Antibiotic Prophylaxis or Granulocyte-Colony Stimulating Factor Support in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Anti-Infective Strategies
2.2. Patient Cohort
2.3. Data Collection
2.4. Data Extraction of Bacterial Isolates and Resistance Rates in the Hematology Department and Transplant Unit between 2015 and 2019
2.5. Statistical Analyses
- (I)
- Antibiotic prophylaxis: median duration of inpatient stay in days x standard rate per day on general ward (EUR 450) + median duration of inpatient stay in days x costs ciprofloxacin/cotrimoxazole per day (EUR 0.12/EUR 0.14).
- (II)
- G-CSF support: median duration of inpatient stay in days x standard rate per day on general ward (EUR 450) + duration from ASCT to leukocytes ≥1/nL in days x costs G-CSF (EUR 5.75) + median duration of inpatient stay in days x cost cotrimoxazole (EUR 0.07)/2.
3. Results
3.1. Baseline Characteristics
3.2. Recovery of Blood Counts and Duration of Inpatient Stay
3.3. Infections
3.4. Detection of Multidrug Resistant (MDR) Bacteria
3.5. Hospital Readmission after Discharge
3.6. Omitting Antibiotic Prophylaxis and G-CSF
3.7. Outcome
3.8. Results of the Cost Analysis
3.9. Resistance Rates of Bacterial Isolates in the Entire Hematology Department and Transplant Unit between 2015 and 2019
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blimark, C.; Holmberg, E.; Mellqvist, U.-H.; Landgren, O.; Bjorkholm, M.; Hultcrantz, M.; Kjellander, C.; Turesson, I.; Kristinsson, S.Y. Multiple Myeloma and Infections: A Population-Based Study on 9253 Multiple Myeloma Patients. Haematologica 2015, 100, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Anaissie, E. Infections in Patients with Multiple Myeloma in the Era of High-Dose Therapy and Novel Agents. Clin. Infect. Dis. 2009, 49, 1211–1225. [Google Scholar] [CrossRef] [Green Version]
- Garderet, L.; Morris, C.; Beksac, M.; Gahrton, G.; Schönland, S.; Yakoub-Agha, I.; Hayden, P.J. Are Autologous Stem Cell Transplants Still Required to Treat Myeloma in the Era of Novel Therapies? A Review from the Chronic Malignancies Working Party of the EBMT. Biol. Blood Marrow Transplant. 2020, 26, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Engelhardt, M.; Terpos, E.; Wäsch, R.; Giaccone, L.; Auner, H.W.; Caers, J.; Gramatzki, M.; van de Donk, N.; Oliva, S.; et al. From Transplant to Novel Cellular Therapies in Multiple Myeloma: European Myeloma Network Guidelines and Future Perspectives. Haematologica 2018, 103, 197–211. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Vehreschild, J.J.; Moritz, G.; Vehreschild, M.J.G.T.; Arenz, D.; Mahne, M.; Bredenfeld, H.; Chemnitz, J.; Klein, F.; Cremer, B.; Böll, B.; et al. Efficacy and Safety of Moxifloxacin as Antibacterial Prophylaxis for Patients Receiving Autologous Haematopoietic Stem Cell Transplantation: A Randomised Trial. Int. J. Antimicrob. Agents 2012, 39, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Vardhana, S.; Soave, R.; Shore, T.B.; Mark, T.M.; Jacobs, S.E.; Walsh, T.J.; Gergis, U. Impact of Prophylactic Levofloxacin on Rates of Bloodstream Infection and Fever in Neutropenic Patients with Multiple Myeloma Undergoing Autologous Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1808–1814. [Google Scholar] [CrossRef] [Green Version]
- Yeshurun, M.; Vaxman, I.; Shargian, L.; Yahav, D.; Bishara, J.; Pasvolsky, O.; Wolach, O.; Lahav, M.; Gurion, R.; Magen, H.; et al. Antibacterial Prophylaxis with Ciprofloxacin for Patients with Multiple Myeloma and Lymphoma Undergoing Autologous Haematopoietic Cell Transplantation: A Quasi-Experimental Single-Centre before-after Study. Clin. Microbiol. Infect. 2018, 24, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucaneve, G.; Micozzi, A.; Menichetti, F.; Martino, P.; Dionisi, M.S.; Martinelli, G.; Allione, B.; D’Antonio, D.; Buelli, M.; Nosari, A.M.; et al. Levofloxacin to Prevent Bacterial Infection in Patients with Cancer and Neutropenia. N. Engl. J. Med. 2005, 353, 977–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhomberg, P.R.; Jones, R.N. Summary Trends for the Meropenem Yearly Susceptibility Test Information Collection Program: A 10-Year Experience in the United States (1999–2008). Diagn. Microbiol. Infect. Dis. 2009, 65, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Maakaron, J.E.; Liscynesky, C.; Boghdadly, Z.E.; Huang, Y.; Agyeman, A.; Brammer, J.; Penza, S.; Efebera, Y.; Benson, D.; Rosko, A.; et al. Fluoroquinolone Prophylaxis in Autologous Stem Cell Transplantation: Worthy of a Second Look. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2020, 26, e198–e201. [Google Scholar] [CrossRef] [PubMed]
- McQuaker, I.G.; Hunter, A.E.; Pacey, S.; Haynes, A.P.; Iqbal, A.; Russell, N.H. Low-Dose Filgrastim Significantly Enhances Neutrophil Recovery Following Autologous Peripheral-Blood Stem-Cell Transplantation in Patients with Lymphoproliferative Disorders: Evidence for Clinical and Economic Benefit. J. Clin. Oncol. 1997, 15, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Ljungman, P.; Cordonnier, C.; Kempf, C.; Linkesch, W.; Alegre, A.; Solano, C.; Simonsson, B.; Sonnen, R.; Diehl, V.; et al. Lenograstim after Autologous Peripheral Blood Progenitor Cell Transplantation: Results of a Double-Blind, Randomized Trial. Bone Marrow Transplant. 2004, 34, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Cibrian, N.; Magnano, L.; Gutiérrez-García, G.; Andrade, X.; Correa, J.G.; Suárez-Lledó, M.; Martínez, C.; Rovira, M.; Carreras, E.; Rosiñol, L.; et al. At-Home Autologous Stem Cell Transplantation in Multiple Myeloma with and without G-CSF Administration: A Comparative Study. Bone Marrow Transplant. 2016, 51, 593–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, M.; Martinez, S.; Corringham, S.; Medley, K.; Ball, E.D. Optimal Use of G-CSF Administration after Hematopoietic SCT. Bone Marrow Transplant. 2009, 43, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.-A.H.; Boeckh, M.A. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: A Global Perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K.; Feyer, P.; Höller, U.; Link, H.; Wörmann, B.; Jahn, F. Supportive Treatments for Patients with Cancer. Dtsch. Aerzteblatt Online 2017, 114, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J.; Caserta, C.; Roila, F. Hematopoietic Growth Factors: ESMO Clinical Practice Guidelines for the Applications. Ann. Oncol. 2010, 21, v248–v251. [Google Scholar] [CrossRef]
- Heinz, W.J.; Buchheidt, D.; Christopeit, M.; von Lilienfeld-Toal, M.; Cornely, O.A.; Einsele, H.; Karthaus, M.; Link, H.; Mahlberg, R.; Neumann, S.; et al. Diagnosis and Empirical Treatment of Fever of Unknown Origin (FUO) in Adult Neutropenic Patients: Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2017, 96, 1775–1792. [Google Scholar] [CrossRef] [Green Version]
- Clemons, M.J.; Fergusson, D.; Joy, A.A.; Meza-Junco, J.; Price Hiller, J.A.; Mackey, J.R.; Zhu, X.; Ibrahim, M.F.K.; Basulaiman, B.M.; Awan, A.A.; et al. A multicenter study comparing granulocyte-colony stimulating factors to antibiotics for primary prophylaxis of taxotere/cyclophosphamide-induced febrile neutropenia in patients with early-stage breast cancer. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Sculier, J.P.; Paesmans, M.; Lecomte, J.; Van Cutsem, O.; Lafitte, J.J.; Berghmans, T.; Koumakis, G.; Florin, M.C.; Thiriaux, J.; Michel, J.; et al. A Three-Arm Phase III Randomised Trial Assessing, in Patients with Extensive-Disease Small-Cell Lung Cancer, Accelerated Chemotherapy with Support of Haematological Growth Factor or Oral Antibiotics. Br. J. Cancer 2001, 85, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.P.; de Vries, E.G.; Mulder, N.H.; Willemse, P.H.; Sleijfer, D.T.; Hospers, G.A.; van der Graaf, W.T. Prevention of Febrile Leucopenia after Chemotherapy in High-Risk Breast Cancer Patients: No Significant Difference between Granulocyte-Colony Stimulating Growth Factor or Ciprofloxacin plus Amphotericin B. J. Antimicrob. Chemother. 1999, 43, 741–743. [Google Scholar] [CrossRef] [Green Version]
- Skoetz, N.; Bohlius, J.; Engert, A.; Monsef, I.; Blank, O.; Vehreschild, J.-J. Prophylactic Antibiotics or G(M)-CSF for the Prevention of Infections and Improvement of Survival in Cancer Patients Receiving Myelotoxic Chemotherapy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavrelova, A.; Paterova, P.; Gabalec, F.; Zak, P.; Radocha, J. Ciprofloxacin Prophylaxis during Autologous Stem Cell Transplantation for Multiple Myeloma in Patients with a High Rate of Fluoroquinolone-Resistant Gram-Negative Bacteria Colonization. Biomed. Pap. 2019, 163, 161–165. [Google Scholar] [CrossRef]
- Drayson, M.T.; Bowcock, S.; Planche, T.; Iqbal, G.; Pratt, G.; Yong, K.; Wood, J.; Raynes, K.; Higgins, H.; Dawkins, B.; et al. Prophylactic Levofloxacin to Prevent Infections in Newly Diagnosed Symptomatic Myeloma: The TEAMM RCT. Health Technol. Assess. Winch. Engl. 2019, 23, 1–94. [Google Scholar] [CrossRef]
- Klumpp, T.R.; Mangan, K.F.; Goldberg, S.L.; Pearlman, E.S.; Macdonald, J.S. Granulocyte Colony-Stimulating Factor Accelerates Neutrophil Engraftment Following Peripheral-Blood Stem-Cell Transplantation: A Prospective, Randomized Trial. J. Clin. Oncol. 2016, 13, 1323–1327. [Google Scholar] [CrossRef]
- Piccirillo, N.; Sica, S.; Laurenti, L.; Chiusolo, P.; La Barbera, E.; Sorà, F.; Leone, G. Optimal Timing of G-CSF Administration after CD34+immunoselected Peripheral Blood Progenitor Cell Transplantation. Bone Marrow Transplant. 1999, 23, 1245–1250. [Google Scholar] [CrossRef] [Green Version]
- Gertz, M.A.; Gastineau, D.A.; Lacy, M.Q.; Dispenzieri, A.; Hayman, S.R.; Kumar, S.K.; Dingli, D.; Leung, N.; Wolf, R.C.; Hogan, W.J.; et al. SCT without Growth Factor in Multiple Myeloma: Engraftment Kinetics, Bacteremia and Hospitalization. Bone Marrow Transplant. 2011, 46, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Alkharabsheh, O.; Sidiqi, M.H.; Aljama, M.A.; Gertz, M.A.; Frankel, A.E. The Human Microbiota in Multiple Myeloma and Proteasome Inhibitors. Acta Haematol. 2020, 143, 118–123. [Google Scholar] [CrossRef] [PubMed]
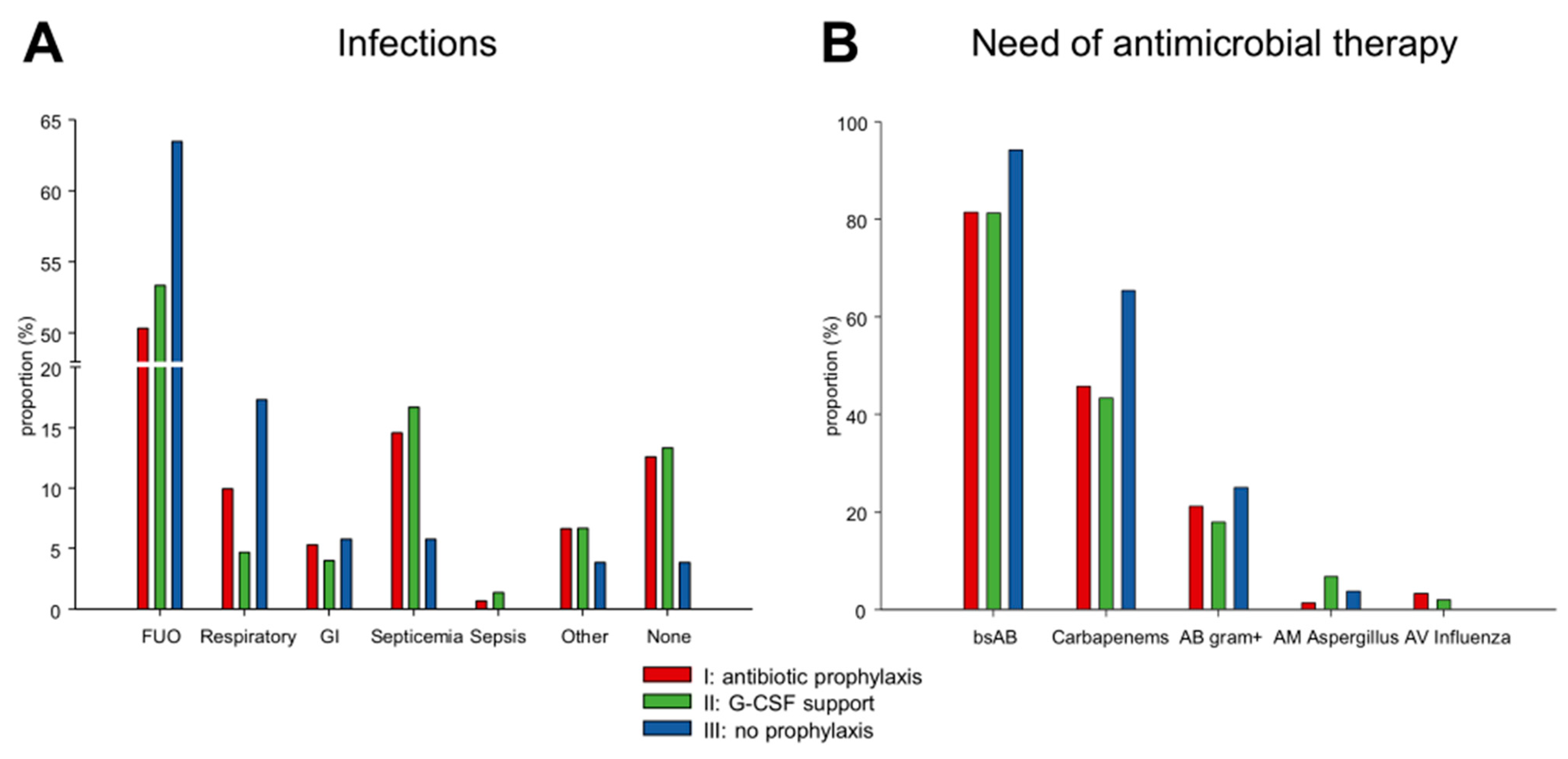
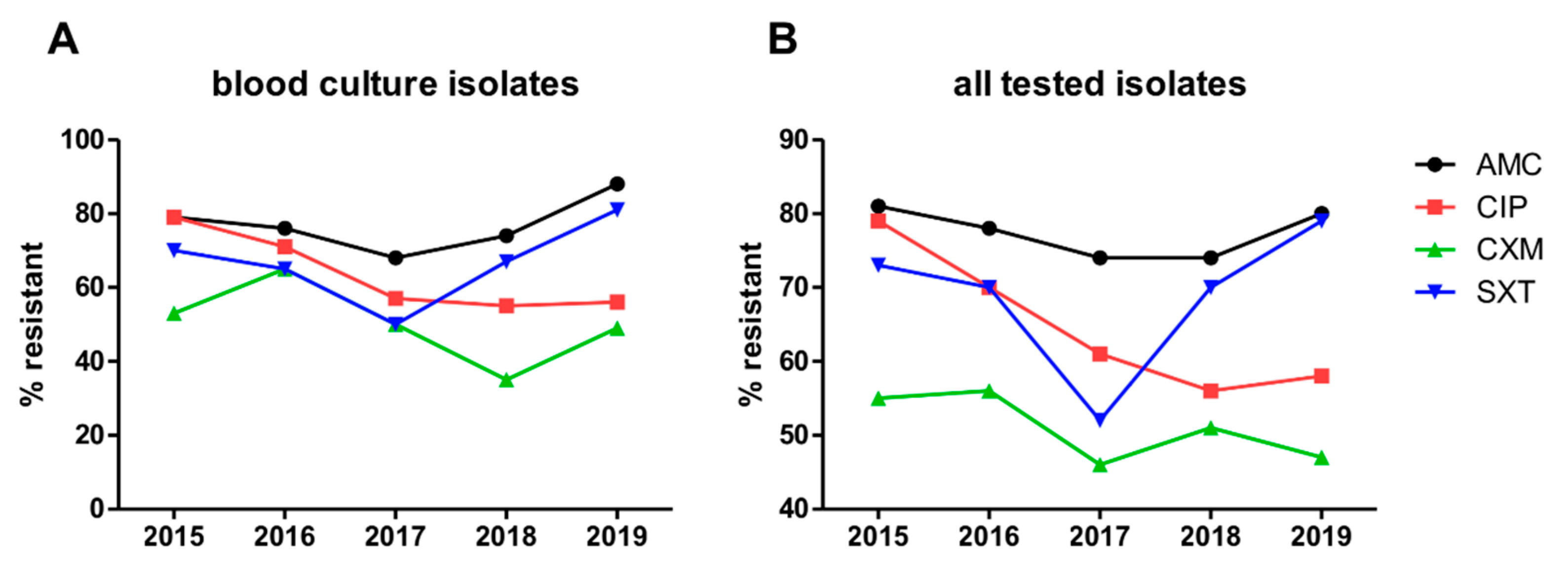
| Variable | (I) Antibiotic Prophylaxis | (II) G-CSF Support | (III) No Prophylaxis |
|---|---|---|---|
| Period A.D. | March 2016–January 2017 | March 2017–July 2018 | January 2017–March 2017 |
| Ciprofloxacin 500 mg or cotrimoxazole 960 mg twice a day | yes | no | no |
| Filgrastim 5 μg/kg of BW daily until leukocytes > 2/nL | no | yes | no |
| PCP prophylaxis with cotrimoxazole 960 mg thrice weekly | not uniform * | yes | no |
| Acyclovir 400 mg twice a day | yes | yes | yes |
| Variable | (I) | (II) | (III) | All | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| n (n = 151 ASCT) | % | n (n = 150 ASCT) | % | n (n = 52 ASCT) | % | n (n = 353 ASCT) | % | ||
| Age at ASCT in years | 0.06 | ||||||||
| Median (range) | 59 (53–66) | 62 (55–68) | 62 (57–66) | 61 (54–67) | |||||
| Response before ASCT | 0.47 | ||||||||
| ≥VGPR | 85 | 56.3 | 83 | 55.3 | 34 | 65.4 | 202 | 57.2 | |
| ≤PR | 60 | 39.7 | 63 | 42.0 | 17 | 32.7 | 140 | 39.7 | |
| Not assessable | 6 | 4.0 | 4 | 2.7 | 1 | 1.9 | 11 | 3.1 | |
| Time point of ASCT | 0.41 | ||||||||
| First-line | 126 | 83.4 | 116 | 77.3 | 42 | 80.8 | 284 | 80.5 | |
| Relapse | 25 | 16.6 | 34 | 22.7 | 10 | 19.2 | 69 | 19.5 | |
| Melphalan dose | 0.37 | ||||||||
| 200 mg/m2 | 138 | 91.4 | 143 | 95.3 | 49 | 94.2 | 330 | 93.5 | |
| Other | 13 | 8.6 | 7 | 4.7 | 3 | 5.8 | 23 | 6.5 | |
| Stem cell amount | 0.06 | ||||||||
| ≥2.5 × 10 6/kg of BW | 123 | 81.5 | 109 | 72.7 | 45 | 86.5 | 277 | 78.5 | |
| <2.5 × 10 6/kg of BW | 28 | 18.5 | 41 | 27.3 | 7 | 13.5 | 76 | 21.5 | |
| Variable | Time to Leukocytes ≥ 1/nL | Duration of Inpatient Stay | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | Estimate (95% CI) | p-Value | |
| G-CSF support (vs. antibiotic prophylaxis) | 16.22 (10.88–24.18) | <0.001 | −0.19 (−0.25–−0.12) | <0.001 |
| No prophylaxis (vs. antibiotic prophylaxis) | 1.10 (0.72–1.69) | 0.65 | 0.06 (−0.03–0.15) | 0.16 |
| Age (per ten years) | 1.00 (0.82–1.21) | 0.97 | 0.07 (0.04–0.11) | <0.001 |
| ASCT at relapse (vs. first-line treatment) | 0.86 (0.60–1.25) | 0.44 | 0.06 (−0.02–0.13) | 0.13 |
| ≥VGPR before ASCT (vs. ≤PR) | 1.15 (0.85–1.55) | 0.38 | 0.05 (−0.01–0.11) | 0.12 |
| Stem cell amount ≥ 2.5 * (vs. <2.5) | 1.36 (0.94–1.96) | 0.10 | −0.07 (−0.14–0.00) | 0.06 |
| Variable | Need of Carbapenems | |
|---|---|---|
| OR (95% CI) | p-Value | |
| G-CSF support (vs. antibiotic prophylaxis) | 0.76 (0.32–1.80) | 0.54 |
| No prophylaxis (vs. antibiotic prophylaxis) | 5.64 (1.24–25.63) | 0.03 |
| Age (per ten years) | 1.20 (0.72–2.02) | 0.48 |
| ASCT at relapse (vs. first-line treatment) | 2.08 (0.73–5.96) | 0.17 |
| ≥VGPR before ASCT (vs. ≤PR) | 1.40 (0.61–3.21) | 0.42 |
| Stem cell amount ≥ 2.5 * (vs. <2.5) | 0.42 (0.14–1.23) | 0.11 |
| Variable | Detection of VRE | |
|---|---|---|
| OR (95% CI) | p-Value | |
| Antibiotic prophylaxis (vs. G-CSF support) | 17.38 (2.24–134.68) | 0.01 |
| No prophylaxis (vs. G-CSF support) | 10.75 (1.12–103.43) | 0.04 |
| Age (per ten years) | 1.53 (0.68–3.44) | 0.30 |
| ASCT at relapse (vs. first-line treatment) | 2.79 (0.62–12.51) | 0.18 |
| ≥VGPR before ASCT (vs. ≤PR) | 2.12 (0.58–7.83) | 0.26 |
| Stem cell amount ≥ 2.5 * (vs. <2.5) | 0.46 (0.11–1.99) | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, E.-M.; Sauer, S.; Klein, S.; Tichy, D.; Benner, A.; Bertsch, U.; Brandt, J.; Kimmich, C.; Goldschmidt, H.; Müller-Tidow, C.; et al. Antibiotic Prophylaxis or Granulocyte-Colony Stimulating Factor Support in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation. Cancers 2021, 13, 3439. https://doi.org/10.3390/cancers13143439
Klein E-M, Sauer S, Klein S, Tichy D, Benner A, Bertsch U, Brandt J, Kimmich C, Goldschmidt H, Müller-Tidow C, et al. Antibiotic Prophylaxis or Granulocyte-Colony Stimulating Factor Support in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation. Cancers. 2021; 13(14):3439. https://doi.org/10.3390/cancers13143439
Chicago/Turabian StyleKlein, Eva-Maria, Sandra Sauer, Sabrina Klein, Diana Tichy, Axel Benner, Uta Bertsch, Juliane Brandt, Christoph Kimmich, Hartmut Goldschmidt, Carsten Müller-Tidow, and et al. 2021. "Antibiotic Prophylaxis or Granulocyte-Colony Stimulating Factor Support in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation" Cancers 13, no. 14: 3439. https://doi.org/10.3390/cancers13143439






