Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Risk of Bias
2.2. Prognostic Factors
2.3. Predictive Factors
3. Discussion
3.1. Prognostic Factors for Overall Survival in Gastric and Esophageal Cancer
3.2. Predictive Factors for Overall Survival in Gastric Cancer
3.3. Predictive Factors for Overall Survival in Esophageal Cancer
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Literature Search
4.2. Study Selection and Quality Assessment
4.3. Data Extraction and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagen, P.; Hulshof, M.C.; Lanschot, J.J.; Steyerberg, E.W.; Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase III trial. J. Clin. Oncol. 2017, 35, 4004. [Google Scholar]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Park, S.R.; Yang, H.K.; Chung, H.C.; Chung, I.J.; Kim, S.W.; Kim, H.H.; Choi, J.H.; Kim, H.K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [PubMed]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar]
- Shapiro, J.; van Lanschot, J.J.; Hulshof, M.C.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; van Laarhoven, H.W.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Tepper, J.; Krasna, M.J.; Niedzwiecki, D.; Hollis, D.; Reed, C.E.; Goldberg, R.; Kiel, K.; Willett, C.; Sugarbaker, D.; Mayer, R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 2008, 26, 1086–1092. [Google Scholar] [CrossRef]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar]
- Italiano, A. Prognostic or Predictive? It’s Time to Get Back to Definitions! J. Clin. Oncol. 2011, 29, 4718. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Van den Boorn, H.G.; Engelhardt, E.G.; van Kleef, J.; Sprangers, M.A.G.; van Oijen, M.G.H.; Abu-Hanna, A.; Zwinderman, A.H.; Coupe, V.M.H.; van Laarhoven, H.W.M. Prediction models for patients with esophageal or gastric cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192310. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.W.; Karpeh, M.S.; Mazumdar, M.; Brennan, M.F. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J. Clin. Oncol. 2003, 21, 3647–3650. [Google Scholar] [CrossRef]
- Stillwell, A.P.; Ho, Y.H.; Veitch, C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J. Surg. 2011, 35, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Cuyun Carter, G.; Barrett, A.M.; Kaye, J.A.; Liepa, A.M.; Winfree, K.B.; John, W.J. A comprehensive review of nongenetic prognostic and predictive factors influencing the heterogeneity of outcomes in advanced non-small-cell lung cancer. Cancer Manag. Res. 2014, 6, 437–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pietrantonio, F.; Raimondi, A.; Choi, Y.Y.; Kang, W.; Langley, R.E.; Kim, Y.W.; Kim, K.-M.; Nankivell, M.G.; Perrone, F.; Kook, M.-C.; et al. MSI-GC-01: Individual patient data (IPD) meta-analysis of microsatellite instability (MSI) and gastric cancer (GC) from four randomized clinical trials (RCTs). J. Clin. Oncol. 2019, 37, 66. [Google Scholar] [CrossRef]
- The Italian Gastrointestinal Tumor Study Group. Adjuvant treatments following curative resection for gastric cancer. Br. J. Surg. 1988, 75, 1100–1104. [Google Scholar] [CrossRef]
- Allum, W.H.; Hallissey, M.T.; Kelly, K.A. Adjuvant chemotherapy in operable gastric cancer. 5 year follow-up of first British Stomach Cancer Group trial. Lancet 1989, 1, 571–574. [Google Scholar] [CrossRef]
- Bajetta, E.; Buzzoni, R.; Mariani, L.; Beretta, E.; Bozzetti, F.; Bordogna, G.; Aitini, E.; Fava, S.; Schieppati, G.; Pinotti, G.; et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann. Oncol. 2002, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Karina, M.; Papakostas, P.; Kostopoulos, I.; Bobos, M.; Vourli, G.; Samantas, E.; Christodoulou, C.; Pentheroudakis, G.; Pectasides, D.; et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother. Pharmacol. 2010, 65, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Basi, A.; Sohrabkhani, S.; Zamani, F.; Baghai-Wadji, M.; Rabiei, N.; Razavi, S.M.; Ajdarkosh, H. Comparing Efficacy of Preoperative neo-Adjuvant Chemotherapy and Surgery versus Surgery Alone in Patients with Resectable Gastroesophageal Cancer. Int. J. Hematol. Oncol. Stem Cell Res. 2013, 7, 24–28. [Google Scholar]
- Chang, H.M.; Jung, K.H.; Kim, T.Y.; Kim, W.S.; Yang, H.K.; Lee, K.U.; Choe, K.J.; Heo, D.S.; Bang, Y.J.; Kim, N.K. A phase III randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil and mitomycin C versus 5-fluorouracil alone in curatively resected gastric cancer. Ann. Oncol. 2002, 13, 1779–1785. [Google Scholar] [CrossRef]
- De Vita, F.; Giuliani, F.; Orditura, M.; Maiello, E.; Galizia, G.; Di Martino, N.; Montemurro, F.; Carteni, G.; Manzione, L.; Romito, S.; et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: A randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann. Oncol. 2007, 18, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, F.; Gasperoni, S.; Manzione, L.; Bisagni, G.; Labianca, R.; Bravi, S.; Cortesi, E.; Carlini, P.; Bracci, R.; Tomao, S.; et al. Adjuvant chemotherapy in completely resected gastric cancer: A randomized phase III trial conducted by GOIRC. J. Natl. Cancer Inst. 2008, 100, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Lise, M.; Nitti, D.; Marchet, A.; Sahmoud, T.; Buyse, M.; Duez, N.; Fiorentino, M.; Dos Santos, J.G.; Labianca, R.; Rougier, P.; et al. Final results of a phase III clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J. Clin. Oncol. 1995, 13, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yao, S.; Li, X.S.; Kang, H.R.; Yao, F.F.; Du, N. Neoadjuvant Therapy of DOF Regimen Plus Bevacizumab Can Increase Surgical Resection Ratein Locally Advanced Gastric Cancer: A Randomized, Controlled Study. Medicine (Baltimore) 2015, 94, e1489. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Kinoshita, T.; Nashimoto, A.; Sairenji, M.; Yamaguchi, T.; Sakamoto, J.; Fujiya, T.; Inada, T.; Sasako, M.; Ohashi, Y.; et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br. J. Surg. 2007, 94, 1468–1476. [Google Scholar] [CrossRef]
- Neri, B.; Cini, G.; Andreoli, F.; Boffi, B.; Francesconi, D.; Mazzanti, R.; Medi, F.; Mercatelli, A.; Romano, S.; Siliani, L.; et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br. J. Cancer 2001, 84, 878–880. [Google Scholar] [CrossRef]
- Popiela, T.; Kulig, J.; Czupryna, A.; Szczepanik, A.M.; Zembala, M. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer 2004, 7, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Sautner, T.; Hofbauer, F.; Depisch, D.; Schiessel, R.; Jakesz, R. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J. Clin. Oncol. 1994, 12, 970–974. [Google Scholar] [CrossRef]
- Nio, Y.; Koike, M.; Omori, H.; Hashimoto, K.; Itakura, M.; Yano, S.; Higami, T.; Maruyama, R. A randomized consent design trial of neoadjuvant chemotherapy with tegafur plus uracil (UFT) for gastric cancer--a single institute study. Anticancer Res. 2004, 24, 1879–1887. [Google Scholar] [PubMed]
- Park, S.H.; Sohn, T.S.; Lee, J.; Lim, D.H.; Hong, M.E.; Kim, K.M.; Sohn, I.; Jung, S.H.; Choi, M.G.; Lee, J.H.; et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J. Clin. Oncol. 2015, 33, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Bouche, O.; Ychou, M.; Burtin, P.; Bedenne, L.; Ducreux, M.; Lebreton, G.; Baulieux, J.; Nordlinger, B.; Martin, C.; Seitz, J.F.; et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann. Oncol. 2005, 16, 1488–1497. [Google Scholar] [CrossRef]
- Jeung, H.C.; Moon, Y.W.; Rha, S.Y.; Yoo, N.C.; Roh, J.K.; Noh, S.H.; Min, J.S.; Kim, B.S.; Chung, H.C. Phase III trial of adjuvant 5-fluorouracil and adriamycin versus 5-fluorouracil, adriamycin, and polyadenylic-polyuridylic acid (poly A:U) for locally advanced gastric cancer after curative surgery: Final results of 15-year follow-up. Ann. Oncol. 2008, 19, 520–526. [Google Scholar] [CrossRef]
- Krook, J.E.; O’Connell, M.J.; Wieand, H.S.; Beart, R.W., Jr.; Leigh, J.E.; Kugler, J.W.; Foley, J.F.; Pfeifle, D.M.; Twito, D.I. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer 1991, 67, 2454–2458. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, K.H.; Ahn, M.J.; Lee, J.S.; Lee, J.H.; Zang, D.Y.; Suh, C.W.; Kim, S.W.; Kim, W.G.; Kim, J.C.; et al. A randomized trial comparing cisplatin plus 5-fluorouracil with or without levamisole in operable gastric cancer. Korean J. Intern. Med. 1997, 12, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, H.C.; Yoon, C.; Yoon, H.J.; Choi, Y.M.; Cho, K.S. OK-432 and 5-fluorouracil, doxorubicin, and mitomycin C (FAM-P) versus FAM chemotherapy in patients with curatively resected gastric carcinoma: A randomized Phase III trial. Cancer 1998, 83, 2054–2059. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, S.Y.; Shin, I.; Cho, K.S.; Joo, H.Z.; Yoon, C. Randomized Phase III Trial of Cisplatin, Epirubicin, Leucovorin, 5-Fluorouracil (PELF) Combination versus 5-fluorouracil Alone as Adjuvant Chemotherapy in Curative Resected Stage III Gastric Cancer. Cancer Res. Treat. 2004, 36, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.G.; Xua, D.F.; Pu, J.; Zong, C.D.; Li, T.; Tao, G.Z.; Ji, F.Z.; Zhou, X.L.; Han, J.H.; Wang, C.S.; et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother. Oncol. 2012, 104, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, M.; Shimada, S.; Ikeshima, S.; Matsuo, A.; Yagi, Y.; Matsuda, M.; Yonemura, Y.; Baba, H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann. Surg. 2009, 250, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Stenning, S.P.; Smyth, E.C.; Okines, A.F.; Allum, W.H.; Rowley, S.; Stevenson, L.; Grabsch, H.I.; Alderson, D.; Crosby, T.; et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): Primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017, 18, 357–370. [Google Scholar] [CrossRef]
- Bajetta, E.; Floriani, I.; Di Bartolomeo, M.; Labianca, R.; Falcone, A.; Di Costanzo, F.; Comella, G.; Amadori, D.; Pinto, C.; Carlomagno, C.; et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann. Oncol. 2014, 25, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Morita, S.; Tanabe, K.; Nishikawa, K.; Ito, Y.; Matsui, T.; Fujitani, K.; Kimura, Y.; Fujita, J.; Aoyama, T.; et al. Survival results of a randomised two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of S-1 plus cisplatin (SC) and paclitaxel plus cisplatin (PC) followed by D2 gastrectomy for resectable advanced gastric cancer. Eur. J. Cancer 2016, 62, 103–111. [Google Scholar] [PubMed]
- Fuchs, C.S.; Niedzwiecki, D.; Mamon, H.J.; Tepper, J.E.; Ye, X.; Swanson, R.S.; Enzinger, P.C.; Haller, D.G.; Dragovich, T.; Alberts, S.R.; et al. Adjuvant Chemoradiotherapy with Epirubicin, Cisplatin, and Fluorouracil Compared with Adjuvant Chemoradiotherapy with Fluorouracil and Leucovorin after Curative Resection of Gastric Cancer: Results from CALGB 80101 (Alliance). J. Clin. Oncol. 2017, 35, 3671–3677. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.M.; Tang, C.W.; Guo, H.H.; Bao, Y.; Fei, M.Y. Prolonged adjuvant capecitabine chemotherapy improved survival of stage IIIA gastric cancer after D2 gastrectomy. Biomed. Pharmacother. 2015, 72, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Hallissey, M.T.; Dunn, J.A.; Ward, L.C.; Allum, W.H. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: Five-year follow-up. Lancet 1994, 343, 1309–1312. [Google Scholar] [CrossRef]
- Lee, C.K.; Jung, M.; Kim, H.S.; Jung, I.; Shin, D.B.; Kang, S.Y.; Zang, D.Y.; Kim, K.H.; Lee, M.H.; Kim, B.S.; et al. S-1 Based Doublet as an Adjuvant Chemotherapy for Curatively Resected Stage III Gastric Cancer: Results from the Randomized Phase III POST Trial. Cancer Res. Treat. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Cats, A.; Jansen, E.P.M.; van Grieken, N.C.T.; Sikorska, K.; Lind, P.; Nordsmark, M.; Meershoek-Klein Kranenbarg, E.; Boot, H.; Trip, A.K.; Swellengrebel, H.A.M.; et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 616–628. [Google Scholar] [CrossRef]
- Nakajima, T.; Nashimoto, A.; Kitamura, M.; Kito, T.; Iwanaga, T.; Okabayashi, K.; Goto, M. Adjuvant mitomycin and fluorouracil followed by oral uracil plus tegafur in serosa-negative gastric cancer: A randomised trial. Gastric Cancer Surgical Study Group. Lancet 1999, 354, 273–277. [Google Scholar] [CrossRef]
- Grau, J.J.; Estape, J.; Fuster, J.; Filella, X.; Visa, J.; Teres, J.; Soler, G.; Albiol, S.; Garcia-Valdecasas, J.C.; Grande, L.; et al. Randomized trial of adjuvant chemotherapy with mitomycin plus ftorafur versus mitomycin alone in resected locally advanced gastric cancer. J. Clin. Oncol. 1998, 16, 1036–1039. [Google Scholar] [CrossRef]
- Boonstra, J.J.; Kok, T.C.; Wijnhoven, B.P.; van Heijl, M.; van Berge Henegouwen, M.I.; Ten Kate, F.J.; Siersema, P.D.; Dinjens, W.N.; van Lanschot, J.J.; Tilanus, H.W.; et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: Long-term results of a randomized controlled trial. BMC Cancer 2011, 11, 181. [Google Scholar] [CrossRef]
- Alderson, D.; Cunningham, D.; Nankivell, M.; Blazeby, J.M.; Griffin, S.M.; Crellin, A.; Grabsch, H.I.; Langer, R.; Pritchard, S.; Okines, A.; et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): An open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1249–1260. [Google Scholar] [CrossRef]
- Stahl, M.; Walz, M.K.; Riera-Knorrenschild, J.; Stuschke, M.; Sandermann, A.; Bitzer, M.; Wilke, H.; Budach, W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer 2017, 81, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Galais, M.P.; Raoul, J.L.; Bouche, O.; Gourgou-Bourgade, S.; Douillard, J.Y.; Etienne, P.L.; Boige, V.; Martel-Lafay, I.; Michel, P.; et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): Final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014, 15, 305–314. [Google Scholar] [CrossRef]
- Mariette, C.; Dahan, L.; Mornex, F.; Maillard, E.; Thomas, P.A.; Meunier, B.; Boige, V.; Pezet, D.; Robb, W.B.; Le Brun-Ly, V.; et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J. Clin. Oncol. 2014, 32, 2416–2422. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, X. Perioperative versus preoperative chemotherapy with surgery in patients with resectable squamous-cell carcinoma of esophagus: A phase III randomized trial. J. Clin. Oncol. 2014, 32. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; Martz, K.; Herskovic, A.; Leichman, L.; Brindle, J.S.; Vaitkevicius, V.K.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: An intergroup study. J. Clin. Oncol. 1997, 15, 277–284. [Google Scholar] [CrossRef]
- Arnott, S.J.; Duncan, W.; Kerr, G.R.; Walbaum, P.R.; Cameron, E.; Jack, W.J.; Mackillop, W.J. Low dose preoperative radiotherapy for carcinoma of the oesophagus: Results of a randomized clinical trial. Radiother. Oncol. 1992, 24, 108–113. [Google Scholar] [CrossRef]
- Baba, M.; Natsugoe, S.; Shimada, M.; Nakano, S.; Kusano, C.; Fukumoto, T.; Aikou, T.; Akazawa, K. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Dis. Esophagus. 2000, 13, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Badwe, R.A.; Sharma, V.; Bhansali, M.S.; Dinshaw, K.A.; Patil, P.K.; Dalvi, N.; Rayabhattanavar, S.G.; Desai, P.B. The quality of swallowing for patients with operable esophageal carcinoma: A randomized trial comparing surgery with radiotherapy. Cancer 1999, 85, 763–768. [Google Scholar] [CrossRef]
- Bass, G.A.; Furlong, H.; O’Sullivan, K.E.; Hennessy, T.P.; Walsh, T.N. Chemoradiotherapy, with adjuvant surgery for local control, confers a durable survival advantage in adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur. J. Cancer 2014, 50, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, B.H.; Smithers, B.M.; Gebski, V.; Fitzgerald, L.; Simes, R.J.; Devitt, P.; Ackland, S.; Gotley, D.C.; Joseph, D.; Millar, J.; et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol. 2005, 6, 659–668. [Google Scholar] [CrossRef]
- Iizuka, T.; Ide, H.; Kakegawa, T.; Sasaki, K.; Takagi, I.; Ando, N.; Mori, S.; Arimori, M.; Tsugane, S. Preoperative radioactive therapy for esophageal carcinoma. Randomized evaluation trial in eight institutions. Chest 1988, 93, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, K.; Satou, H.; Isono, K.; Mitomi, T.; Endoh, M.; Sugita, M. Immunotherapy for esophageal cancer. A randomized trial in combination with radiotherapy and radiochemotherapy. Cooperative Study Group for Esophageal Cancer in Japan. Am. J. Clin. Oncol. 1995, 18, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.H.; He, S.Q.; Yao, W.Q.; Wang, Y.; Guo, X.M.; Wu, G.D.; Zhu, L.X.; Liu, T.F. Comparison between continuous accelerated hyperfractionated and late-course accelerated hyperfractionated radiotherapy for esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 131–136. [Google Scholar]
- Stahl, M.; Stuschke, M.; Lehmann, N.; Meyer, H.J.; Walz, M.K.; Seeber, S.; Klump, B.; Budach, W.; Teichmann, R.; Schmitt, M.; et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 2005, 23, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Urba, S.G.; Orringer, M.B.; Turrisi, A.; Iannettoni, M.; Forastiere, A.; Strawderman, M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J. Clin. Oncol. 2001, 19, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Chiu, P.W.Y.; Yeung, W.K.; Liu, S.Y.W.; Wong, S.K.H.; Ng, E.K.W. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: Results from a randomized controlled trial. Ann. Oncol. 2013, 24, 165–171. [Google Scholar] [CrossRef]
- Kumar, S.; Dimri, K.; Khurana, R.; Rastogi, N.; Das, K.J.M.; Lal, P. A randomised trial of radiotherapy compared with cisplatin chemo-radiotherapy in patients with unresectable squamous cell cancer of the esophagus. Radiother. Oncol. 2007, 83, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Park, S.I.; Kim, S.B.; Jung, H.Y.; Lee, G.H.; Kim, J.H.; Song, H.Y.; Cho, K.J.; Kim, W.K.; Lee, J.S.; et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann. Oncol. 2004, 15, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Gignoux, M.; Triboulet, J.P.; Tiret, E.; Mantion, G.; Elias, D.; Lozach, P.; Ollier, J.C.; Pavy, J.J.; Mercier, M.; et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N. Engl. J. Med. 1997, 337, 161–167. [Google Scholar] [CrossRef]
- Ma, D.Y.; Tan, B.X.; Liu, M.; Li, X.F.; Zhou, Y.Q.; Lu, Y. Concurrent three-dimensional conformal radiotherapy and chemotherapy for postoperative recurrence of mediastinal lymph node metastases in patients with esophageal squamous cell carcinoma: A phase 2 single-institution study. Radiat. Oncol. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, D.P.; Winter, K.A.; Gunderson, L.L.; Mortimer, J.; Estes, N.C.; Haller, D.G.; Ajani, J.A.; Kocha, W.; Minsky, B.D.; Roth, J.A.; et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): A random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J. Clin. Oncol. 2007, 25, 3719–3725. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yang, Z.; Liu, Y.; Liu, X.; Shang, B.; Jiang, W.P. Postoperative Radiotherapy Improves Survival in Stage pT2N0M0 Esophageal Squamous Cell Carcinoma with High Risk of Poor Prognosis. Ann. Surg. Oncol. 2016, 23, 265–272. [Google Scholar] [CrossRef]
- Crosby, T.; Hurt, C.N.; Falk, S.; Gollins, S.; Mukherjee, S.; Staffurth, J.; Ray, R.; Bashir, N.; Bridgewater, J.A.; Geh, J.I.; et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013, 14, 627–637. [Google Scholar] [CrossRef]
- Xiao, Z.F.; Yang, Z.Y.; Liang, J.; Miao, Y.J.; Wang, M.; Yin, W.B.; Gu, X.Z.; Zhang, D.C.; Zhang, R.G.; Wang, L.J. Value of radiotherapy after radical surgery for esophageal carcinoma: A report of 495 patients. Ann. Thorac. Surg. 2003, 75, 331–336. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; Wang, J.; et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Thuss-Patience, P.; Hayoz, S.; Schacher, S.; Knorrenschild, J.R.; Schnider, A.; Plasswilm, L.; Budach, W.; Eisterer, W.; Hawle, H.; et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: A randomized, open-label, phase III trial (SAKK 75/08). Ann. Oncol. 2018, 29, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, M.; Winter, K.; Ilson, D.; Dicker, A.P.; Kachnic, L.; Konski, A.; Chakravarthy, A.B.; Anker, C.J.; Thakrar, H.; Horiba, N.; et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients with Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1520–1528. [Google Scholar] [CrossRef]
- Von Dobeln, G.A.; Klevebro, F.; Jacobsen, A.B.; Johannessen, H.O.; Nielsen, N.H.; Johnsen, G.; Hatlevoll, I.; Glenjen, N.I.; Friesland, S.; Lundell, L.; et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: Long-term results of a randomized clinical trial. Dis. Esophagus 2019, 32. [Google Scholar] [CrossRef]
- Yu, C.C.; Levison, D.A.; Dunn, J.A.; Ward, L.C.; Demonakou, M.; Allum, W.H.; Hallisey, M.T. Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resectable gastric cancer. Br. J. Cancer 1995, 71, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, M.; Pietrantonio, F.; Pellegrinelli, A.; Martinetti, A.; Mariani, L.; Daidone, M.G.; Bajetta, E.; Pelosi, G.; de Braud, F.; Floriani, I.; et al. Osteopontin, E-cadherin, and beta-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer 2016, 19, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Macdonald, J.S.; Benedetti, J.; Fitzsimmons, T.; Southwest Oncology Group; The Gastric Intergroup. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: The effect of undertreatment. Ann. Surg. Oncol. 2002, 9, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Nakajima, T.; Ohta, K.; Nashimoto, A.; Arai, K.; Hiratsuka, M.; Sasako, M.; Kodera, Y.; Goto, M. Determining prognostic factors for gastric cancer using the regression tree method. Gastric Cancer 2002, 5, 201–207. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.H.; Kim, K.M.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, S.J.; Kim, S.T.; et al. The influence of metastatic lymph node ratio on the treatment outcomes in the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) trial: A phase III trial. J. Gastric Cancer 2016, 16, 105–110. [Google Scholar] [CrossRef]
- Ichikawa, W.; Terashima, M.; Ochiai, A.; Kitada, K.; Kurahashi, I.; Sakuramoto, S.; Katai, H.; Sano, T.; Imamura, H.; Sasako, M. Impact of insulin-like growth factor-1 receptor and amphiregulin expression on survival in patients with stage II/III gastric cancer enrolled in the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer. Gastric Cancer 2017, 20, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Kitada, K.; Ochiai, A.; Ichikawa, W.; Kurahashi, I.; Sakuramoto, S.; Katai, H.; Sano, T.; Imamura, H.; Sasako, M.; et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin. Cancer Res. 2012, 18, 5992–6000. [Google Scholar] [CrossRef] [PubMed]
- Okines, A.F.; Thompson, L.C.; Cunningham, D.; Wotherspoon, A.; Reis-Filho, J.S.; Langley, R.E.; Waddell, T.S.; Noor, D.; Eltahir, Z.; Wong, R.; et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann. Oncol. 2013, 24, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Terashima, M.; Ichikawa, W.; Ochiai, A.; Kitada, K.; Kurahashi, I.; Sakuramoto, S.; Katai, H.; Sano, T.; Imamura, H. Impact of the expression of thymidylate synthase and dihydropyrimidine dehydrogenase genes on survival in stage II/III gastric cancer. Gastric Cancer 2015, 18, 538–548. [Google Scholar] [CrossRef][Green Version]
- Smyth, E.C.; Fassan, M.; Cunningham, D.; Allum, W.H.; Okines, A.F.; Lampis, A.; Hahne, J.C.; Rugge, M.; Peckitt, C.; Nankivell, M.; et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J. Clin. Oncol. 2016, 34, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.A.; Gundacker, H.M.; Benedetti, J.; Macdonald, J.S.; Baranda, J.C.; Levin, W.J.; Blanke, C.D.; Elatre, W.; Weng, P.; Zhou, J.Y.; et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann. Oncol. 2013, 24, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Grau, J.J.; Domingo-Domenech, J.; Morente, V.; Pera, M.; Garcia-Valdecasas, J.C.; Fuster, J.; Bombi, A.; Mellado, B.; Albanell, J.; Gascon, P. Low thymidylate synthase expression in the primary tumor predicts favorable clinical outcome in resected gastric cancer patients treated with adjuvant tegafur. Oncology 2004, 66, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, H.; Liu, X.; Wang, Q.; Zhang, X.; He, J.; Sun, K.; Liu, X.; Zhou, Z.; Xu, N.; et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann. Thorac. Surg. 2014, 98, 513–519. [Google Scholar] [CrossRef]
- Robb, W.B.; Dahan, L.; Mornex, F.; Maillard, E.; Thomas, P.A.; Meunier, B.; Boige, V.; Pezet, D.; Brun-Ly, V.; Bosset, J.F.; et al. Impact of neoadjuvant chemoradiation on lymph node status in esophageal cancer: Post hoc analysis of a randomized controlled trial. Ann. Surg. 2015, 261, 902–908. [Google Scholar] [CrossRef]
- Crosby, T.; Hurt, C.N.; Falk, S.; Gollins, S.; Staffurth, J.; Ray, R.; Bridgewater, J.A.; Geh, J.I.; Cunningham, D.; Blazeby, J.; et al. Long-term results and recurrence patterns from SCOPE-1: A phase II/III randomised trial of definitive chemoradiotherapy+/−cetuximab in oesophageal cancer. Br. J. Cancer 2017, 116, 709–716. [Google Scholar] [CrossRef]
- Cox, S.; Hurt, C.; Grenader, T.; Mukherjee, S.; Bridgewater, J.; Crosby, T. The prognostic value of derived neutrophil to lymphocyte ratio in oesophageal cancer treated with definitive chemoradiotherapy. Radiother. Oncol. 2017, 125, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Powell, C.; Carter, B.; Hurt, C.; Mukherjee, S.; Crosby, T. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: Outcomes from SCOPE1. Br. J. Cancer 2016, 115, 172–177. [Google Scholar] [CrossRef]
- Bascoul-Mollevi, C.; Gourgou, S.; Galais, M.P.; Raoul, J.L.; Bouche, O.; Douillard, J.Y.; Adenis, A.; Etienne, P.L.; Juzyna, B.; Bedenne, L.; et al. Health-related quality of life results from the PRODIGE 5/ACCORD 17 randomised trial of FOLFOX versus fluorouracil-cisplatin regimen in oesophageal cancer. Eur. J. Cancer 2017, 84, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Allum, W.H.; Stenning, S.P.; Bancewicz, J.; Clark, P.I.; Langley, R.E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009, 27, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Claassen, Y.H.M.; van Amelsfoort, R.M.; Hartgrink, H.H.; Dikken, J.L.; de Steur, W.O.; van Sandick, J.W.; van Grieken, N.C.T.; Cats, A.; Boot, H.; Trip, A.K.; et al. Effect of Hospital Volume with Respect to Performing Gastric Cancer Resection on Recurrence and Survival: Results from the CRITICS Trial. Ann. Surg. 2018, 7, 10. [Google Scholar] [CrossRef]
- Davarzani, N.; Hutchins, G.G.A.; West, N.P.; Hewitt, L.C.; Nankivell, M.; Cunningham, D.; Allum, W.H.; Smyth, E.; Valeri, N.; Langley, R.E.; et al. Prognostic value of pathological lymph node status and primary tumour regression grading following neoadjuvant chemotherapy—Results from the MRC OE02 oesophageal cancer trial. Histopathology 2018, 72, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Zhang, X.; Jung, M.; Jung, I.; Park, H.S.; Beom, S.H.; Kim, H.S.; Rha, S.Y.; Kim, H.; Choi, Y.Y.; et al. Immunohistochemistry Biomarkers Predict Survival in Stage II/III Gastric Cancer Patients: From a Prospective Clinical Trial. Cancer Res. 2018, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.A.; Heo, S.J.; et al. Marked Loss of Muscle, Visceral Fat, or Subcutaneous Fat After Gastrectomy Predicts Poor Survival in Advanced Gastric Cancer: Single-Center Study from the CLASSIC Trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Takeuchi, H.; Mizusawa, J.; Igaki, H.; Ozawa, S.; Abe, T.; Nakamura, K.; Kato, K.; Ando, N.; Kitagawa, Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann. Surg. 2016, 8, 8. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.H.; Lee, J.; Kang, W.K. Prognostic value of the metastatic lymph node (N) ratio in the adjuvant chemoradiotherapy in stomach tumors (ARTIST) phase III trial. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Han, D.S.; Suh, Y.S.; Kong, S.H.; Lee, H.J.; Choi, Y.; Aikou, S.; Sano, T.; Park, B.J.; Kim, W.H.; Yang, H.K. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J. Clin. Oncol. 2012, 30, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Dikken, J.L.; Baser, R.E.; Gonen, M.; Kattan, M.W.; Shah, M.A.; Verheij, M.; van de Velde, C.J.; Brennan, M.F.; Coit, D.G. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann. Surg. Oncol. 2013, 20, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Eom, B.W.; Ryu, K.W.; Nam, B.H.; Park, Y.; Lee, H.J.; Kim, M.C.; Cho, G.S.; Kim, C.Y.; Ryu, S.W.; Shin, D.W.; et al. Survival nomogram for curatively resected Korean gastric cancer patients: Multicenter retrospective analysis with external validation. PLoS ONE 2015, 10, e0119671. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Kosugi, S.; Isobe, Y.; Nashimoto, A.; Oda, I.; Hayashi, K.; Miyashiro, I.; Tsujitani, S.; Kodera, Y.; Seto, Y.; et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann. Oncol. 2014, 25, 1179–1184. [Google Scholar] [CrossRef]
- Kim, Y.; Spolverato, G.; Ejaz, A.; Squires, M.H.; Poultsides, G.; Fields, R.C.; Bloomston, M.; Weber, S.M.; Votanopoulos, K.; Acher, A.W.; et al. A nomogram to predict overall survival and disease-free survival after curative resection of gastric adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 1828–1835. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Q.; Chen, S.; Liu, X.; Kong, P.; Zhou, Z.; Zhan, Y.; Xu, D. Nomogram based on systemic inflammatory response markers predicting the survival of patients with resectable gastric cancer after D2 gastrectomy. Oncotarget 2016, 7, 37556–37565. [Google Scholar] [CrossRef]
- Song, K.Y.; Park, Y.G.; Jeon, H.M.; Park, C.H. A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer 2014, 17, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Berenato, R.; Turati, L.; Mennitto, A.; Steccanella, F.; Caporale, M.; Dallera, P.; de Braud, F.; Pezzica, E.; Di Bartolomeo, M.; et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2017, 8, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yuan, P.; Wang, L.; Wang, Y.; Ma, H.; Yuan, X.; Lv, W.; Hu, J. Clinical Nomogram for Predicting Survival of Esophageal Cancer Patients after Esophagectomy. Sci. Rep. 2016, 6, 26684. [Google Scholar] [CrossRef]
- Duan, J.; Deng, T.; Ying, G.; Huang, D.; Zhang, H.; Zhou, L.; Bai, M.; Li, H.; Yang, H.; Qu, Y.; et al. Prognostic nomogram for previously untreated patients with esophageal squamous cell carcinoma after esophagectomy followed by adjuvant chemotherapy. Jpn. J. Clin. Oncol. 2016, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Eil, R.; Diggs, B.S.; Wang, S.J.; Dolan, J.P.; Hunter, J.G.; Thomas, C.R. Nomogram for predicting the benefit of neoadjuvant chemoradiotherapy for patients with esophageal cancer: A SEER-Medicare analysis. Cancer 2014, 120, 492–498. [Google Scholar] [CrossRef]
- Lagarde, S.M.; Reitsma, J.B.; de Castro, S.M.; Ten Kate, F.J.; Busch, O.R.; van Lanschot, J.J. Prognostic nomogram for patients undergoing oesophagectomy for adenocarcinoma of the oesophagus or gastro-oesophageal junction. Br. J. Surg. 2007, 94, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhou, X.; Chen, Q.; Jiang, Y.; Yang, X.; Zheng, W.; Tao, K.; Wu, J.; Yan, Z.; Liu, L.; et al. Prognostic Nomogram for Thoracic Esophageal Squamous Cell Carcinoma after Radical Esophagectomy. PLoS ONE 2015, 10, e0124437. [Google Scholar] [CrossRef][Green Version]
- Coebergh van den Braak, R.R.J.; van Rijssen, L.B.; van Kleef, J.J.; Vink, G.R.; Berbee, M.; van Berge Henegouwen, M.I.; Bloemendal, H.J.; Bruno, M.J.; Burgmans, M.C.; Busch, O.R.C.; et al. Nationwide comprehensive gastro-intestinal cancer cohorts: The 3P initiative. Acta Oncol. 2018, 57, 195–202. [Google Scholar] [CrossRef]
- Verheij, M.; Cats, A.; Jansen Edwin, P.M.; Van Grieken Nicole, C.T.; Aaronson Neil, K.; Boot, H.; Lind Pehr, A.; Meershoek-Klein Kranenbarg, E.; Nordsmark, M.; Putter, H.; et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. Ann. Oncol. 2016, 27, ii140. [Google Scholar] [CrossRef]
- Ge, S.; Xia, X.; Ding, C.; Zhen, B.; Zhou, Q.; Feng, J.; Yuan, J.; Chen, R.; Li, Y.; Ge, Z.; et al. A proteomic landscape of diffuse-type gastric cancer. Nat. Commun. 2018, 9, 1012. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202. [Google Scholar]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Wallmark, J.M.; Lorente, D.; Elez, E.; Raimbourg, J.; Gomez-Roca, C.; Ejadi, S.; Piha-Paul, S.A.; Stein, M.N.; Abdul Razak, A.R.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS ONE 2017, 12, e0189848. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nature Medicine 2018, 24, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Roh, C.K.; Choi, Y.Y.; Choi, S.; Seo, W.J.; Cho, M.; Jang, E.; Son, T.; Kim, H.I.; Kim, H.; Hyung, W.J.; et al. Single Patient Classifier Assay, Microsatellite Instability, and Epstein-Barr Virus Status Predict Clinical Outcomes in Stage II/III Gastric Cancer: Results from CLASSIC Trial. Yonsei Med. J. 2019, 60, 132–139. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Kim, J.; Bowlby, R.; Mungall, A.J.; Robertson, A.G.; Odze, R.D.; Cherniack, A.D.; Shih, J.; Pedamallu, C.S.; Cibulskis, C.; et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169. [Google Scholar] [CrossRef]
- Nakamura, K.; Kato, K.; Igaki, H.; Ito, Y.; Mizusawa, J.; Ando, N.; Udagawa, H.; Tsubosa, Y.; Daiko, H.; Hironaka, S.; et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn. J. Clin. Oncol. 2013, 43, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Attwood, K.; Shah, R.; Nurkin, S.; Hochwald, S.; Kukar, M. Novel Calculator to Estimate Overall Survival Benefit from Neoadjuvant Chemoradiation in Patients with Esophageal Adenocarcinoma. J. Am. Coll. Surg. 2017, 224, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bohanes, P.; Yang, D.; Chhibar, R.S.; Labonte, M.J.; Winder, T.; Ning, Y.; Gerger, A.; Benhaim, L.; Paez, D.; Wakatsuki, T.; et al. Influence of sex on the survival of patients with esophageal cancer. J. Clin. Oncol. 2012, 30, 2265–2272. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
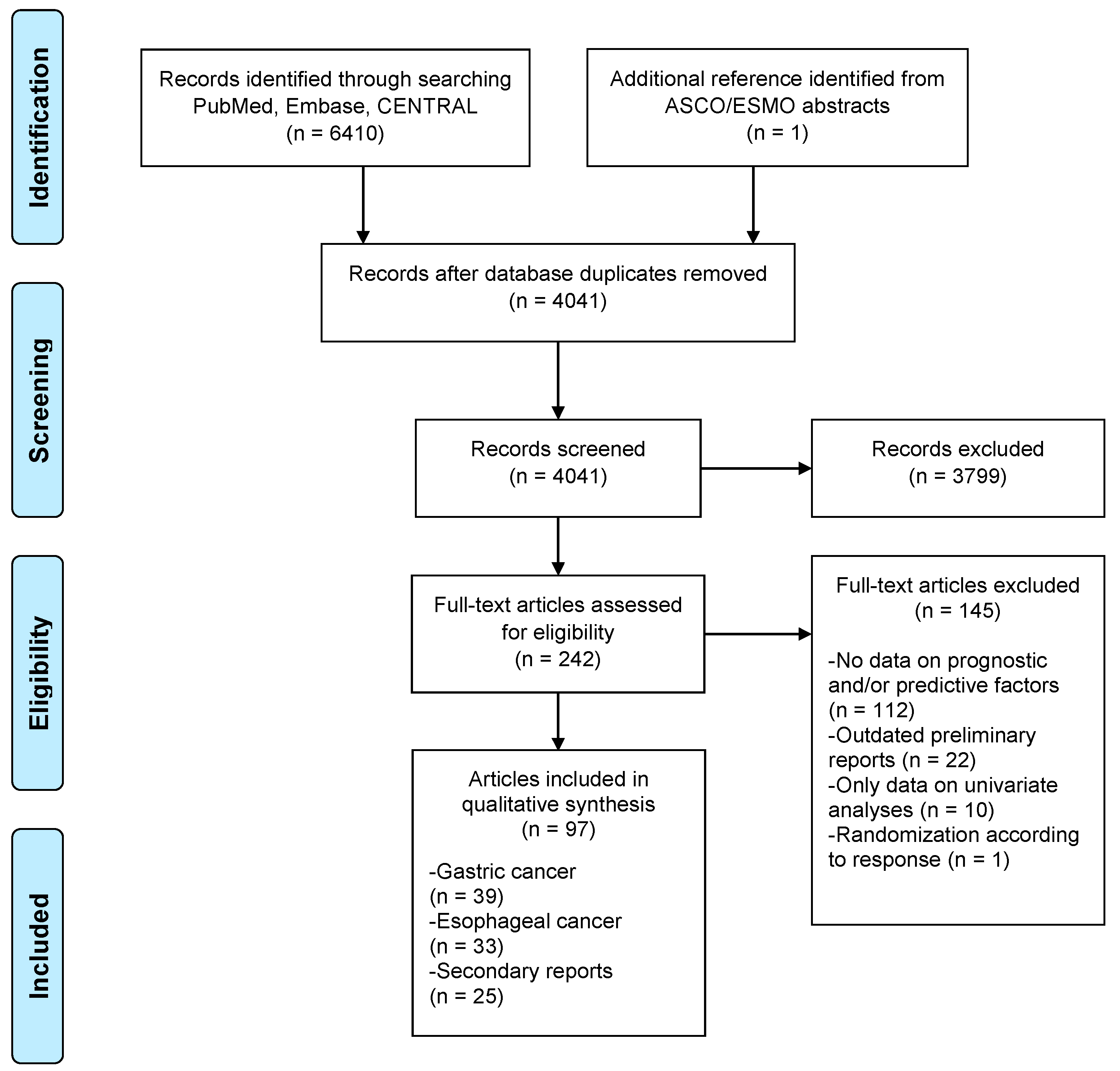


| Gastric Cancer | ||
|---|---|---|
| Prognostic Factor | Subgroup | Strategy |
| Age (years) | <65 vs. ≥65, <60 vs. 60–69, <60 vs. 70–80, <74 vs. ≥74, Increasing age | Neo [103] Adj [4,5,22,26,31,45,87] |
| AREG expression | High vs. Low | Adj [89] |
| Center size (No. trial patients) | Large (≥20) vs. Small (≤5) | Adj [35] |
| Comorbidity | None vs. 1–2 or ≥3 | Neo [103] |
| Country of origin | South Korea vs. China/Taiwan | Adj [5] |
| EGFR expression | Negative vs. Positive | Adj [90] |
| Hemoglobin | NR | Adj [27] |
| Hospital resection volume (per year) | ≥20 vs. 1–20 | Neo [103] |
| IGFR1R expression | Low vs. High | Adj [89] |
| Lymph node ratio invaded/removed | ≤0.3 vs. >0.3, 0–25% vs. >25% | Adj [35,108] |
| M stage | M0 vs. M1 | Neo, Adj [20,33] |
| Maruyama index | <5 vs. ≥5 | Adj [86] |
| Microsatellite instability | MSI vs. MSS | Neo and Adj [18] |
| N stage | N− vs. N+, N0 vs. N1 or N1–2, pN0 vs. pN1–3, N0–N1 vs. N2–N3, N1 vs. N2, N ≤ 6 vs. N > 6, N 0–7 vs. N 8–15, N 0–7 vs. N > 15 | Neo [28,33,103], Adj [5,19,20,21,22,24,25,26,27,42,43,84,87] |
| Number of nodes examined | >15 vs. ≤15 | Adj [26] |
| Osteopontin expression | 0/1+ vs. 3+ | Adj [85] |
| Pathological R stage | R0 vs. R1, R0 vs. R1, R2 | Neo [23,28,103], Adj [20,84] |
| Relative dose intensity (MMC+5-FU+UFT) | >0.98 vs. <0.98 | Adj [87] |
| Race | Asian vs. Caucasian (benefit in subgroup NR) | Neo and Adj [18] |
| Stage | II vs. IIIA, II vs. IIIB, IB/IIA vs. IIB/IIIA, IB/IIA vs. IIIB, IB/IIA vs. IIIC, IB/II vs. III/IV | Adj [4,5,20,24,34,37,40,42,45,53] |
| T stage | pT0/pTis/pT1 vs. pT3-4, pT1 vs. pT2, pT1 vs. pT3-T4, pT1-2 vs. pT3-4, pT3 vs. pT4, Mucosa versus Serosa | Neo [33,103], Adj [5,19,21,22,24,25,26,27,32,35,84,87] |
| Weight | ≥57 kg vs. <57 kg | Adj [5] |
| Weight loss (% of normal weight) | Before surgery | Adj [20] |
| Esophageal cancer | |||
|---|---|---|---|
| Prognostic factor | Histology | Subgroup | Strategy |
| Age (years) | AC and SCC | <60 vs. ≥60, or >70 | Neo [65,70] |
| AC and SCC | 60–69 vs. <60 | dCRT [60] | |
| SCC | <70 vs. ≥70 | Neo [62] | |
| Albumin level | SCC | ≥4 vs. <4, pretreatment level | Neo, Adj, dCRT [71,107] |
| Cisplatin intensity | AC and SCC | ≥75% vs. <75% | dCRT [98] |
| Derived neutrophil to lymphocyte ratio | AC and SCC | <2 vs. ≥2 | dCRT [98] |
| Dose of pre-operative chemotherapy | SCC | ≥90% vs. <90% | Neo [107] |
| EGFR expression | SCC | Low vs. High | Adj [96] |
| Full radiation dose | AC and SCC | Yes vs. No | dCRT [98] |
| Histological grade | AC and SCC | Well/moderate vs. Poor | Neo [61,65] |
| SCC | Well vs. Poor, Well vs. Moderate, Well/moderate vs. Poor | Neo [66,107] Adj [77] | |
| Lymph node involvement | AC and SCC | No vs. Yes | Neo [61] |
| AC and SCC | 0 vs. ≥1 | Neo [58] | |
| SCC | No vs. Yes, Recurrence 1 node vs. >1 node | Neo, dCRT [66,75] | |
| N stage | AC and SCC | cN0 vs. cN1, pN− vs. pN+ | Neo [64], dCRT [60] |
| SCC | cN0 vs. cN1 | Neo [74] | |
| Nutritional Risk Index baseline | AC and SCC | ≥100 vs. <100 | dCRT [100] |
| Nutritional intervention baseline NRI <100 | AC and SCC | Dietary advice vs. None, Oral supplements vs. None, Major intervention vs. None | dCRT [100] |
| R0 resection | AC and SCC | R0 vs. R1/R2/R3 | Neo [76] |
| SCC | R0 vs. R1/R2 | Neo [74] | |
| Stage | AC and SCC | I/II vs. III, I/II vs. III+IV | dCRT [98,101] |
| SCC | I/II vs. III, I/II vs. III+IV, IIA vs. IIB | dCRT [71,75], Adj [77] | |
| T stage | AC and SCC | cT1/T2 vs. cT3 or cT3–4 | Neo [83], dCRT [82] |
| SCC | cT1/T2 vs. cT3 or cT4, pT1–2 vs. pT3 | Neo [10,66,80] dCRT [72] | |
| Tumor size (cm) | AC and SCC | <5 vs. ≥5 | Neo [70] dCRT [82] |
| SCC | <3 vs. ≥3, <5 vs. ≥5, <6 vs. ≥6 | Neo [62], dRT [63] Adj [77] | |
| Gastric Cancer | ||||||
|---|---|---|---|---|---|---|
| Factor | Study | Experimental | vs. | Comparator | HR (95%CI) | N |
| Age (years) | ||||||
| ≥ 70 (vs. <70) | Cunningham 2017 [44] | Peri+Epi+Cis+Cap+BEV | < | Peri+Epi+Cis+Cap | 1.67 (1.10–2.52) | 1063 |
| Gender | ||||||
| Male (vs. female) | Noh 2014 [5] | Ox+Cap | > | Surg | 0.60 (0.45–0.81) | 1035 |
| Female (vs. male) | Bajetta 2014 [45] | Dtx+IRI+Cis+5-FU/Lv | > | 5-FU/Lv | 0.73 (0.54–0.98) | 1100 |
| Male (vs. female) | Smalley 2012 [6] | 5-FU/Lv+RT | > | Surg | 0.69 (0.55–0.86) | 559 |
| T stage | ||||||
| T1, T2 (vs. T3, T4) | Noh 2014 [5] | Ox+Cap | > | Surg | 0.49 (0.33–0.74) | 1035 |
| Nodal stage | ||||||
| N0 (vs. N1, N2) | Sasako 2011 [4] | S-1 | > | Surg | 0.32 (0.13–0.79) | 1034 |
| N1 (vs. N0, N2) | Sasako 2011 [4] | S-1 | > | Surg | 0.61 (0.44–0.84) | 1034 |
| Histopathological grade | ||||||
| G1, G2 (vs. G3, G4, GX) | Noh 2014 [5] | Ox+Cap | > | Surg | 0.50 (0.31–0.82) | 1035 |
| No. of examined lymph nodes | ||||||
| 15–24 (vs. <15 and ≥25) | Bajetta 2014 [45] | Dtx+IRI+Cis+5-FU/Lv | < | 5-FU/Lv | 1.48 (1.09–2.01) | 1100 |
| Race | ||||||
| African American (vs. other) | Smalley 2012 [6] | 5-FU/Lv+RT | > | Surg | 0.56 (0.33–0.95) | 559 |
| Histology | ||||||
| Intestinal (vs. diffuse) | Smalley 2012 [6] | 5-FU/Lv+RT | > | Surg | 0.71 (0.54–0.94) | 559 |
| Gender and histology | ||||||
| Men intestinal (vs. women intestinal, women diffuse, men diffuse) | Smalley 2012 [6] | 5-FU/Lv+RT | > | Surg | 0.72 (0.52–0.98) | 559 |
| Woman diffuse (vs. women intestinal, men intestinal, men diffuse) | Smalley 2012 [6] | 5-FU/Lv+RT | < | Surg | 2.22 (1.14–4.35) | 559 |
| No. nodal metastasis | ||||||
| 0 vs. (>1) | Sasako 2011 [6] | S-1 | > | Surg | 0.32 (0.13–0.79) | 1034 |
| 1–2 vs. (0 and ≥3) | Sasako 2011 [6] | S-1 | > | Surg | 0.45 (0.28–0.75) | 1034 |
| Stage | ||||||
| Stage III (vs. stage II) | Jeung 2008 [36] | Doxo+5-FU+PAU | > | Doxo+5-FU | 0.70 (0.51–0.97) | 292 |
| TS expression | ||||||
| High (vs. low) | Sasako 2011 [92] | S-1 | > | Surg | 0.37 (0.22–0.62) | 808 |
| DPD expression | ||||||
| High (vs. low) | Sasako 2011 [92] | S-1 | > | Surg | 0.52 (0.38–0.72) | 807 |
| HER2 expression FISH | ||||||
| Non-amplified (vs. amplified) | Smalley 2012 [94] | 5-FU/Lv+RT | > | Surg | 0.63 (0.47–0.85) | 258 |
| Microsatellite instability | ||||||
| MSS (vs. MSI) | Pietrantonio 2019 [18] (IPD of MAGIC, ARTIST, ITACA-S and CLASSIC) | Perioperative or adjuvant chemotherapy | > | Surg | 0.73 (0.61–0.86) | 1552 |
| MSS (vs. MSI) | Pietrantonio 2019 [18] (IPD of MAGIC and CLASSIC) | Perioperative or adjuvant chemotherapy | > | Surg | 0.71 (0.58–0.88) | 1552 |
| Esophageal Cancer | ||||||
|---|---|---|---|---|---|---|
| Factor | Study | Experimental | vs. | Comparator | HR (95%CI) | N (histology) |
| Age (years) | ||||||
| >60 (vs. <60) | Boonstra 2011 [54] | Neo+Eto+Cis | > | Surg | 0.63 (0.39–1.00) | 169 (SCC) |
| >70 (vs. <69) | MRC 2002 [11] | Neo+Cis+5-FU | > | Surg | 0.64 (0.44–0.91) | 802 (AC, SCC) |
| <60 (vs. >60) | MRC 2002 [11] | Neo+Cis+5-FU | > | Surg | 0.71 (0.55–0.94) | 802 (AC, SCC) |
| 60–69 (<60 and ≥70) | Alderson 2017 [44] | Neo+Epi+Cis+Cap | > | Neo+Cis+5-FU | 0.72 (0.57–0.91) | 629 (AC) |
| Gender | ||||||
| Male (vs. female) | Crosby 2017 [98] | dCRT-Cis+Cap+CTX+RT | < | dCRT-Cis+Cap+RT | 1.87 (1.26–2.77) | 432 (AC, SCC) |
| Female (vs. male) | Stahl 2017 [56] | Neo+Eto+Cis+5-FU/Lv+RT | > | Neo+Cis+5-FU/Lv | 0.18 (0.03–0.95) | 119 (AC) |
| Female (vs. male) | Liu 2018 [80] | Neo+Vin+Cis+RT | > | Surg | 0.34 (0.15–0.80) | 451 (SCC) |
| Histology | ||||||
| SCC (vs. AC) | Shapiro 2015 [8] | Neo+Ptx+Car+RT | > | Surg | 0.46 (0.26–0.79) | 235 (AC, SCC) |
| Stage | ||||||
| II (vs. III) | Ando 2012 [10] | Neo+Cis+5-FU | > | Cis+5-FU | 0.60 (0.36–0.96) | 329 (SCC) |
| cT stage | ||||||
| cT1-2 (vs. cT3) | Ando 2012 [10] | Neo+Cis+5-FU | > | Cis+5-FU | 0.36 (0.17–0.80) | 330 (SCC) |
| cT3 (vs. cT1-2 and cT4) | Liu 2018 [80] | Neo+Vin+Cis+RT | > | Surg | 0.56 (0.38–0.82) | 451 (SCC) |
| N stage | ||||||
| cN0 (vs. cN1) | Shapiro 2015 [8] | Neo+Ptx+Car+RT | > | Surg | 0.49 (0.30–0.80) | 231 (AC, SCC) |
| N0 (vs. N1) | Alderson 2017 [55] | Neo+Epi+Cis+Cap | > | Neo+Cis+5-FU | 0.63 (0.45–0.90) | 624 (AC) |
| Pretreatment weight loss | ||||||
| >10% (vs. 6–10% and <5%) | Boonstra 2011 [54] | Neo+Eto+Cis | > | Surg | 0.40 (0.22–0.72) | 147 (SCC) |
| Tumor location | ||||||
| Middle (vs. upper-distal third) | Boonstra 2011 [54] | Neo+Eto+Cis | > | Surg | 0.47 (0.29–0.77) | 154 (SCC) |
| Lower third (vs. upper/middle and cardia) | MRC 2002 [11] | Neo+Cis+5-FU | > | Surg | 0.74 (0.61–0.90) | 802 (AC, SCC) |
| Dysphagia score | ||||||
| 1 (vs. 0 and >2) | MRC 2002 [11] | Neo+Cis+5-FU | > | Surg | 0.66 (0.61–0.85) | 754 (AC, SCC) |
| Reasons for no surgery | ||||||
| Comorbidity/poor PS (vs. patient choice and local extensive disease) | Crosby 2017 [98] | dCRT-Cis+Cap+CTX+RT | < | dCRT-Cis+Cap+RT | 3.00 (1.20–7.50) | 432 (AC, SCC) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Ende, T.; ter Veer, E.; Mali, R.M.A.; van Berge Henegouwen, M.I.; Hulshof, M.C.C.M.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 530. https://doi.org/10.3390/cancers11040530
van den Ende T, ter Veer E, Mali RMA, van Berge Henegouwen MI, Hulshof MCCM, van Oijen MGH, van Laarhoven HWM. Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Cancers. 2019; 11(4):530. https://doi.org/10.3390/cancers11040530
Chicago/Turabian Stylevan den Ende, Tom, Emil ter Veer, Rosa M. A. Mali, Mark I. van Berge Henegouwen, Maarten C. C. M. Hulshof, Martijn G. H. van Oijen, and Hanneke W. M. van Laarhoven. 2019. "Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis" Cancers 11, no. 4: 530. https://doi.org/10.3390/cancers11040530
APA Stylevan den Ende, T., ter Veer, E., Mali, R. M. A., van Berge Henegouwen, M. I., Hulshof, M. C. C. M., van Oijen, M. G. H., & van Laarhoven, H. W. M. (2019). Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Cancers, 11(4), 530. https://doi.org/10.3390/cancers11040530






