] | Cohort study (31 cHL patients) | MRI in patients treated with mediastinal RT | Pathologic findings were reduced LVEF (<55%) in 23%of patients, hemodynamically relevant VHD in 42%, late myocardial enhancement in 29%, and any perfusion deficit in 68% of patients | - |
| Nolan M.T. 2016 [48] | Systematic review (21 studies, total of 1659 patients) | 10 studies used transthoracic echocardiography (TTE), 8 used radionuclide ventriculography (RNV), 2 used TTE and RNV, and 1 used cardiac MRI | LVEF presented limitations for the identification of mild or subclinical LV systolic dysfunction | 2D speckle tracking strain, showed more accuracy and reproducibility in diagnosing subclinical systolic dysfunction |
| Tsai H.R. 2010 [49] | Cohort study (47 cHL patients) | Left ventricular function assessed by 2D speckle tracking echocardiography | The global longitudinal strain was reduced in patients receiving anthracycline with mediastinal RT compared to the other group receiving mediastinal RT alone or combined RT and regimens without anthracyclines (16.1 1.9% vs. 17.5 1.7%, respectively, p < 0.05). Both patient groups had reduced strain compared to the healthy controls (20.4 1.7%, both p < 0.001). The circumferential strain was also reduced in the treatment groups (18.3 3.2% and 17.8 3.6% vs. 22.5 2.1%, both p < 0.001) | The LV ejection fraction did not differ between the patient groups but was reduced compared to that of the controls |
| Heidenreich P.A. 2007 [50] | Cohort study (294 cHL patients) | Stress echocardiography (97% exercise, 3% dobutamina) in all patients; in 274 patients, radionuclide perfusion imaging | A 2.7% prevalence of severe, three-vessel, or left main coronary artery disease, and a 7.5% prevalence of coronary stenosis greater than 50% in patients treated with mediastinal RT in doses of ≥35 Gy for HL at a mean of 15 years following irradiation | - |
| Murbreach K. 2015 [51] | Prospective cross-sectional study (274 cHL patients) | Echocardiographic imaging; LVEF assessed by Simpson’s biplane rule | In the multivariable analysis, only doxorubicin ≥ 300 mg/m2 (OR, 3.3; 95% CI, 1.2 to 8.9; p = 0.02) and cardiac-RT more than 30 Gy (OR, 4.3; 95% CI, 1.7 to 11.4; p = 0.003) remained significantly associated with LV systolic dysfunction | - |
| Armenian S.H. 2018 [52] | Cohort study (155 cHL and NHL patients) | Monodimensional/2D echocardiographic imaging; 2D speckle tracking echocardiography | At a median follow-up of 9.4 years from diagnosis, one in five (20.6%) lymphoma survivors had cardiac dysfunction; The prevalence of reduced LVEF, diastolic dysfunction, and abnormal GLS was 8.4%, 5.2%, and 14.2%, respectively | A dose-dependent association with cumulative exposure to anthracycline was shown in comparison to not exposed controls: 1–249 mg/m2, OR = 4.7 (95% CI 1.0–17.4), p = 0.05; ≥250 mg/m2, OR = 7.6 (95% CI 2.7–24.3), p < 0.01 |
| Andersen R. 2010 [53] | Cohort study (47 cHL patients) | CAC-score CTA | The prevalence rate of significant CHD on CTA was 20% (n = 9, p = 0.01, 95% confidence interval 8.3% to 31.7%) | Patients with a CAC-score >200 often have clinically important CAD (55% compared with 17%) |
| Daniels L.A. 2014 [54] | Cohort study (52 cHL patients) | CAC-score CTA | Patients with relevant CHD on CTA more often had high CAC scores (75th–100th percentile) than patients who had no severe anomalies on the CTA scan (55% compared with 17%) | - |
| Wethal T. 2009 [55] | Cohort study (116 cHL patients) | 2D echocardiographic imaging | cHL patients treated with mediastinal RT were at a high risk of developing growing valvular impairment and aortic stenosis 20 years after initial therapy | - |
| Bijl J.M. 2016 [56] | Cross-sectional study (82 cHL patients) | 2D echocardiographic imaging | ≥mild valvular disease was present in 61.2% of HL survivors with mediastinal RT (n = 30), compared with 31.0% of HL survivors without MRT (n = 9; odds ratio (OR) 3.51, 95% CI 1.32 to 9.30, p = 0.01) | - |
| Heidenreich P.A. 2003 [57] | Cohort study (254 cHL patients) | Monodimensional/2D echocardiographic imaging | 21% had a thickened pericardium, and small pericardial effusions were present in 3%, no patients had wall-motion abnormalities or Doppler findings suggestive of constrictive pericarditis | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, S.; Puzzovivo, A.; Gerardi, C.; Allocati, E.; De Sanctis, V.; Minoia, C.; Skrypets, T.; Guarini, A.; Gini, G. Late Cardiological Sequelae and Long-Term Monitoring in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2022, 14, 61. https://doi.org/10.3390/cancers14010061
Oliva S, Puzzovivo A, Gerardi C, Allocati E, De Sanctis V, Minoia C, Skrypets T, Guarini A, Gini G. Late Cardiological Sequelae and Long-Term Monitoring in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi. Cancers. 2022; 14(1):61. https://doi.org/10.3390/cancers14010061
Chicago/Turabian StyleOliva, Stefano, Agata Puzzovivo, Chiara Gerardi, Eleonora Allocati, Vitaliana De Sanctis, Carla Minoia, Tetiana Skrypets, Attilio Guarini, and Guido Gini. 2022. "Late Cardiological Sequelae and Long-Term Monitoring in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi" Cancers 14, no. 1: 61. https://doi.org/10.3390/cancers14010061
APA StyleOliva, S., Puzzovivo, A., Gerardi, C., Allocati, E., De Sanctis, V., Minoia, C., Skrypets, T., Guarini, A., & Gini, G. (2022). Late Cardiological Sequelae and Long-Term Monitoring in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi. Cancers, 14(1), 61. https://doi.org/10.3390/cancers14010061






