A Fenofibrate Diet Prevents Paclitaxel-Induced Peripheral Neuropathy in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
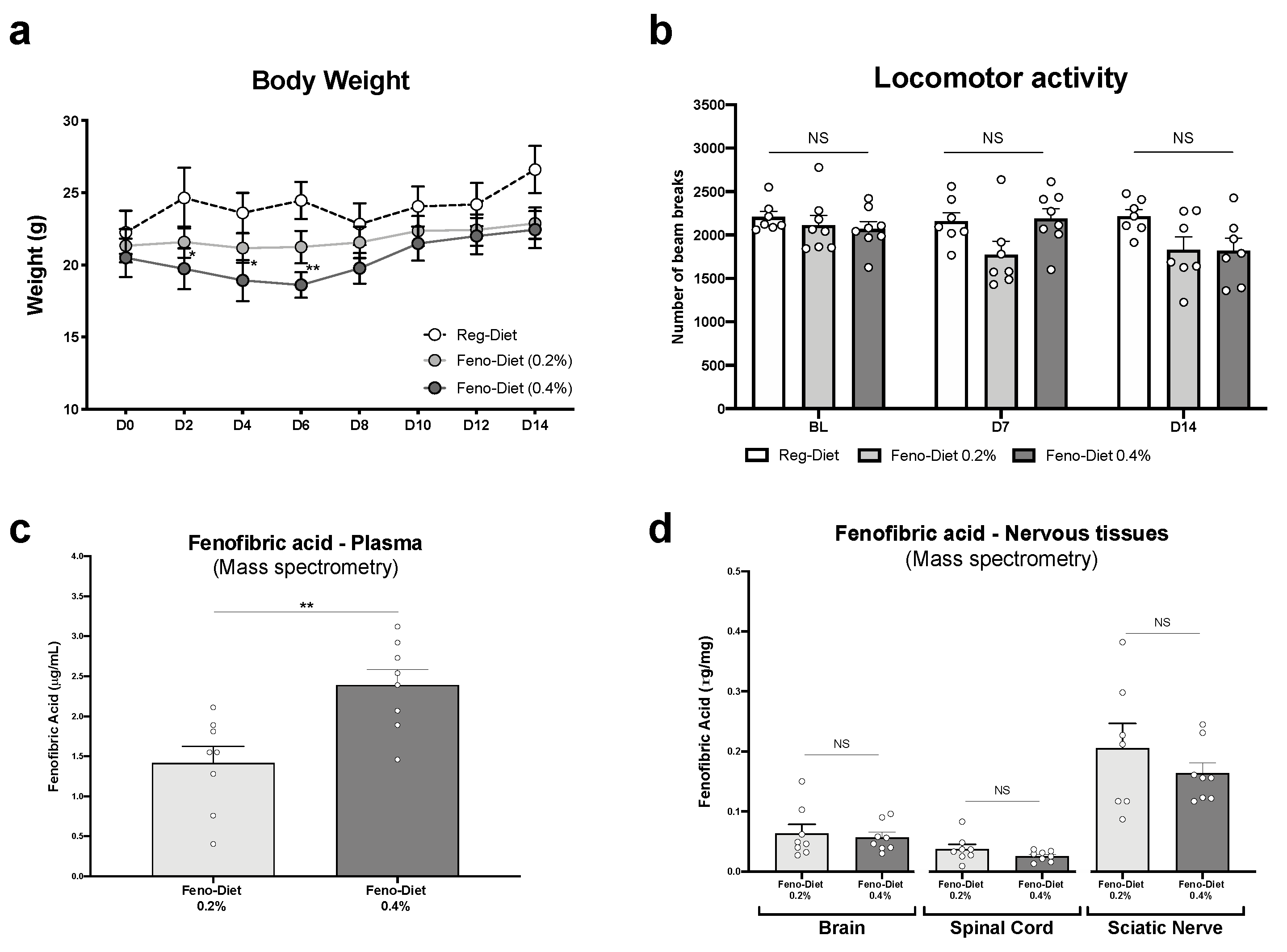
2.1. Study of Bioavailability and Tolerability of Fenofibrate Diets
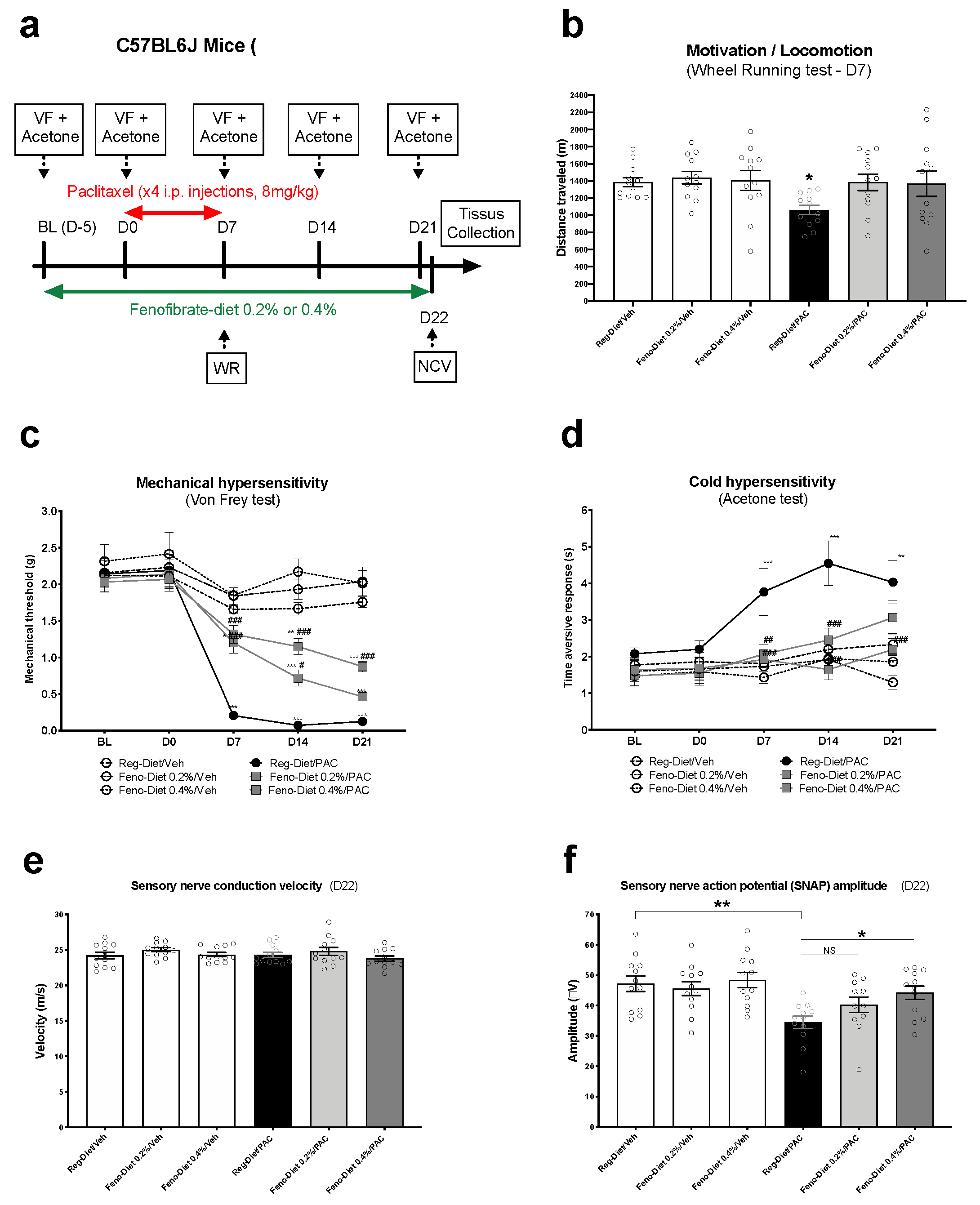
2.2. Fenofibrate Diets Reduce Signs of Paclitaxel-Induced Peripheral Neuropathy
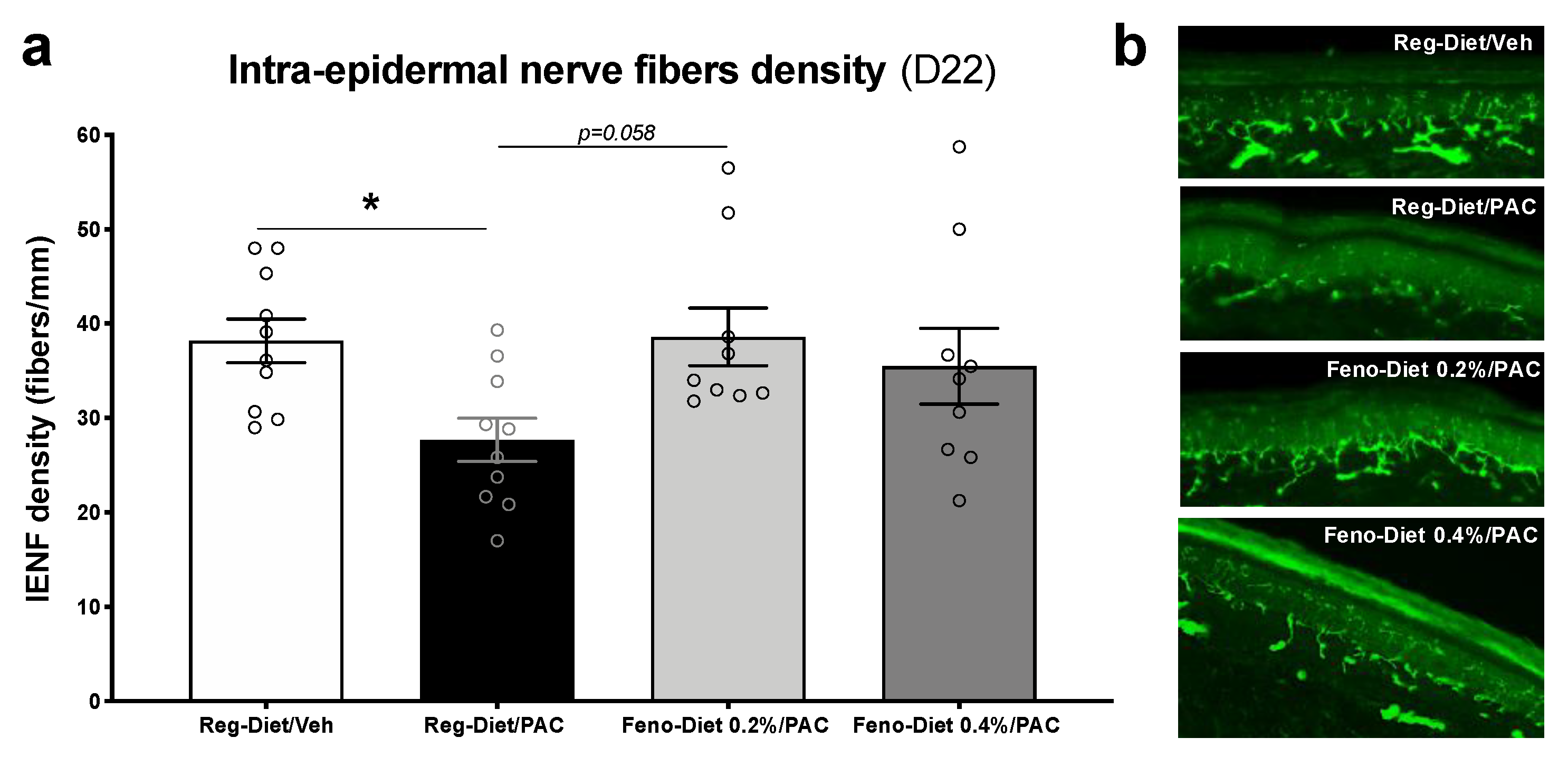
2.3. Fenofibrate Diet Prevents the Decrease in Density of Intra-Epidermal Nerve Fibers
2.4. Fenofibrate Diet Reduces Mitochondria Damage
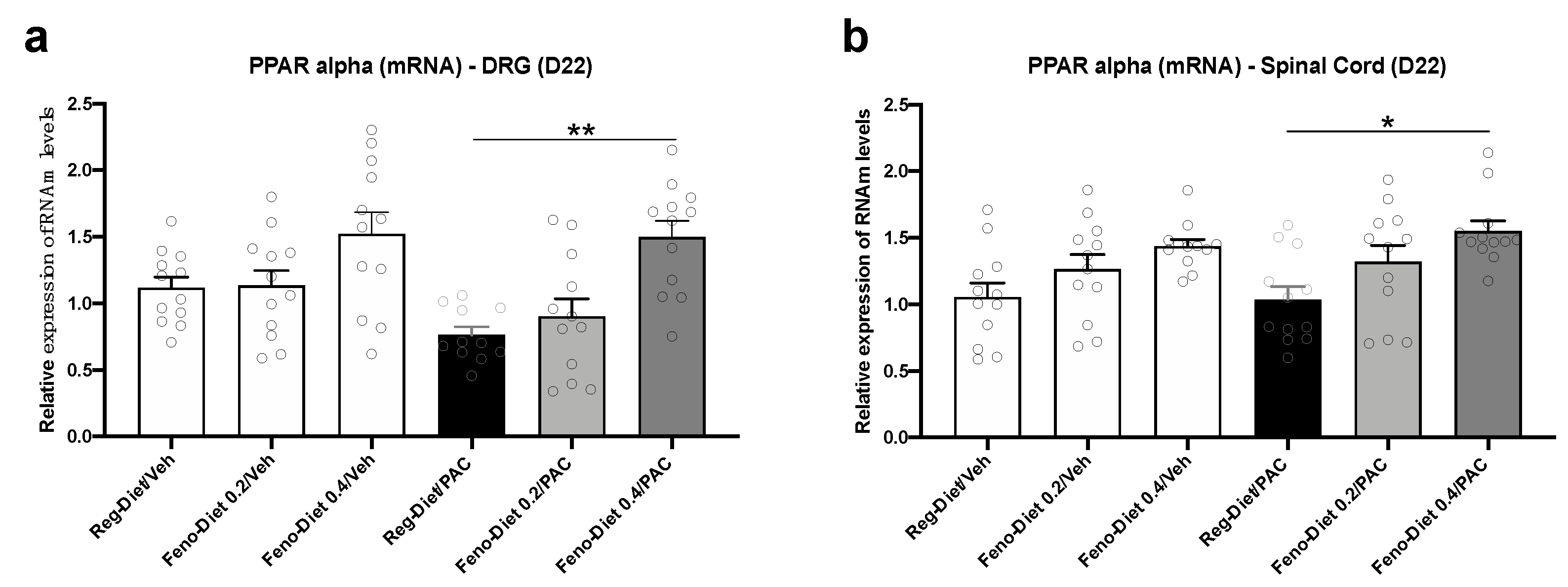
2.5. Fenofibrate Diet Increases PPAR-α mRNA Expression in DRG and Spinal Cord
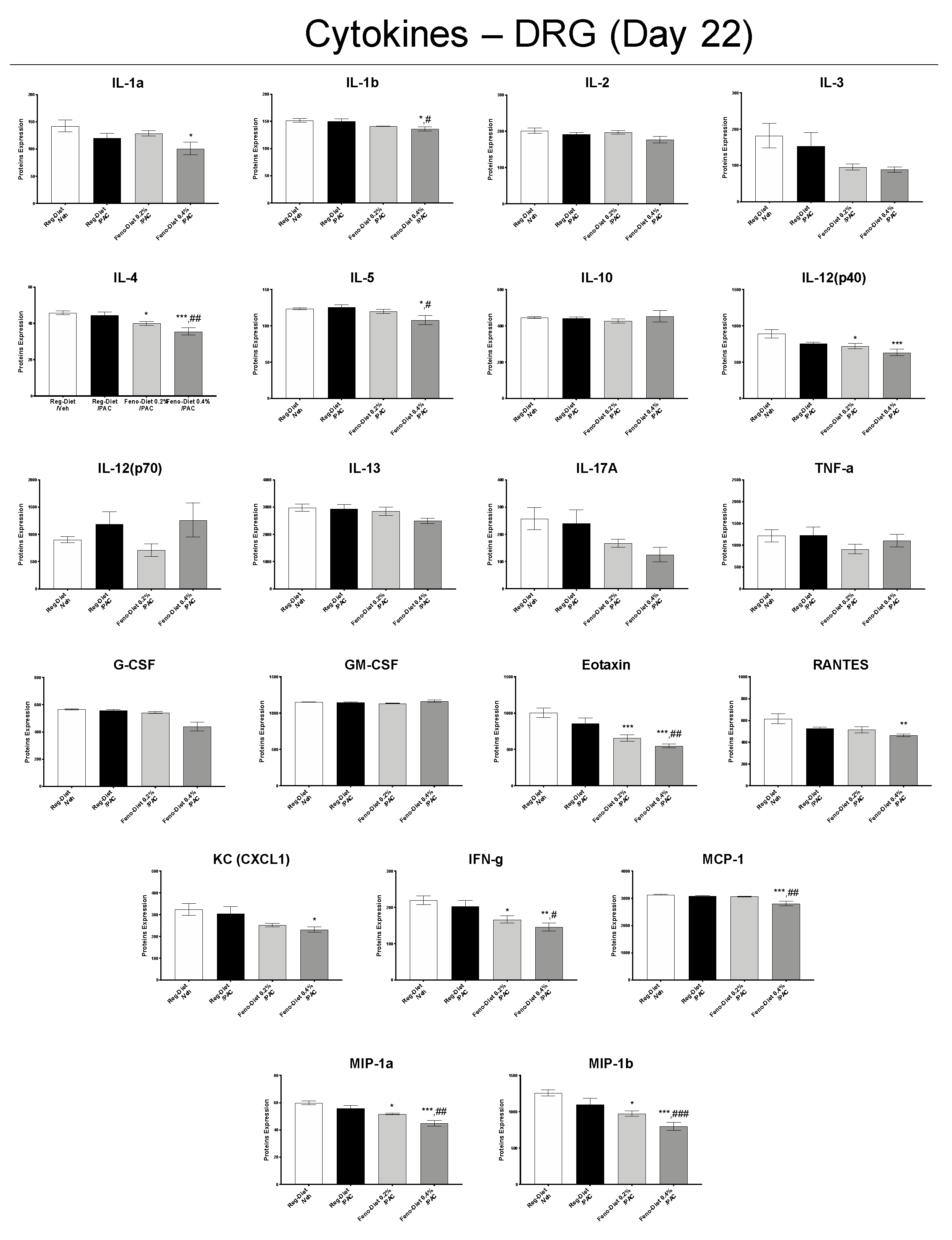
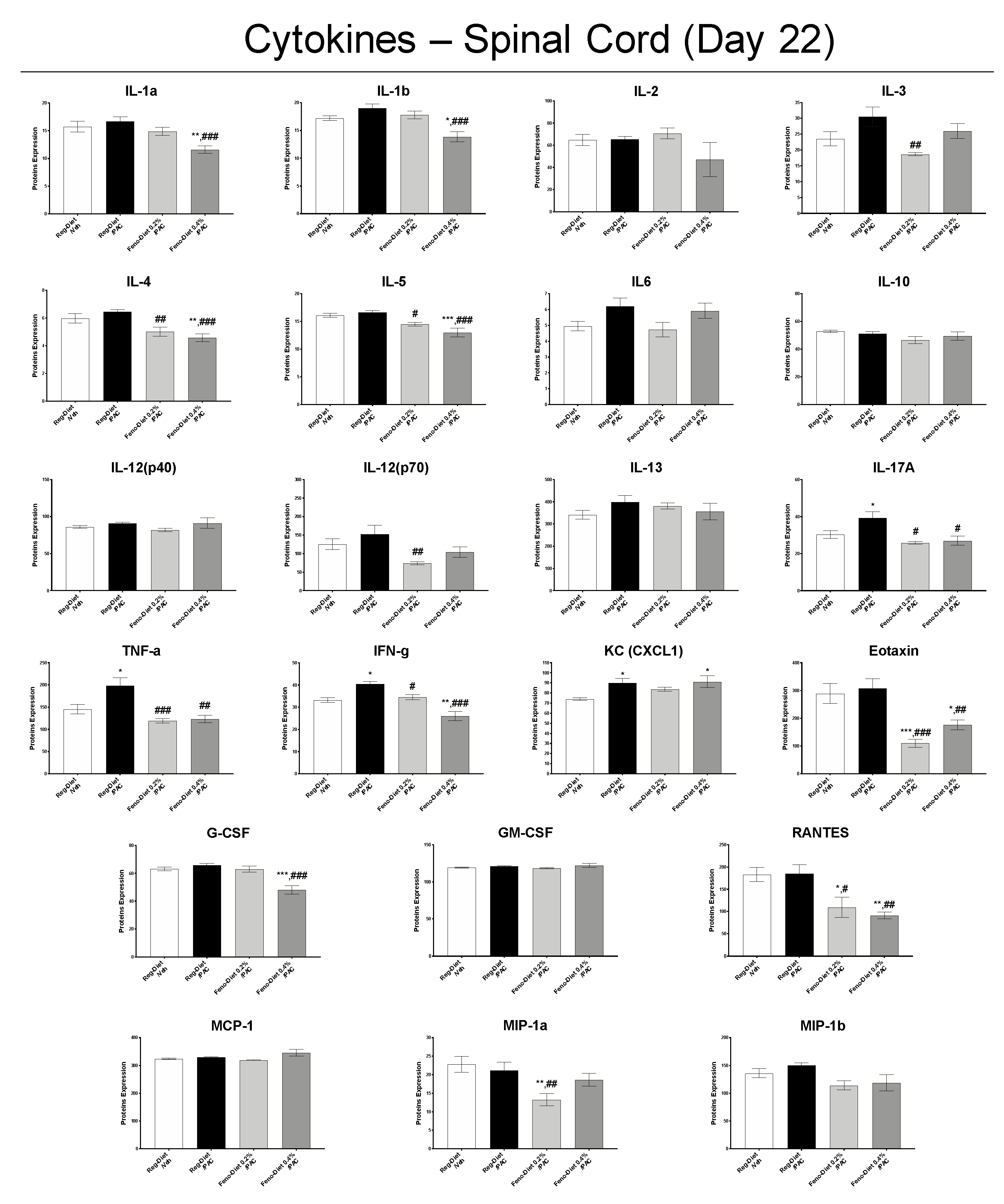
2.6. Fenofibrate Diet Reduces Inflammatory Markers in DRG and Spinal Cord
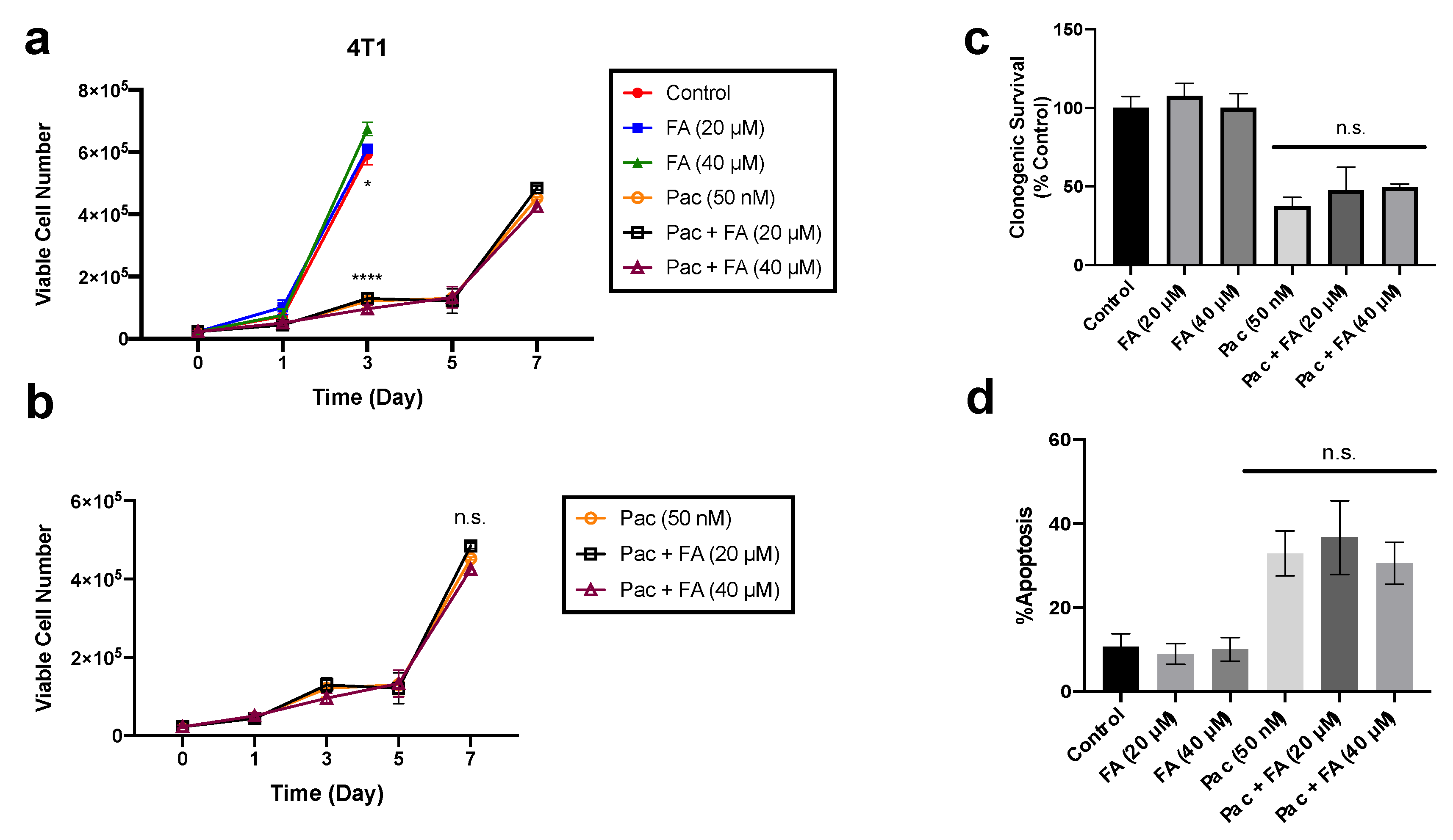
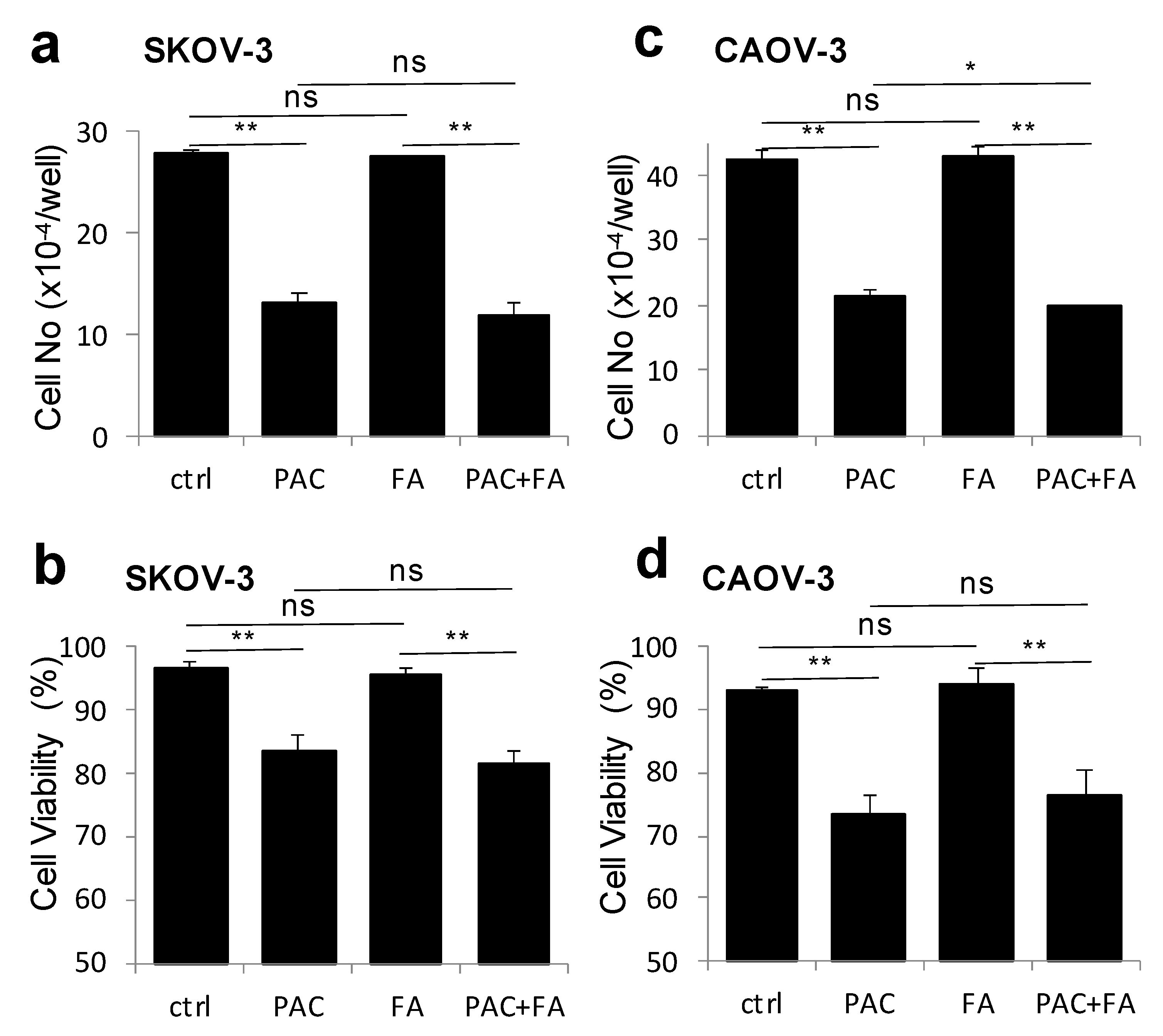
2.7. Fenofibric Acid Did Not Alter Paclitaxel-Induced Cytotoxicity in Breast and Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug and Treatments
4.3. Von Frey Filaments Test
4.4. Acetone Test
4.5. Locomotor Activity
4.6. Wheel Running Test
4.7. Measurement of Caudal Nerve Conduction
4.8. Quantification of Intra-Epidermal Nerve Fibers (IENFs) by Immunohistochemistry
4.9. Electronic Microscopy of Sciatic Nerve
4.10. Cytokines Analysis by Multiplex Assay
4.11. qRT-PCR
4.12. UPLC-MS/MS Method for the Analysis of Fenofibrate and Fenofibric Acid
4.13. Cell Culture
4.14. Cell Viability Assay
4.15. Cell Growth Assay
4.16. Clonogenic Survival Assay
4.17. Assessment of Apoptosis
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilkington, G.; Boland, A.; Brown, T.; Oyee, J.; Bagust, A.; Dickson, R. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015, 70, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Lin, C.S.-Y.; Krishnan, A.V.; Friedlander, M.L.; Lewis, C.R.; Kiernan, M.C. Early, progressive, and sustained dysfunction of sensory axons underlies paclitaxel-induced neuropathy. Muscle Nerve 2011, 43, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Weng, H.R.; Lee, B.N.; Reuben, J.M.; Dougherty, P.M. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006, 72, 151–169. [Google Scholar]
- Windebank, A.J.; Grisold, W. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 2008, 13, 27–46. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Deal, A.M.; Shachar, S.S.; Basch, E.; Reeve, B.B.; Choi, S.K.; Lee, J.T.; Wood, W.A.; Anders, C.K.; Carey, L.A.; et al. Patient-Reported Toxicities During Chemotherapy Regimens in Current Clinical Practice for Early Breast Cancer. Oncologist 2019, 24, 762–771. [Google Scholar] [CrossRef]
- Miaskowski, C.; Mastick, J.; Paul, S.M.; Abrams, G.; Cheung, S.; Sabes, J.H.; Kober, K.M.; Schumacher, M.; Conley, Y.P.; Topp, K.; et al. Impact of chemotherapy-induced neurotoxicities on adult cancer survivors’ symptom burden and quality of life. J. Cancer Surviv. 2018, 12, 234–245. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Yadav, S.; Gupta, R.K.; Waggoner, G.R.; Deloach, A.; Calingasan, N.Y.; Beal, M.F.; Kiaei, M. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Hum. Mol. Genet. 2016, 25, 317–327. [Google Scholar] [CrossRef]
- Othman, A.; Benghozi, R.; Alecu, I.; Wei, Y.; Niesor, E.; von Eckardstein, A.; Hornemann, T. Fenofibrate lowers atypical sphingolipids in plasma of dyslipidemic patients: A novel approach for treating diabetic neuropathy? J. Clin. Lipidol. 2015, 9, 568–575. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.; Bertollo, C.M.; Rocha, L.T.S.; Nascimento, E.B.; Costa, K.A.; Coelho, M.M. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR alpha, and pioglitazone, an agonist of PPAR gamma. Eur. J. Pharm. 2007, 561, 194–201. [Google Scholar] [CrossRef]
- Kostadinova, R.; Wahli, W.; Michalik, L. PPARs in diseases: Control mechanisms of inflammation. Curr. Med. Chem. 2005, 12, 2995–3009. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Xiong, Y.; Coicou, L.G.; Piomelli, D.; Seybold, V. Peroxisome proliferator-activated receptor α mediates acute effects of palmitoylethanolamide on sensory neurons. J. Neurosci. 2012, 32, 12735–12743. [Google Scholar] [CrossRef] [PubMed]
- LoVerme, J.; Russo, R.; La Rana, G.; Fu, J.; Farthing, J.; Mattace-Raso, G.; Meli, R.; Hohmann, A.; Calignano, A.; Piomelli, D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J. Pharm. Exp. 2006, 319, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharm. 2016, 82, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Donvito, G.; Wilkerson, J.L.; Damaj, M.I.; Lichtman, A.H. Palmitoylethanolamide Reverses Paclitaxel-Induced Allodynia in Mice. J. Pharm. Exp. 2016, 359, 310–318. [Google Scholar] [CrossRef]
- Avraham, O.; Deng, P.-Y.; Jones, S.; Kuruvilla, R.; Semenkovich, C.F.; Klyachko, V.A.; Cavalli, V. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 2020, 11, 4891. [Google Scholar] [CrossRef]
- Alagona, P. Fenofibric acid: A new fibrate approved for use in combination with statin for the treatment of mixed dyslipidemia. Vasc. Health Risk Manag. 2010, 6, 351–362. [Google Scholar] [CrossRef]
- Baer, A.N.; Wortmann, R.L. Myotoxicity associated with lipid-lowering drugs. Curr. Opin. Rheumatol. 2007, 19, 67–73. [Google Scholar] [CrossRef]
- Contreras, K.M.; Caillaud, M.; Neddenriep, B.; Bagdas, D.; Roberts, J.L.; Ulker, E.; White, A.B.; Aboulhosn, R.; Toma, W.; Khalefa, T.; et al. Deficit in voluntary wheel running in chronic inflammatory and neuropathic pain models in mice: Impact of sex and genotype. Behav. Brain Res. 2020, 113009. [Google Scholar] [CrossRef]
- Bruna, J.; Alberti, P.; Calls-Cobos, A.; Caillaud, M.; Damaj, M.I.; Navarro, X. Methods for in vivo studies in rodents of chemotherapy induced peripheral neuropathy. Exp. Neurol. 2020, 325, 113154. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.L.; Bennett, G.J. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain 2006, 122, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Dougherty, P.M.; Abdi, S. Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol. Oncol. 2015, 136, 453–459. [Google Scholar] [CrossRef] [PubMed]
- De Frias, F.T.; Rocha, K.C.E.; de Mendonça, M.; Murata, G.M.; Araujo, H.N.; de Sousa, L.G.O.; de Sousa, É.; Hirabara, S.M.; de Leite, N.C.; Carneiro, E.M.; et al. Fenofibrate reverses changes induced by high-fat diet on metabolism in mice muscle and visceral adipocytes. J. Cell Physiol. 2018, 233, 3515–3528. [Google Scholar] [CrossRef]
- Jeong, S.; Han, M.; Lee, H.; Kim, M.; Kim, J.; Nicol, C.J.; Kim, B.H.; Choi, J.H.; Nam, K.-H.; Oh, G.T.; et al. Effects of fenofibrate on high-fat diet-induced body weight gain and adiposity in female C57BL/6J mice. Metabolism 2004, 53, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Slipchenko, M.N.; Cheng, J.-X.; Buhman, K.K. Fenofibrate, a peroxisome proliferator-activated receptor α agonist, alters triglyceride metabolism in enterocytes of mice. Biochim. Biophys. Acta 2011, 1811, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Raslová, K.; Dubovská, D.; Mongiellová, V.; Trnovec, T. Relationship between plasma fenofibric acid levels and the effect of micronized fenofibrate on cholesterol, low-density-lipoprotein cholesterol and apolipoprotein B in patients with primary hypercholesterolemia. Eur. J. Clin. Pharm. 1997, 52, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Toma, W.; Kyte, S.L.; Bagdas, D.; Alkhlaif, Y.; Alsharari, S.D.; Lichtman, A.H.; Chen, Z.-J.; Del Fabbro, E.; Bigbee, J.W.; Gewirtz, D.A.; et al. Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 2017, 117, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Boehmerle, W.; Huehnchen, P.; Peruzzaro, S.; Balkaya, M.; Endres, M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Quasthoff, S.; Hartung, H.P. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002, 249, 9–17. [Google Scholar] [CrossRef]
- Kemp, S.W.P.; Cederna, P.S.; Midha, R. Comparative outcome measures in peripheral regeneration studies. Exp. Neurol. 2017, 287, 348–357. [Google Scholar] [CrossRef]
- Caillaud, M.; Msheik, Z.; Ndong-Ntoutoume, G.M.-A.; Vignaud, L.; Richard, L.; Favreau, F.; Faye, P.-A.; Sturtz, F.; Granet, R.; Vallat, J.-M.; et al. Curcumin-cyclodextrin/cellulose nanocrystals improve the phenotype of Charcot-Marie-Tooth-1A transgenic rats through the reduction of oxidative stress. Free Radic. Biol. Med. 2020, 161, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jang, S.Y.; Shin, Y.K.; Koh, H.; Suh, D.J.; Shinji, T.; Araki, T.; Park, H.T. Mitochondrial swelling and microtubule depolymerization are associated with energy depletion in axon degeneration. Neuroscience 2013, 238, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Cendán, C.M.; Cañizares, F.J.; Cubero, M.A.; Vela, J.M.; Fernández-Segura, E.; Baeyens, J.M. Genetic inactivation and pharmacological blockade of sigma-1 receptors prevent paclitaxel-induced sensory-nerve mitochondrial abnormalities and neuropathic pain in mice. Mol. Pain 2014, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Siau, C.; Bennett, G.J. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 2006, 102, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Park, C.; Lee, J.N.; Park, R. Protective roles of fenofibrate against cisplatin-induced ototoxicity by the rescue of peroxisomal and mitochondrial dysfunction. Toxicol. Appl. Pharm. 2018, 353, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; D’Agostino, G.; Pacini, A.; Russo, R.; Zanardelli, M.; Ghelardini, C.; Calignano, A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: Pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediat. Inflamm. 2013, 2013, 328797. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Sasso, O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef]
- Ba, X.; Wang, J.; Zhou, S.; Luo, X.; Peng, Y.; Yang, S.; Hao, Y.; Jin, G. Cinobufacini protects against paclitaxel-induced peripheral neuropathic pain and suppresses TRPV1 up-regulation and spinal astrocyte activation in rats. Biomed. Pharm. 2018, 108, 76–84. [Google Scholar] [CrossRef]
- Burgos, E.; Gómez-Nicola, D.; Pascual, D.; Martín, M.I.; Nieto-Sampedro, M.; Goicoechea, C. Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur. J. Pharm. 2012, 682, 62–72. [Google Scholar] [CrossRef]
- Kalynovska, N.; Diallo, M.; Sotakova-Kasparova, D.; Palecek, J. Losartan attenuates neuroinflammation and neuropathic pain in paclitaxel-induced peripheral neuropathy. J. Cell Mol. Med. 2020, 24, 7949–7958. [Google Scholar] [CrossRef]
- Lian, X.; Wang, G.; Zhou, H.; Zheng, Z.; Fu, Y.; Cai, L. Anticancer Properties of Fenofibrate: A Repurposing Use. J. Cancer 2018, 9, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Zhao, F.; Xuan, Q.; Shen, Z.; Xiao, J.; Shen, Q. Fenofibrate inhibits the growth of prostate cancer through regulating autophagy and endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2018, 503, 2685–2689. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Schmeel, F.C.; Schmidt-Wolf, I.G.H. In Vitro Apoptosis Induction by Fenofibrate in Lymphoma and Multiple Myeloma. Anticancer Res. 2017, 37, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Ellen, R.L.; McPherson, R. Long-term efficacy and safety of fenofibrate and a statin in the treatment of combined hyperlipidemia. Am. J. Cardiol. 1998, 81, 60B–65B. [Google Scholar] [CrossRef]
- Keating, G.M. Fenofibrate: A review of its lipid-modifying effects in dyslipidemia and its vascular effects in type 2 diabetes mellitus. Am. J. Cardiovasc. Drugs 2011, 11, 227–247. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. NC3Rs Reporting Guidelines Working Group Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharm. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Kreisler, A.; Duhamel, A.; Vanbesien-Mailliot, C.; Destée, A.; Bordet, R. Differing short-term neuroprotective effects of the fibrates fenofibrate and bezafibrate in MPTP and 6-OHDA experimental models of Parkinson’s disease. Behav. Pharm. 2010, 21, 194–205. [Google Scholar] [CrossRef]
- Puligheddu, M.; Pillolla, G.; Melis, M.; Lecca, S.; Marrosu, F.; De Montis, M.G.; Scheggi, S.; Carta, G.; Murru, E.; Aroni, S.; et al. PPAR-alpha agonists as novel antiepileptic drugs: Preclinical findings. PLoS ONE 2013, 8, e64541. [Google Scholar] [CrossRef]
- Chachad, S.S.; Gole, M.; Malhotra, G.; Naidu, R. Comparison of pharmacokinetics of two fenofibrate tablet formulations in healthy human subjects. Clin. Ther. 2014, 36, 967–973. [Google Scholar] [CrossRef]
- Gordon, L.A.; Malati, C.Y.; Hadigan, C.; McLaughlin, M.; Alfaro, R.M.; Calderón, M.M.; Kovacs, J.A.; Penzak, S.R. Lack of an Effect of Ritonavir Alone and Lopinavir-Ritonavir on the Pharmacokinetics of Fenofibric Acid in Healthy Volunteers. Pharmacotherapy 2016, 36, 49–56. [Google Scholar] [CrossRef]
- Godfrey, A.R.; Digiacinto, J.; Davis, M.W. Single-dose bioequivalence of 105-mg fenofibric acid tablets versus 145-mg fenofibrate tablets under fasting and fed conditions: A report of two phase I, open-label, single-dose, randomized, crossover clinical trials. Clin. Ther. 2011, 33, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Wabaidur, S.M.; Kazi, M.; Zeid, A.A. Development of a Stability Indicating UPLC-MS/MS Method for Rapid and Reliable Determination of Fenofibrate in Marketed Product (Lypanthyl 200M) and Human Plasma. J. Pharm. Drug Dev. 2013, 1, 1. [Google Scholar] [CrossRef]
- Scientific Working Group for Forensic Toxicology. Standard Practices for Method Validation in Forensic Toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caillaud, M.; Patel, N.H.; Toma, W.; White, A.; Thompson, D.; Mann, J.; Tran, T.H.; Roberts, J.L.; Poklis, J.L.; Bigbee, J.W.; et al. A Fenofibrate Diet Prevents Paclitaxel-Induced Peripheral Neuropathy in Mice. Cancers 2021, 13, 69. https://doi.org/10.3390/cancers13010069
Caillaud M, Patel NH, Toma W, White A, Thompson D, Mann J, Tran TH, Roberts JL, Poklis JL, Bigbee JW, et al. A Fenofibrate Diet Prevents Paclitaxel-Induced Peripheral Neuropathy in Mice. Cancers. 2021; 13(1):69. https://doi.org/10.3390/cancers13010069
Chicago/Turabian StyleCaillaud, Martial, Nipa H. Patel, Wisam Toma, Alyssa White, Danielle Thompson, Jared Mann, Tammy H. Tran, Jane L. Roberts, Justin L. Poklis, John W. Bigbee, and et al. 2021. "A Fenofibrate Diet Prevents Paclitaxel-Induced Peripheral Neuropathy in Mice" Cancers 13, no. 1: 69. https://doi.org/10.3390/cancers13010069
APA StyleCaillaud, M., Patel, N. H., Toma, W., White, A., Thompson, D., Mann, J., Tran, T. H., Roberts, J. L., Poklis, J. L., Bigbee, J. W., Fang, X., Gewirtz, D. A., & Damaj, M. I. (2021). A Fenofibrate Diet Prevents Paclitaxel-Induced Peripheral Neuropathy in Mice. Cancers, 13(1), 69. https://doi.org/10.3390/cancers13010069






