Safety and Efficacy of Robotic Radiosurgery for Visceral and Lymph Node Metastases of Renal Cell Carcinoma: A Retrospective, Single Center Analysis
Abstract
Simple Summary
Abstract
1. Introduction
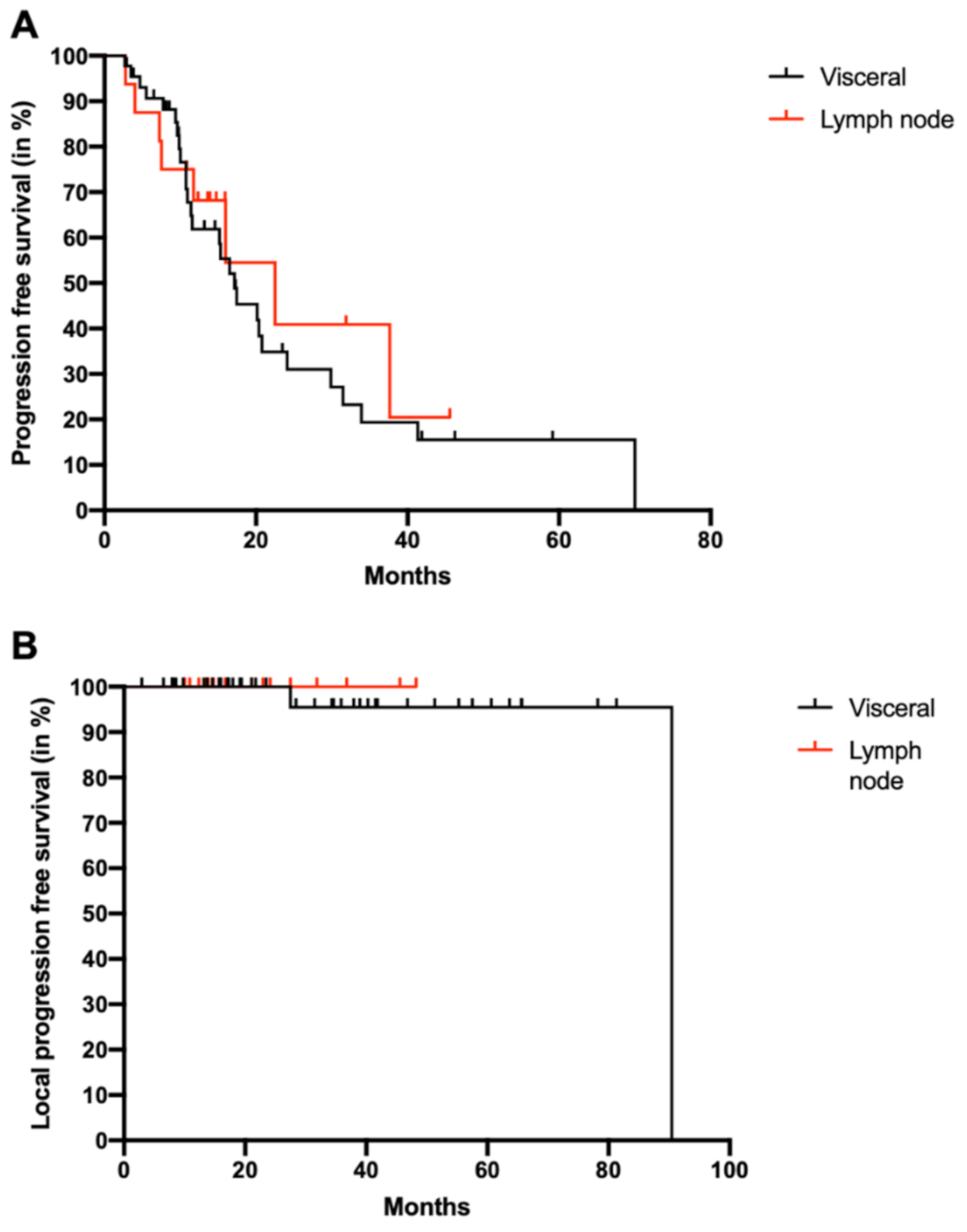
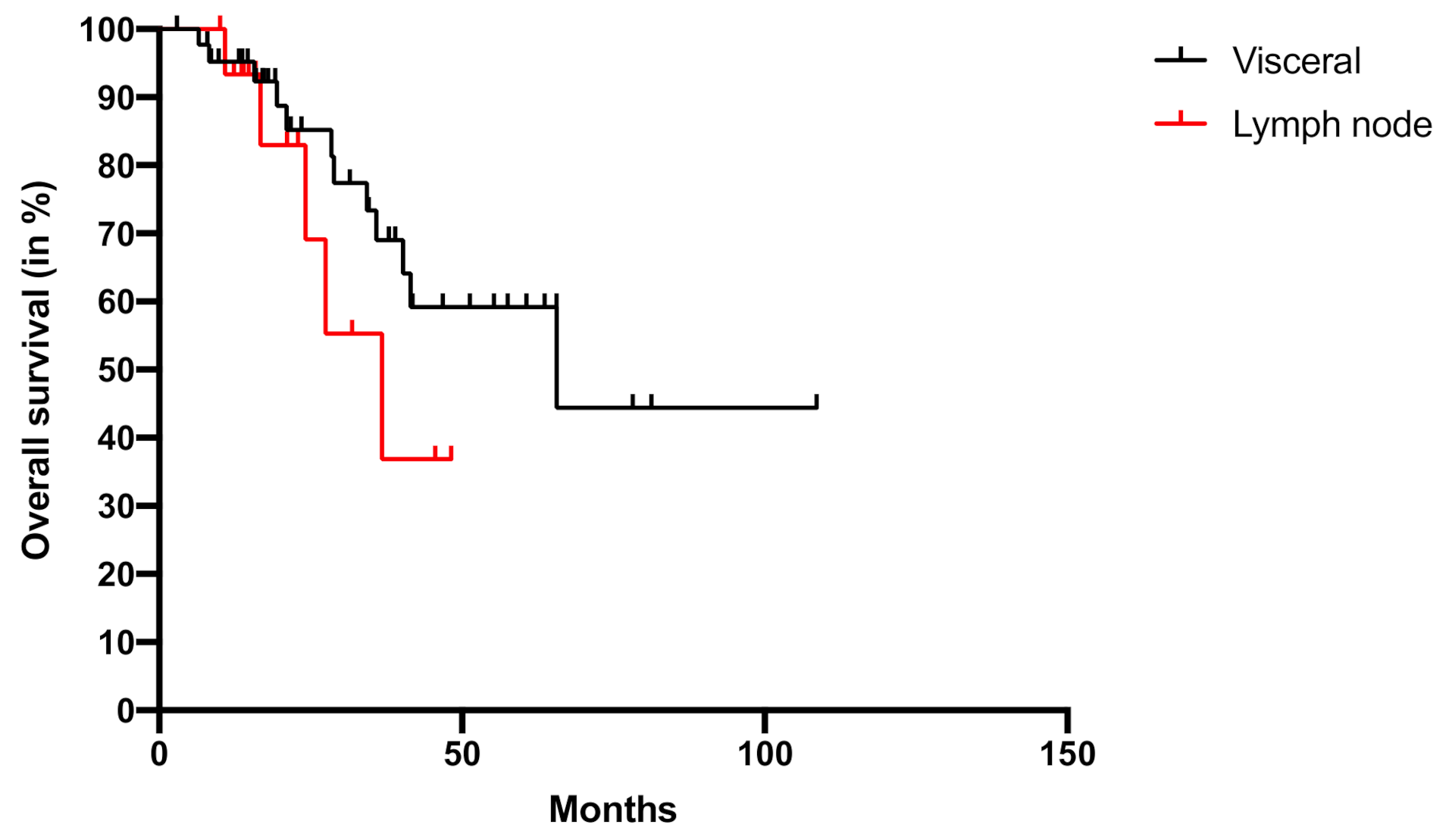
2. Results
3. Discussion
3.1. RRS in Primary RCC
3.2. RRS in Metastatic RCC
3.3. RRS as Combination Therapy
3.4. Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.-D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann. Oncol. 2011, 23, 973–980. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- El Dib, R.; Touma, N.J.; Kapoor, A. Cryoablation vs. radiofrequency ablation for the treatment of renal cell carcinoma: A meta-analysis of case series studies. BJU Int. 2012, 110, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Deschavanne, P.J.; Fertil, B. A review of human cell radiosensitivity in vitro. Int. J. Radiat. Oncol. 1996, 34, 251–266. [Google Scholar] [CrossRef]
- Ning, S.; Trisler, K.; Wessels, B.W.; Knox, S.J. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997, 80, 2519–2528. [Google Scholar] [CrossRef]
- Siva, S.; Kothari, G.; Muacevic, A.; Louie, A.V.; Slotman, B.J.; Teh, B.S.; Lo, S.S. Radiotherapy for renal cell carcinoma: Renaissance of an overlooked approach. Nat. Rev. Urol. 2017, 14, 549–563. [Google Scholar] [CrossRef]
- Hara, W.Y.; Tran, P.T.; Li, G.; Su, Z.; Puataweepong, P.; Adler, J.R.; Soltys, S.G.; Chang, S.D.; Gibbs, I.C. Cyberknife for brain metastases of malignant melanoma and renal cell carcinoma. Neurosurgery 2009, 64, A26–A32. [Google Scholar] [CrossRef]
- Adler, J.R., Jr.; Chang, S.D.; Murphy, M.J.; Doty, J.; Geis, P.; Hancock, S.L. The Cyberknife: A frameless robotic system for radiosurgery. Stereotact. Funct. Neurosurg. 1997, 69, 124–128. [Google Scholar] [CrossRef]
- Staehler, M.; Bader, M.; Schlenker, B.; Casuscelli, J.; Karl, A.; Roosen, A.; Stief, C.G.; Bex, A.; Wowra, B.; Muacevic, A. Single Fraction Radiosurgery for the Treatment of Renal Tumors. J. Urol. 2015, 193, 771–775. [Google Scholar] [CrossRef]
- Bruno, J.J., 2nd; Snyder, M.E.; Motzer, R.J.; Russo, P. Renal cell carcinoma local recurrences: Impact of surgical treatment and concomitant metastasis on survival. BJU Int. 2006, 97, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Tselis, N.; Chatzikonstantinou, G. Treating the Chameleon: Radiotherapy in the management of Renal Cell Cancer. Clin. Transl. Radiat. Oncol. 2019, 16, 7–14. [Google Scholar] [CrossRef] [PubMed]
- DiBiase, S.J.; Valicenti, R.K.; Schultz, D.; Xie, Y.; Gomella, L.G.; Corn, B.W. Palliative Irradiation for Focally Symptomatic Metastatic Renal Cell Carcinoma: Support for Dose Escalation Based on a Biological Model. J. Urol. 1997, 158, 746–749. [Google Scholar] [CrossRef]
- Muacevic, A.; Kufeld, M.; Rist, C.; Wowra, B.; Stief, C.; Staehler, M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 455–460. [Google Scholar] [CrossRef]
- Correa, R.J.; Louie, A.V.; Zaorsky, N.G.; Lehrer, E.J.; Ellis, R.; Ponsky, L.; Kaplan, I.; Mahadevan, A.; Chu, W.; Swaminath, A.; et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2019, 5, 958–969. [Google Scholar] [CrossRef]
- Correa, R.J.M.; Rodrigues, G.B.; Chen, H.; Warner, A.; Ahmad, B.; Louie, A.V. Stereotactic Ablative Radiotherapy (SABR) for Large Renal Tumors: A Retrospective Case Series Evaluating Clinical Outcomes, Toxicity, and Technical Considerations. Am. J. Clin. Oncol. 2018, 41, 568–575. [Google Scholar] [CrossRef]
- Funayama, S.; Onishi, H.; Kuriyama, K.; Komiyama, T.; Marino, K.; Araya, M.; Saito, R.; Aoki, S.; Maehata, Y.; Nonaka, H.; et al. Renal Cancer is Not Radioresistant: Slowly but Continuing Shrinkage of the Tumor After Stereotactic Body Radiation Therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033818822329. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Ozhasoglu, C.; Burton, S.A.; Vogel, W.J.; Atkins, B.A.; Kalnicki, S.; Welch, W.C. CyberKnife frameless stereotactic radiosurgery for spinal lesions: Clinical experience in 125 cases. Neurosurgery 2004, 55, 89–99. [Google Scholar] [CrossRef]
- Staehler, M.; Haseke, N.; Nuhn, P.; Tüllmann, C.; Karl, A.; Siebels, M.; Stief, C.G.; Wowra, B.; Muacevic, A. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int. 2010, 108, 673–678. [Google Scholar] [CrossRef]
- Kothari, G.; Foroudi, F.; Gill, S.; Corcoran, N.M.; Siva, S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol. 2014, 54, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Greco, C.; Motzer, R.J.; Magsanoc, J.M.; Pei, X.; Lovelock, M.; Mechalakos, J.G.; Zatcky, J.; Fuks, Z.; Yamada, Y. Tumor Control Outcomes After Hypofractionated and Single-Dose Stereotactic Image-Guided Intensity-Modulated Radiotherapy for Extracranial Metastases From Renal Cell Carcinoma. Int. J. Radiat. Oncol. 2012, 82, 1744–1748. [Google Scholar] [CrossRef]
- Svedman, C.; Sandström, P.; Pisa, P.; Blomgren, H.; Lax, I.; Kälkner, K.M.; Nilsson, S.; Wersäll, P. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006, 45, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Zaorsky, N.G.; Lehrer, E.J.; Kothari, G.; Louie, A.V.; Siva, S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis of 28 studies. Eur. Urol. Oncol. 2019, 2, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Dabestani, S.; Marconi, L.; Hofmann, F.; Stewart, F.; Lam, T.B.L.; Canfield, E.S.; Staehler, M.; Powles, T.; Ljungberg, B.; Bex, A. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol. 2014, 15, e549–e561. [Google Scholar] [CrossRef]
- Tanis, P.J.; Van Der Gaag, N.A.; Busch, O.R.C.; Van Gulik, T.M.; Gouma, D.J. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. BJS 2009, 96, 579–592. [Google Scholar] [CrossRef]
- Staehler, M.; Kruse, J.; Haseke, N.; Stadler, T.; Roosen, A.; Karl, A.; Stief, C.G.; Jauch, K.W.; Bruns, C.J. Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J. Urol. 2010, 28, 543–547. [Google Scholar] [CrossRef]
- Siva, S.; Lobachevsky, P.N.; MacManus, M.P.; Kron, T.; Möller, A.; Lobb, R.J.; Ventura, J.; Best, N.; Smith, J.; Ball, D.; et al. Radiotherapy for Non–Small Cell Lung Cancer Induces DNA Damage Response in Both Irradiated and Out-of-field Normal Tissues. Clin. Cancer Res. 2016, 22, 4817–4826. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic Radiosurgery for Melanoma Brain Metastases in Patients Receiving Ipilimumab: Safety Profile and Efficacy of Combined Treatment. Int. J. Radiat. Oncol. 2015, 92, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.; Brown, P.D.; Sheehan, J.P.; Quiñones-Hinojosa, A.; Zaorsky, N.G.; Trifiletti, D.M. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother. Oncol. 2019, 130, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hammers, H.J.; VonMerveldt, D.; Ahn, C.; Nadal, R.M.; Drake, C.G.; Folkert, M.R.; Laine, A.M.; Courtney, K.D.; Brugarolas, J.; Song, D.Y.; et al. Combination of dual immune checkpoint inhibition (ICI) with stereotactic radiation (SBRT) in metastatic renal cell carcinoma (mRCC) (RADVAX RCC). J. Clin. Oncol. 2020, 38, 614. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5%20%C3%97%207.pdf (accessed on 5 October 2020).
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; Choueiri, T.K.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Visceral Metastases | Lymph Node Metastases | p Value | ||
|---|---|---|---|---|---|
| (n = 44) | (n = 16) | ||||
| Age at diagnosis | 0.275 | ||||
| Median (Years) | 55 | 60 | |||
| Range (Years) | 37–77 | 38–81 | |||
| Age at RRS treatment | 0.996 | ||||
| Median (Years) | 64 | 64 | |||
| Range (Years) | 45–80 | 42–83 | |||
| % | n | % | n | ||
| Gender | 0.730 | ||||
| Male | 70 | 31 | 75 | 12 | |
| Female | 30 | 13 | 25 | 4 | |
| IMDC | 0.731 | ||||
| Favorable | 61 | 27 | 50 | 8 | |
| Intermediate | 34 | 15 | 44 | 7 | |
| Poor | 5 | 2 | 6 | 1 | |
| Histology | 0.171 | ||||
| Clear cell | 86 | 38 | 94 | 15 | |
| Papillary Typ 2 | 7 | 3 | 0 | 0 | |
| Chromophob | 7 | 3 | 0 | 0 | |
| TFE-3 Translocation | 0 | 0 | 6 | 1 | |
| Prior therapies | 0.939 | ||||
| Surgery | 100 | 44 | 100 | 16 | |
| TKI | 36 | 16 | 31 | 5 | |
| Immunotherapy | 16 | 7 | 13 | 2 | |
| Therapy at RRS | 0.934 | ||||
| No systemic therapy | 59 | 26 | 63 | 10 | |
| TKI | 25 | 11 | 25 | 4 | |
| Immunotherapy | 16 | 7 | 13 | 2 | |
| Parameter | Visceral Metastases | Lymph Node Metastases | p Value | ||
|---|---|---|---|---|---|
| (n = 44) | (n = 16) | ||||
| Median | Range | Median | Range | ||
| Fractions | 1 | 1–5 | 1 | 1–5 | 0.003 |
| Prescription Dose (Gy) | 24 | 8–26 | 18 | 7–26 | <0.001 |
| Prescription Isodose (Gy) | 70 | 60–75 | 70 | 65–70 | 0.434 |
| Target volume cm3 | 26.3 | 1.4–97.4 | 18.6 | 2.9–120 | 0.434 |
| Number of metastases | 1 | 1–2 | 1 | 1–2 | 0.170 |
| Adverse Event | CTCAE 1 | CTCAE 2 | CTCAE 3 | CTCAE >3 |
|---|---|---|---|---|
| Fatigue | 3 | 1 | 0 | 0 |
| Stroke/Thrombosis | 0 | 0 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodler, S.; Schott, M.; Tamalunas, A.; Marcon, J.; Graser, A.; Mumm, J.-N.; Casuscelli, J.; Stief, C.G.; Fürweger, C.; Muacevic, A.; et al. Safety and Efficacy of Robotic Radiosurgery for Visceral and Lymph Node Metastases of Renal Cell Carcinoma: A Retrospective, Single Center Analysis. Cancers 2021, 13, 680. https://doi.org/10.3390/cancers13040680
Rodler S, Schott M, Tamalunas A, Marcon J, Graser A, Mumm J-N, Casuscelli J, Stief CG, Fürweger C, Muacevic A, et al. Safety and Efficacy of Robotic Radiosurgery for Visceral and Lymph Node Metastases of Renal Cell Carcinoma: A Retrospective, Single Center Analysis. Cancers. 2021; 13(4):680. https://doi.org/10.3390/cancers13040680
Chicago/Turabian StyleRodler, Severin, Melanie Schott, Alexander Tamalunas, Julian Marcon, Annabel Graser, Jan-Niclas Mumm, Jozefina Casuscelli, Christian G. Stief, Christoph Fürweger, Alexander Muacevic, and et al. 2021. "Safety and Efficacy of Robotic Radiosurgery for Visceral and Lymph Node Metastases of Renal Cell Carcinoma: A Retrospective, Single Center Analysis" Cancers 13, no. 4: 680. https://doi.org/10.3390/cancers13040680
APA StyleRodler, S., Schott, M., Tamalunas, A., Marcon, J., Graser, A., Mumm, J.-N., Casuscelli, J., Stief, C. G., Fürweger, C., Muacevic, A., & Staehler, M. (2021). Safety and Efficacy of Robotic Radiosurgery for Visceral and Lymph Node Metastases of Renal Cell Carcinoma: A Retrospective, Single Center Analysis. Cancers, 13(4), 680. https://doi.org/10.3390/cancers13040680






