Image-Guided Robotic Radiosurgery for the Management of Intramedullary Spinal Cord Metastases—A Multicenter Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient and Treatment Characteristics
2.2. Outcome and Survival Data
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milano, M.T.; Johnson, M.D.; Sul, J.; Mohile, N.A.; Korones, D.N.; Okunieff, P.; Walter, K.A. Primary spinal cord glioma: A Surveillance, Epidemiology, and End Results database study. J. Neurooncol. 2010, 98, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Barr, M.M.; Huang, K.T.; Moses, Z.B.; Iorgulescu, J.B.; Chi, J.H. Recent advances in intradural spinal tumors. Neuro-Oncology 2017, 20, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Dam-Hieu, P.; Seizeur, R.; Mineo, J.F.; Metges, J.P.; Meriot, P.; Simon, H. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin. Neurol. Neurosurg. 2009, 111, 10–17. [Google Scholar] [CrossRef]
- Payer, S.; Mende, K.C.; Westphal, M.; Eicker, S.O. Intramedullary spinal cord metastases: An increasingly common diagnosis. Neurosurg. Focus 2015, 39, E15. [Google Scholar] [CrossRef]
- Kalayci, M.; Cagavi, F.; Gul, S.; Yenidunya, S.; Acikgoz, B. Intramedullary spinal cord metastases: Diagnosis and treatment - an illustrated review. Acta Neurochir. 2004, 146, 1347–1354. [Google Scholar] [CrossRef]
- Gasser, T.G.; Pospiech, J.; Stolke, D.; Schwechheimer, K. Spinal intramedullary metastases. Report of two cases and review of the literature. Neurosurg. Rev. 2001, 24, 88–92. [Google Scholar] [CrossRef]
- Costigan, D.A.; Winkelman, M.D. Intramedullary spinal cord metastasis. A clinicopathological study of 13 cases. J. Neurosurg. 1985, 62, 227–233. [Google Scholar] [CrossRef]
- Lieberson, R.E.; Veeravagu, A.; Eckermann, J.M.; Doty, J.R.; Jiang, B.; Andrews, R.; Chang, S.D. Intramedullary spinal cord metastasis from prostate carcinoma: A case report. J. Med. Case Rep. 2012, 6, 139. [Google Scholar] [CrossRef]
- Schiff, D.; O’Neill, B.P. Intramedullary spinal cord metastases: Clinical features and treatment outcome. Neurology 1996, 47, 906–912. [Google Scholar] [CrossRef]
- Kumar, J.I.; Yanamadala, V.; Shin, J.H. Intramedullary spinal metastasis of a carcinoid tumor. J. Clin. Neurosci. 2015, 22, 1990–1991. [Google Scholar] [CrossRef]
- O′Neill, A.H.; Phung, T.B.; Lai, L.T. Intramedullary spinal cord metastasis from thyroid carcinoma: Case report and a systematic pooled analysis of the literature. J. Clin. Neurosci. 2018, 49, 7–15. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, M.K.; Sym, S.J.; Kim, S.W.; Kim, W.K.; Kim, S.B.; Ahn, J.H. Intramedullary spinal cord metastases: A single-institution experience. J. Neurooncol. 2007, 84, 85–89. [Google Scholar] [CrossRef]
- Goyal, A.; Yolcu, Y.; Kerezoudis, P.; Alvi, M.A.; Krauss, W.E.; Bydon, M. Intramedullary spinal cord metastases: An institutional review of survival and outcomes. J. Neurooncol. 2019, 142, 347–354. [Google Scholar] [CrossRef]
- Kotecha, R.; Mehta, M.P.; Chang, E.L.; Brown, P.D.; Suh, J.H.; Lo, S.S.; Das, S.; Samawi, H.H.; Keith, J.; Perry, J.; et al. Updates in the management of intradural spinal cord tumors: A radiation oncology focus. Neuro-Oncology 2019, 21, 707–718. [Google Scholar] [CrossRef]
- Hussain, I.; Parker, W.E.; Barzilai, O.; Bilsky, M.H. Surgical Management of Intramedullary Spinal Cord Tumors. Neurosurg. Clin. N. Am. 2020, 31, 237–249. [Google Scholar] [CrossRef]
- Saeed, H.; Patel, R.; Thakkar, J.; Hamoodi, L.; Chen, L.; Villano, J.L. Multimodality therapy improves survival in intramedullary spinal cord metastasis of lung primary. Hematol. Oncol. Stem Cell Ther. 2017, 10, 143–150. [Google Scholar] [CrossRef]
- Goyal, A.; Cajigas, I.; Ibrahim, G.M.; Brathwaite, C.D.; Khatib, Z.; Niazi, T.; Bhatia, S.; Ragheb, J. Surgical Treatment of Intramedullary Spinal Metastasis in Medulloblastoma: Case Report and Review of the Literature. World Neurosurg. 2018, 118, 42–46. [Google Scholar] [CrossRef]
- Shin, D.A.; Huh, R.; Chung, S.S.; Rock, J.; Ryu, S. Stereotactic spine radiosurgery for intradural and intramedullary metastasis. Neurosurg. Focus 2009, 27, E10. [Google Scholar] [CrossRef]
- Hamilton, K.R.; Lee, S.S.; Urquhart, J.C.; Jonker, B.P. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J. Clin. Neurosci. 2019, 63, 168–175. [Google Scholar] [CrossRef]
- Hernandez-Duran, S.; Hanft, S.; Komotar, R.J.; Manzano, G.R. The role of stereotactic radiosurgery in the treatment of intramedullary spinal cord neoplasms: A systematic literature review. Neurosurg. Rev. 2016, 39, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Veeravagu, A.; Lieberson, R.E.; Mener, A.; Chen, Y.R.; Soltys, S.G.; Gibbs, I.C.; Adler, J.R.; Tian, A.G.; Chang, S.D. CyberKnife stereotactic radiosurgery for the treatment of intramedullary spinal cord metastases. J. Clin. Neurosci. 2012, 19, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Heron, D.E. Fractionated radiosurgical management of intramedullary spinal cord metastasis: A case report and review of the literature. Clin. Neurol. Neurosurg. 2009, 111, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.I.; Kim, D.H.; Chang, S.D. Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg. Focus 2003, 15, E10. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Eaton, K.D.; Fink, J.R.; Tredway, T. Intradural intramedullary spinal cord metastasis due to mesothelioma. J. Neurooncol. 2010, 97, 133–136. [Google Scholar] [CrossRef]
- Daly, M.E.; Choi, C.Y.; Gibbs, I.C.; Adler, J.R., Jr.; Chang, S.D.; Lieberson, R.E.; Soltys, S.G. Tolerance of the spinal cord to stereotactic radiosurgery: Insights from hemangioblastomas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 213–220. [Google Scholar] [CrossRef]
- Garcia, R.; Sallabanda, K.; Santa-Olalla, I.; Lopez Guerra, J.L.; Aviles, L.; Sallabanda, M.; Rivin, E.; Samblas, J. Robotic Radiosurgery for the Treatment of Intramedullary Spinal Cord Metastases: A Case Report and Literature Review. Cureus 2016, 8, e609. [Google Scholar] [CrossRef]
- Barrie, U.; Elguindy, M.; Pernik, M.; Adeyemo, E.; Aoun, S.G.; Hall, K.; Reyes, V.P.; El Ahmadieh, T.Y.; Bagley, C.A. Intramedullary Spinal Metastatic Renal Cell Carcinoma: Systematic Review of Disease Presentation, Treatment, and Prognosis with Case Illustration. World Neurosurg. 2020, 134, 584–593. [Google Scholar] [CrossRef]
- Klekamp, J. Treatment of intramedullary tumors: Analysis of surgical morbidity and long-term results. J. Neurosurg. Spine 2013, 19, 12–26. [Google Scholar] [CrossRef]
- Sung, W.S.; Sung, M.J.; Chan, J.H.; Manion, B.; Song, J.; Dubey, A.; Erasmus, A.; Hunn, A. Intramedullary spinal cord metastases: A 20-year institutional experience with a comprehensive literature review. World Neurosurg. 2013, 79, 576–584. [Google Scholar] [CrossRef]
- Phillips, K.A.; Gaughan, E.; Gru, A.; Schiff, D. Regression of an intramedullary spinal cord metastasis with a checkpoint inhibitor: A case report. CNS Oncol. 2017, 6, 275–280. [Google Scholar] [CrossRef]
- Strickland, B.A.; McCutcheon, I.E.; Chakrabarti, I.; Rhines, L.D.; Weinberg, J.S. The surgical treatment of metastatic spine tumors within the intramedullary compartment. J. Neurosurg. Spine 2018, 28, 79–87. [Google Scholar] [CrossRef]
- Diehn, F.E.; Rykken, J.B.; Wald, J.T.; Wood, C.P.; Eckel, L.J.; Hunt, C.H.; Schwartz, K.M.; Lingineni, R.K.; Carter, R.E.; Kaufmann, T.J. Intramedullary Spinal Cord Metastases: Prognostic Value of MRI and Clinical Features from a 13-Year Institutional Case Series. Am. J. Neuroradiol. 2015, 36, 587–593. [Google Scholar] [CrossRef]
- Lv, J.; Liu, B.; Quan, X.; Li, C.; Dong, L.; Liu, M. Intramedullary spinal cord metastasis in malignancies: An institutional analysis and review. OncoTargets Ther. 2019, 12, 4741–4753. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
| Patient and Treatment Characteristics | |||
|---|---|---|---|
| Total number of patients | 33 | ||
| Total number of lesions | 46 | ||
| Sex male (%)/female (%) | 9 (27)/24 (73) | ||
| Median | Mean | Range | |
| Age (years) | 48.4 | 49.2 | 11.3–74.4 |
| Pretreatment KPS (%) | 60 | 65.4 | 30–90 |
| Follow-up (months) | 8.5 | 13.6 | 1–40.8 |
| Tumor volume (cc) | 0.7 | 1.1 | 0.1–5.8 |
| Dose (Gy) | 16 | 16.1 | 6–24 |
| Fractions | 1 | 1.1 | 1–3 |
| Prescription isodose (%) | 70 | 69.0 | 60–80 |
| Conformity index | 1.1 | 1.2 | 1–1.6 |
| Homogeneity index | 1.4 | 1.4 | 1.2–1.67 |
| Coverage | 95 | 92.5 | 71.4–99.8 |
| Tumor location | Cervical | Thoracic | Lumbar |
| Number of lesions (%) | 15 (33) | 20 (43) | 11 (24) |
| Tumor entities | |||
| Histology | Number of patients | ||
| Breast (%) | 16 (48) | ||
| Lung (%) | 4 (12) | ||
| Malignant melanoma (%) | 3 (9) | ||
| Other (%) | 10 (31) | ||
| Comparison of Locally Controlled and Uncontrolled Patients | ||||
|---|---|---|---|---|
| Variable | Local control | Treatment failure | p-value | |
| Mean (±SD) | ||||
| Age | 49.1 (11.2) | 48.8 (11.5) | 0.94 | |
| Tumor volume (cc) | 1.1 (0.9) | 0.8 (0.4) | 0.30 | |
| Dose (Gy) | 15.7 (2.2) | 16.2 (0.9) | 0.56 | |
| Prescription dose | 69.0 (4.4) | 68.8 (3.3) | 0.67 | |
| Conformity index | 1.2 (0.1) | 1.2 (0.1) | 0.63 | |
| Homogeneity index | 1.4 (0.1) | 1.4 (0.1) | 0.69 | |
| Coverage (%) | 91.9 (7.7) | 94.7 (4.8) | 0.31 | |
| Outcome and Survival | ||||
| Variable | Time (in months) | Value (%) | 95% Confidence interval (%) | |
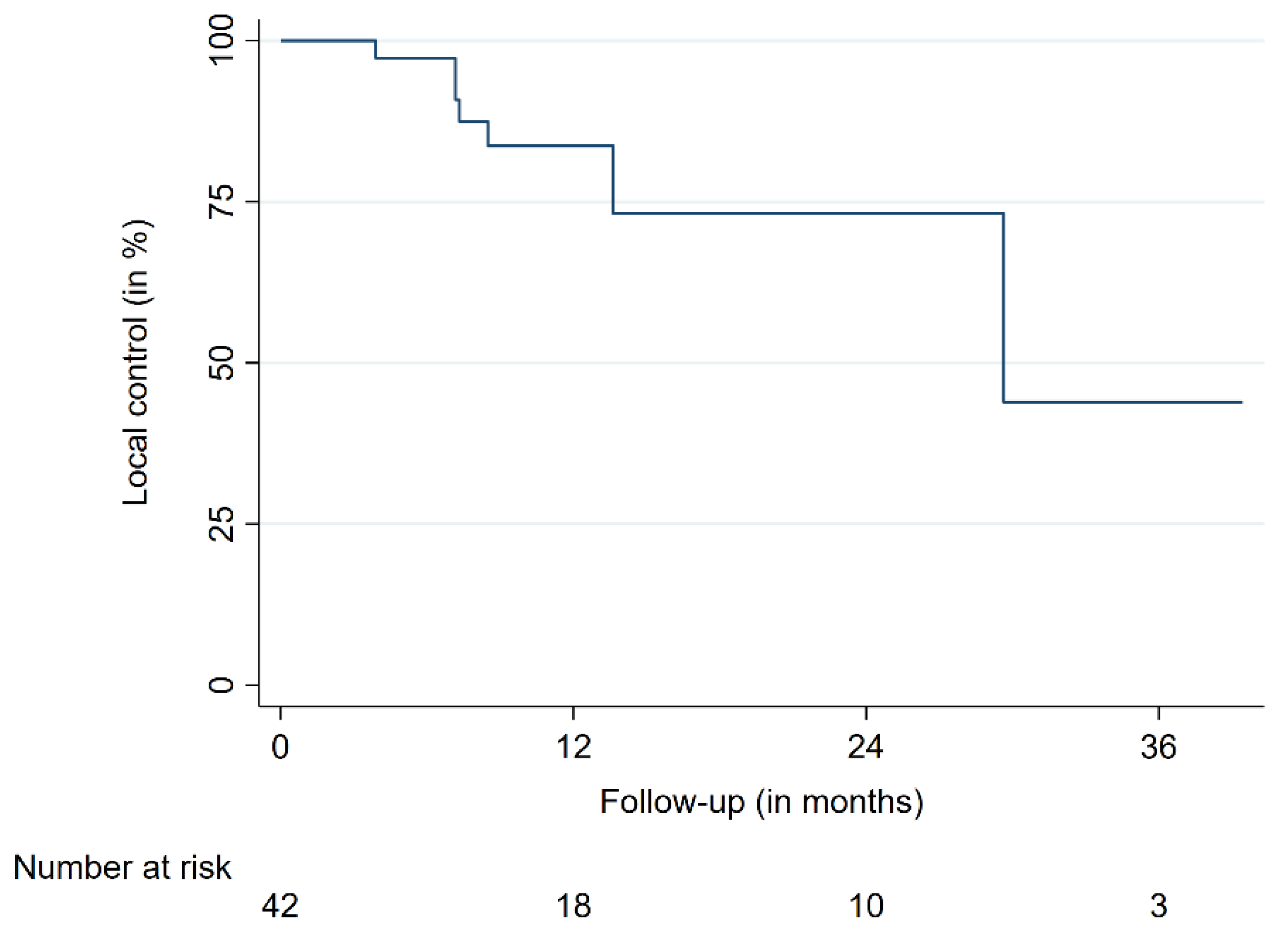
| LC | 12 | 84.6 | 66.9–93.2 | |
| 24 | 73.3 | 50.3–86.9 | ||
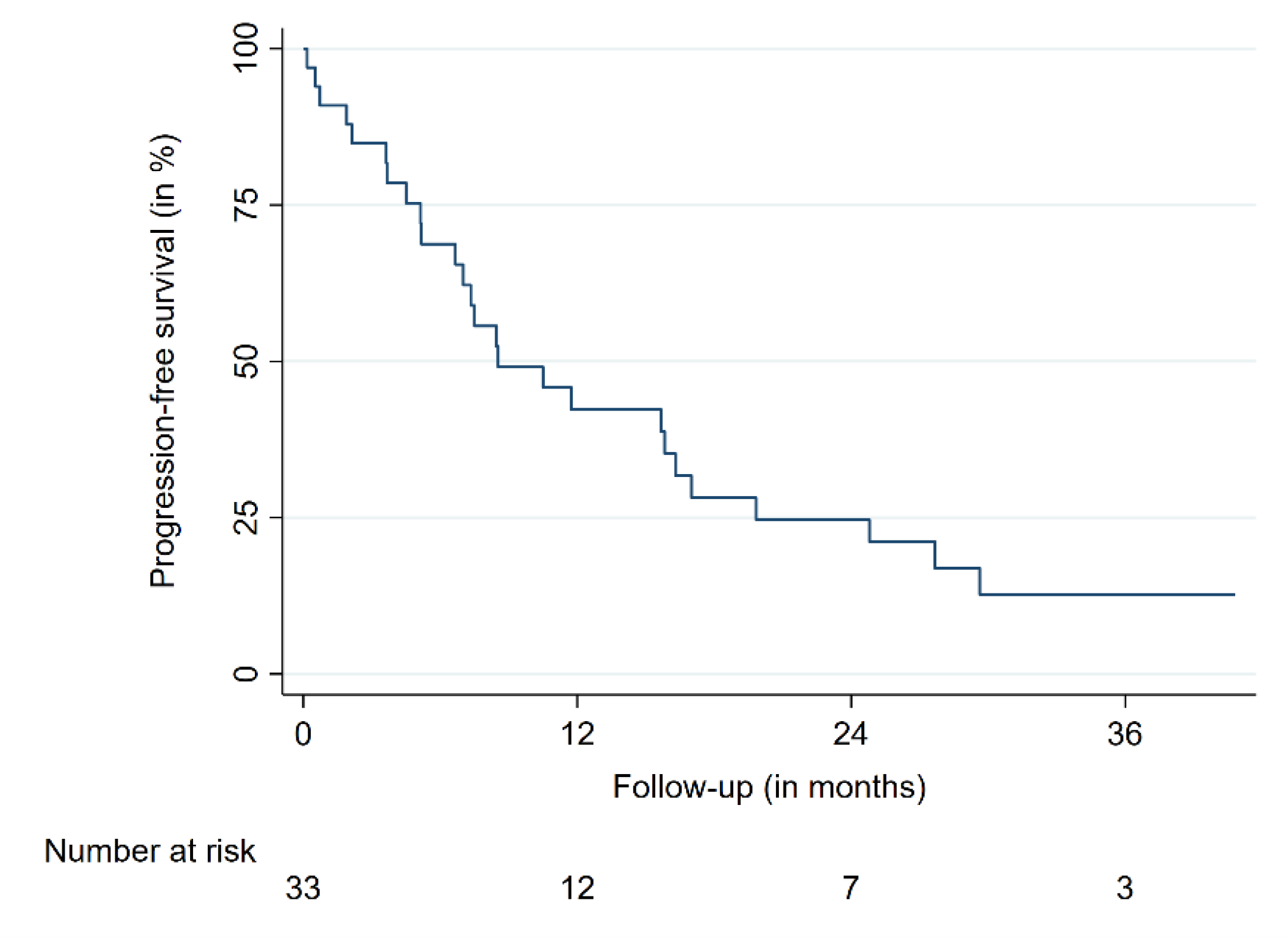
| PFS | 12 | 42.8 | 25.5–59.0 | |
| 24 | 25.0 | 11.3–41.3 | ||
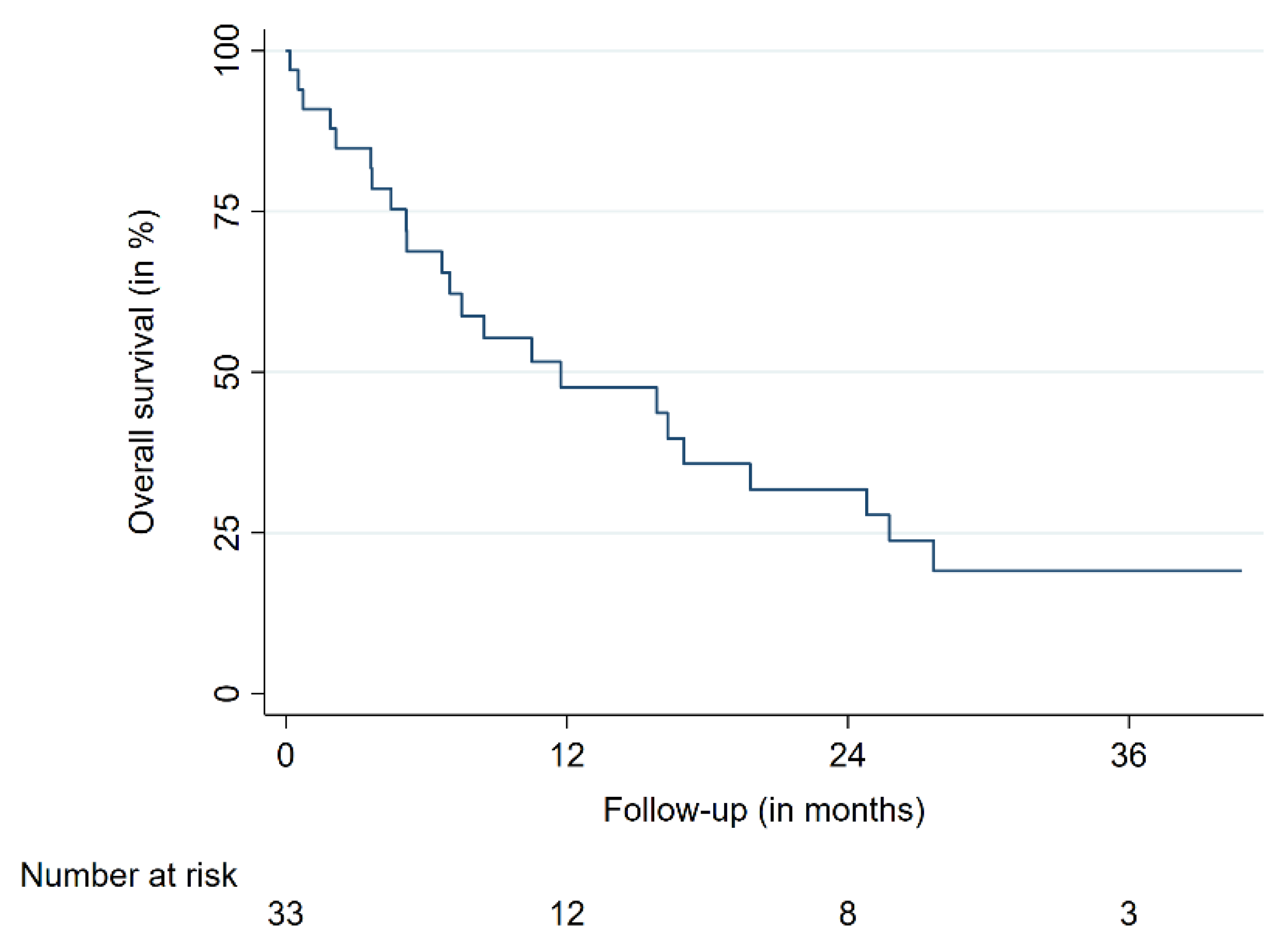
| OS | 12 | 47.5 | 29.3–63.7 | |
| 24 | 31.6 | 15.7–48.9 | ||
| Author | Year | Number of Patients (Metastases) | Number of Primarily Treated Metastasis | Treatment Modality | Median Follow-Up in Months | Dose/Fractions | Clinical Outcome | Radiographic Outcome |
|---|---|---|---|---|---|---|---|---|
| Garcia et al. [27] | 2016 | 1 (1) | 1 | CK | 37 | 14 Gy in 1 fraction | Stable | LC 100% |
| Veeravagu et al. [22] | 2012 | 9 (11) | 11 | CK | NR (median survival: 4.1 months) | Median: 21 Gy in 3 fractions | Improvement: 9% Stable: 36% NA: 54% | LC 100% in four patients with follow-up imaging. |
| Lieberson et al. [9] | 2012 | 1 (1) | 0 | CK | 3 | 27 Gy in 3 fractions | Stable | LC 100% |
| Parikh et al. [23] | 2009 | 1 (1) | 0 | CK | 26 | 15 Gy in 3 fractions | Improvement | LC 100% |
| Shin et al. [19] * | 2009 | 9 (11) | 8 | LINAC | NR (median survival: 8 months) | Median: 14 Gy in 1 fraction | Improvement: 88%, Stable: 11%, Worse: 11% | LC 89% in eight patients with follow-up imaging. |
| Chamberlain et al. [25] | 2010 | 1 (1) | 1 | CK | NR | NR | NR | NR |
| Barrie et al. [28] | 2019 | 1 (1) | 1 | CK | 26 | 25 Gy in 5 fractions | Worse | Progressive disease |
| This series | 2020 | 33 (46) | 41 | CK | 8.5 | Median: 16 Gy in 1 fraction | Improvement: 27%, Stable: 30%, Worse: 21% of patients with available clinical follow-up. | LC 79% in 29 patients with follow-up imaging. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehret, F.; Senger, C.; Kufeld, M.; Fürweger, C.; Kord, M.; Haidenberger, A.; Windisch, P.; Rueß, D.; Kaul, D.; Ruge, M.; et al. Image-Guided Robotic Radiosurgery for the Management of Intramedullary Spinal Cord Metastases—A Multicenter Experience. Cancers 2021, 13, 297. https://doi.org/10.3390/cancers13020297
Ehret F, Senger C, Kufeld M, Fürweger C, Kord M, Haidenberger A, Windisch P, Rueß D, Kaul D, Ruge M, et al. Image-Guided Robotic Radiosurgery for the Management of Intramedullary Spinal Cord Metastases—A Multicenter Experience. Cancers. 2021; 13(2):297. https://doi.org/10.3390/cancers13020297
Chicago/Turabian StyleEhret, Felix, Carolin Senger, Markus Kufeld, Christoph Fürweger, Melina Kord, Alfred Haidenberger, Paul Windisch, Daniel Rueß, David Kaul, Maximilian Ruge, and et al. 2021. "Image-Guided Robotic Radiosurgery for the Management of Intramedullary Spinal Cord Metastases—A Multicenter Experience" Cancers 13, no. 2: 297. https://doi.org/10.3390/cancers13020297
APA StyleEhret, F., Senger, C., Kufeld, M., Fürweger, C., Kord, M., Haidenberger, A., Windisch, P., Rueß, D., Kaul, D., Ruge, M., Schichor, C., Tonn, J.-C., & Muacevic, A. (2021). Image-Guided Robotic Radiosurgery for the Management of Intramedullary Spinal Cord Metastases—A Multicenter Experience. Cancers, 13(2), 297. https://doi.org/10.3390/cancers13020297





