Tumor Marker-Based Definition of the Transarterial Chemoembolization-Refractoriness in Intermediate-Stage Hepatocellular Carcinoma: A Multi-Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Derivation of Prognostic Models from the Training Cohort
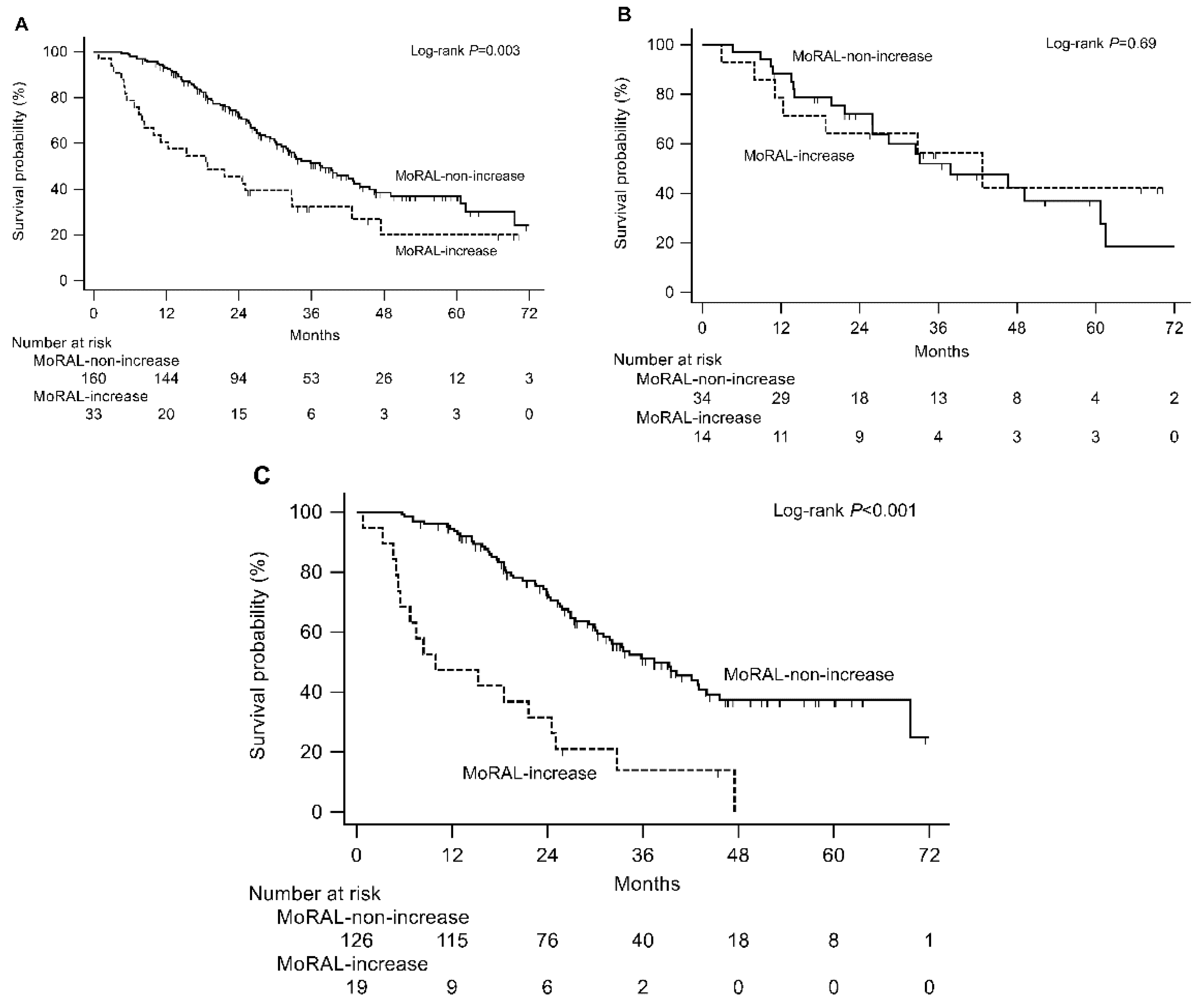
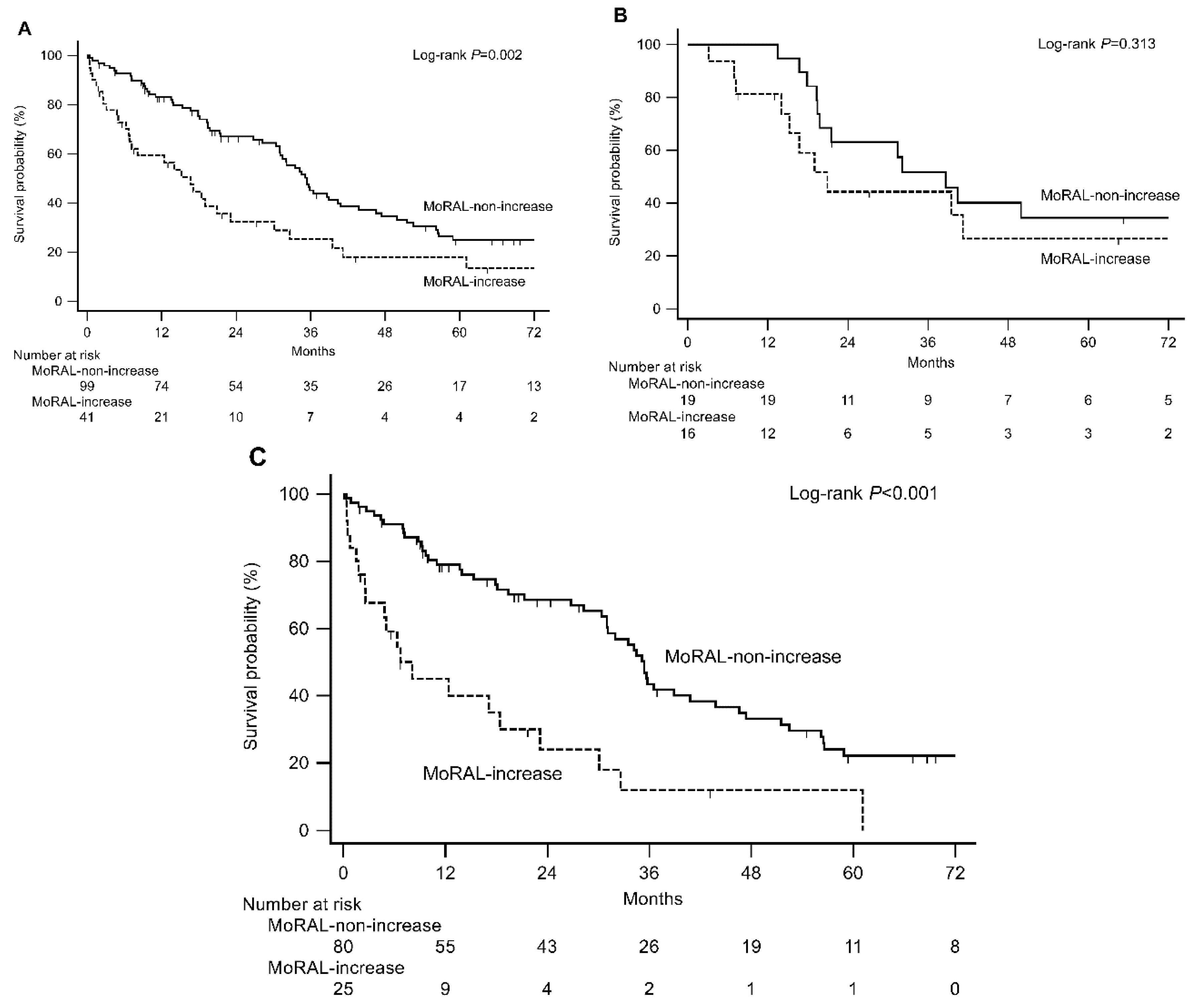
2.3. External Validation in the Hospital and Nationwide Validation Cohorts
2.4. Comparison with Other Indices
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Treatment, Procedures and Assessments
4.3. Endpoint
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.E.; de Lope, C.R.; Bruix, J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Raoul, J.L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. New Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Tovoli, F.; Foerster, F.; Worns, M.A.; Cucchetti, A.; Bolondi, L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J. Hepatol. 2017, 67, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Hucke, F.; Pinter, M.; Graziadei, I.; Vogel, W.; Muller, C.; Heinzl, H.; Trauner, M.; Peck-Radosavljevic, M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology (Baltim. Md.) 2013, 57, 2261–2273. [Google Scholar] [CrossRef]
- Adhoute, X.; Penaranda, G.; Naude, S.; Raoul, J.L.; Perrier, H.; Bayle, O.; Monnet, O.; Beaurain, P.; Bazin, C.; Pol, B.; et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J. Hepatol. 2015, 62, 855–862. [Google Scholar] [CrossRef]
- Kudo, M.; Arizumi, T.; Ueshima, K. Assessment for retreatment (ART) score for repeated transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology (Baltim. Md.) 2014, 59, 2424–2425. [Google Scholar] [CrossRef]
- Arizumi, T.; Ueshima, K.; Iwanishi, M.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; et al. Evaluation of ART Scores for Repeated Transarterial Chemoembolization in Japanese Patients with Hepatocellular Carcinoma. Oncology 2015, 89 (Suppl. 2), 4–10. [Google Scholar] [CrossRef]
- Facciorusso, A.; Bhoori, S.; Sposito, C.; Mazzaferro, V. Repeated transarterial chemoembolization: An overfitting effort? J. Hepatol. 2015, 62, 1440–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloeckner, R.; Pitton, M.B.; Dueber, C.; Schmidtmann, I.; Galle, P.R.; Koch, S.; Worns, M.A.; Weinmann, A. Validation of Clinical Scoring Systems ART and ABCR after Transarterial Chemoembolization of Hepatocellular Carcinoma. J. Vasc. Int. Rad. 2017, 28, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltim. Md.) 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Shim, J.H.; Shin, Y.M.; Kim, K.M.; Lim, Y.S.; Lee, H.C. Clinical significance of the best response during repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma. J. Hepatol. 2014, 60, 1212–1218. [Google Scholar] [CrossRef]
- Jung, E.S.; Kim, J.H.; Yoon, E.L.; Lee, H.J.; Lee, S.J.; Suh, S.J.; Lee, B.J.; Seo, Y.S.; Yim, H.J.; Seo, T.S.; et al. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J. Hepatol. 2013, 58, 1181–1187. [Google Scholar] [CrossRef]
- Prajapati, H.J.; Spivey, J.R.; Hanish, S.I.; El-Rayes, B.F.; Kauh, J.S.; Chen, Z.; Kim, H.S. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann. Oncol. 2013, 24, 965–973. [Google Scholar] [CrossRef]
- Gillmore, R.; Stuart, S.; Kirkwood, A.; Hameeduddin, A.; Woodward, N.; Burroughs, A.K.; Meyer, T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J. Hepatol. 2011, 55, 1309–1316. [Google Scholar] [CrossRef]
- Ronot, M.; Bouattour, M.; Wassermann, J.; Bruno, O.; Dreyer, C.; Larroque, B.; Castera, L.; Vilgrain, V.; Belghiti, J.; Raymond, E.; et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncology 2014, 19, 394–402. [Google Scholar] [CrossRef]
- Donati, O.F.; Do, R.K.; Hotker, A.M.; Katz, S.S.; Zheng, J.; Moskowitz, C.S.; Beattie, C.; Brown, K.T. Interreader and inter-test agreement in assessing treatment response following transarterial embolization for hepatocellular carcinoma. Eur. Radiol. 2015, 25, 2779–2788. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.J.; Lee, K.W.; Kim, S.H.; et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Montal, R.; Andreu-Oller, C.; Bassaganyas, L.; Esteban-Fabro, R.; Moran, S.; Montironi, C.; Moeini, A.; Pinyol, R.; Peix, J.; Cabellos, L.; et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Liu, X.Y. PIVKA-II is an independent prognostic factor for overall survival of HCC patients and maybe associated with epithelial-mesenchymal transition. J. Hepatol. 2015, 63, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Peralvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; Garcia-Caparros, C.; O’Beirne, J.; Poyato-Gonzalez, A.; Ferrin-Sanchez, G.; Montero-Alvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Hoshida, Y.; Toffanin, S.; Lachenmayer, A.; Villanueva, A.; Minguez, B.; Llovet, J.M. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin. Liver Dis. 2010, 30, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, N.; Sato, Y.; Kitao, A.; Ikeda, H.; Sawada-Kitamura, S.; Miyakoshi, M.; Harada, K.; Sasaki, M.; Matsui, O.; Nakanuma, Y. Epidermal growth factor induces cytokeratin 19 expression accompanied by increased growth abilities in human hepatocellular carcinoma. Lab. Investig. 2011, 91, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Uenishi, T.; Ogawa, M.; Ichikawa, T.; Hai, S.; Sakabe, K.; Tanaka, S.; Kato, H.; Mikami, S.; Ikebe, T.; et al. Immunohistologic attempt to find carcinogenesis from hepatic progenitor cell in hepatocellular carcinoma. Dig. Surg. 2005, 22, 364–370. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, Y.B.; Lee, J.H.; Chung, S.W.; Kim, M.S.; Kim, S.W.; Yoon, J.S.; Chang, Y.; Cho, E.J.; Yu, S.J.; et al. O-038: A MoRAL Score Utilizing Serum Tumor Markers Provides Refined Prognostication of Patients with Hepatocellular Carcinoma after Curative Resection: Data from 662 Consecutive Patients. 춘 추 계 학 술 대회 (KASL) 2018, 2018, 29–30. [Google Scholar]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (Lond. Engl.) 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet. Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Cho, E.J.; Kim, S.E.; Suk, K.T.; An, J.; Jeong, S.W.; Chung, W.J.; Kim, Y.J. Current status and strategies for hepatitis B control in Korea. Clin. Mol. Hepatol. 2017, 23, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Do, Y.S.; Choo, S.W.; Lieu, W.C.; Cho, S.K.; Park, K.B.; Yoo, B.C.; Kang, E.H.; Choo, I.W. Diaphragmatic weakness after transcatheter arterial chemoembolization of inferior phrenic artery for treatment of hepatocellular carcinoma. Radiology 2006, 241, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Chung, J.W.; Jae, H.J.; Yoon, J.H.; Lee, J.H.; Kim, Y.J.; Lee, H.S.; Yoon, C.J.; Park, J.H. Caudate lobe hepatocellular carcinoma treated with selective chemoembolization. Radiology 2010, 257, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Arii, S.; Kudo, M.; Ichida, T.; Matsui, O.; Izumi, N.; Matsuyama, Y.; Sakamoto, M.; Nakashima, O.; Ku, Y.; et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J. Hepatol. 2012, 56, 886–892. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.A.; Park, M.S.; Choi, S.Y. MRI Findings and Prediction of Time to Progression of Patients with Hepatocellular Carcinoma Treated with Drug-eluting Bead Transcatheter Arterial Chemoembolization. J. Korean Med Sci. 2015, 30, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodes, J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
| Variables | Training Cohort (n = 193) | Hospital validation Cohort (n = 140) | Nationwide Validation Cohort (n = 149) |
|---|---|---|---|
| Before the first session of TACE | |||
| Age | 63.0 (55.0–70.0) | 60.0 (53.0–68.0) | 61.0 (54.0–68.0) |
| Male sex | 164 (85.0%) | 117 (83.6%) | 127 (85.2%) |
| Etiology | |||
| HBV infection | 132 (68.4%) | 99 (70.7%) | 96 (64.4%) |
| HCV infection | 24 (12.4%) | 12 (8.6%) | 20 (13.4%) |
| Alcohol | 20 (10.4%) | 20 (14.3%) | 24 (16.1%) |
| Others | 17 (8.8%) | 9 (6.4%) | 9 (6.0%) |
| Child-Pugh class | |||
| A | 164 (85%) | 128 (91.4%) | 125 (83.9%) |
| B 7 points | 18 (9.3%) | 9 (6.4%) | 15 (10.1%) |
| ≥B 8 points | 11 (5.7%) | 3 (2.1%) | 9 (6.0%) |
| Tumor number | 4.0 (2.0–5.0) | 4.0 (2.0–5.0) | 4.0 (2.0–5.0) |
| Maximum tumor size | 5.0 (3.3–8.4) | 4.6 (3.5–7.0) | 5.0 (3.4–7.0) |
| AFP, ng/mL | 38.8 (8.2–711.0) | 29.0 (8.8–529.2) | 59.6 (14.6–874.1) |
| PIVKA-II, mAU/mL | 358.0 (48.0–3499.0) | 288.0 (41.5–1200.0) | 693.0 (88.0–2000.0) |
| MoRAL score | 259.2 (89.5–780.6) | 237.8 (93.1–419.3) | 311.9 (133.0–544.6) |
| Before the second session of TACE | |||
| AFP, ng/mL | 15.2 (5.3–85.9) | 14.4 (6.2–120.0) | – |
| PIVKA-II, mAU/mL | 42 (24–236) | 53.8 (22.0–450.0) | – |
| MoRAL score | 93.8 (60.9–231.4) | 103.2 (62.7–263.8) | – |
| Radiological tumor response a | – | ||
| Absent (SD/PD) | 55 (28.5%) | 44 (31.4%) | |
| Present (PR) | 138 (71.5%) | 96 (68.6%) | |
| Dynamics of variables | |||
| Child-Pugh increase | – | ||
| Absent | 145 (75.1%) | 108 (77.1%) | |
| +1 point | 43 (22.3%) | 30 (21.4%) | |
| +2 points or more | 5 (2.6%) | 2 (1.4%) | |
| AST increase >25% | 22 (11.4%) | 26 (18.6%) | – |
| ΔMoRAL | – | ||
| MoRAL-increase | 33 (17.1%) | 41 (29.3%) | |
| MoRAL-non-increase | 163 (82.9%) | 99 (70.7%) |
| Variables | Group Analysis | Overall Survival (Months) | p Value a | ||
|---|---|---|---|---|---|
| n = 193 | Median | 95% CI | |||
| Age | <65 | 109 | 37.8 | 26.9–45.6 | |
| ≥65 | 84 | 32.5 | 26.9–42.9 | 0.685 | |
| Sex | Male | 164 | 33.3 | 26.9–42.1 | |
| Female | 29 | 44 | 29.9–61.5 | 0.331 | |
| Etiology | Viral | 156 | 39.3 | 32.1–45.6 | |
| Other | 37 | 26.9 | 19.7–33.5 | 0.150 | |
| Child–Pugh class | A | 151 | 39.5 | 32.5–45.6 | |
| B | 42 | 23.9 | 17.8–32.8 | 0.020 | |
| Tumor number | <4 | 86 | 40.2 | 28.4–61.5 | |
| ≥4 | 107 | 32.8 | 26.6–39.5 | 0.086 | |
| Tumor size, cm | <5 | 95 | 40.2 | 27.5–46.6 | |
| ≥5 | 98 | 32.8 | 26.9–39.5 | 0.847 | |
| AFP, ng/mL | <200 | 125 | 33.3 | 28.4–60.7 | |
| ≥200 | 68 | 34.3 | 25.6–42.9 | 0.229 | |
| PIVKA–II, mAU/mL | <500 | 105 | 37.8 | 28.4–46.6 | |
| ≥500 | 88 | 33.5 | 25.8–43.0 | 0.991 | |
| Baseline MoRAL score | <314.8 | 105 | 37.8 | 28.4–46.6 | |
| ≥314.8 | 88 | 32.7 | 25.3–43.0 | 0.689 | |
| Radiological tumor response b | Absent (SD/PD) | 55 | 26.9 | 18.5–33.2 | |
| Present (PR) | 138 | 40.2 | 31.8–46.6 | 0.014 | |
| AST increase >25% | Absent | 171 | 37.4 | 31.8–43.0 | |
| Present | 22 | 26.9 | 17.8–29.9 | 0.123 | |
| Child–Pugh increase | Absent | 145 | 42.1 | 33.2–49.1 | |
| Present | 48 | 26.9 | 24.0–32.1 | 0.001 | |
| ΔMoRAL | MoRAL-non-increase | 160 | 37.8 | 31.8–45.6 | |
| MoRAL-increase | 33 | 18.8 | 9.9–32.8 | 0.003 | |
| Variables | Group Analysis | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|
| Overall Survival (Months) | p Value a | Overall Survival (Months) | p Value a | ||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | ||||
| Child-Pugh class | A | 1 (Ref) | – | – | 1 (Ref) | – | – |
| B | 1.57 | 1.02–2.42 | 0.039 | 1.59 | 1.04–2.45 | 0.034 | |
| Child-Pugh score increase | Absent | 1 (Ref) | – | – | 1 (Ref) | – | – |
| Present | 1.84 | 1.23–2.76 | 0.003 | 2.03 | 1.35–3.05 | 0.001 | |
| Radiological tumor response b | Present (PR) | 1 (Ref) | – | – | – | – | – |
| Absent (SD/PD) | 1.63 | 1.08–2.46 | 0.019 | ||||
| ΔMoRAL | MoRAL-non-increase | – | – | – | 1 (Ref) | – | – |
| MoRAL-increase | 2.18 | 1.37–3.46 | 0.001 | ||||
| Prediction Models | Training Cohort | Hospital Validation Cohort |
|---|---|---|
| ΔMoRAL | ||
| c-index (95% CI) | 0.73 (0.62–0.84) | 0.72 (0.62–0.83) |
| Baseline MoRAL score | ||
| c-index (95% CI) | 0.55 (0.48–0.61) | 0.59 (0.53–0.66) |
| pa | 0.005 | 0.04 |
| Baseline AFP | ||
| c-index (95% CI) | 0.55 (0.49–0.61) | 0.59 (0.53–0.66) |
| pa | 0.005 | 0.04 |
| Baseline PIVKA-II | ||
| c-index (95% CI) | 0.52 (0.46–0.59) | 0.59 (0.52–0.66) |
| pa | 0.002 | 0.03 |
| AFP increase | ||
| c-index (95% CI) | 0.60 (0.47–0.74) | 0.64 (0.51–0.77) |
| pa | 0.04 | 0.17 |
| PIVKA-II increase | ||
| c-index (95% CI) | 0.66 (0.53–0.79) | 0.55 (0.41–0.69) |
| pa | 0.02 | 0.002 |
| Radiological tumor response b | ||
| c-index (95% CI) | 0.67 (0.57–0.77) | 0.64 (0.51–0.76) |
| pa | 0.20 | 0.13 |
| ART score of 2.5 | ||
| c-index (95% CI) | 0.68 (0.57–0.79) | 0.61 (0.49–0.73) |
| pa | 0.24 | 0.07 |
| ART score + ΔMoRAL of 2.5 | ||
| c-index (95% CI) | 0.73 (0.63–0.84) | 0.64 (0.52–0.75) |
| pa | 0.52 | 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.S.; Sinn, D.H.; Lee, J.-H.; Kim, H.Y.; Lee, C.-H.; Kim, S.W.; Lee, H.Y.; Nam, J.Y.; Chang, Y.; Lee, Y.B.; et al. Tumor Marker-Based Definition of the Transarterial Chemoembolization-Refractoriness in Intermediate-Stage Hepatocellular Carcinoma: A Multi-Cohort Study. Cancers 2019, 11, 1721. https://doi.org/10.3390/cancers11111721
Yoon JS, Sinn DH, Lee J-H, Kim HY, Lee C-H, Kim SW, Lee HY, Nam JY, Chang Y, Lee YB, et al. Tumor Marker-Based Definition of the Transarterial Chemoembolization-Refractoriness in Intermediate-Stage Hepatocellular Carcinoma: A Multi-Cohort Study. Cancers. 2019; 11(11):1721. https://doi.org/10.3390/cancers11111721
Chicago/Turabian StyleYoon, Jun Sik, Dong Hyun Sinn, Jeong-Hoon Lee, Hwi Young Kim, Cheol-Hyung Lee, Sun Woong Kim, Hyo Young Lee, Joon Yeul Nam, Young Chang, Yun Bin Lee, and et al. 2019. "Tumor Marker-Based Definition of the Transarterial Chemoembolization-Refractoriness in Intermediate-Stage Hepatocellular Carcinoma: A Multi-Cohort Study" Cancers 11, no. 11: 1721. https://doi.org/10.3390/cancers11111721
APA StyleYoon, J. S., Sinn, D. H., Lee, J.-H., Kim, H. Y., Lee, C.-H., Kim, S. W., Lee, H. Y., Nam, J. Y., Chang, Y., Lee, Y. B., Cho, E. J., Yu, S. J., Kim, H.-C., Chung, J. W., Kim, Y. J., & Yoon, J.-H. (2019). Tumor Marker-Based Definition of the Transarterial Chemoembolization-Refractoriness in Intermediate-Stage Hepatocellular Carcinoma: A Multi-Cohort Study. Cancers, 11(11), 1721. https://doi.org/10.3390/cancers11111721





