Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
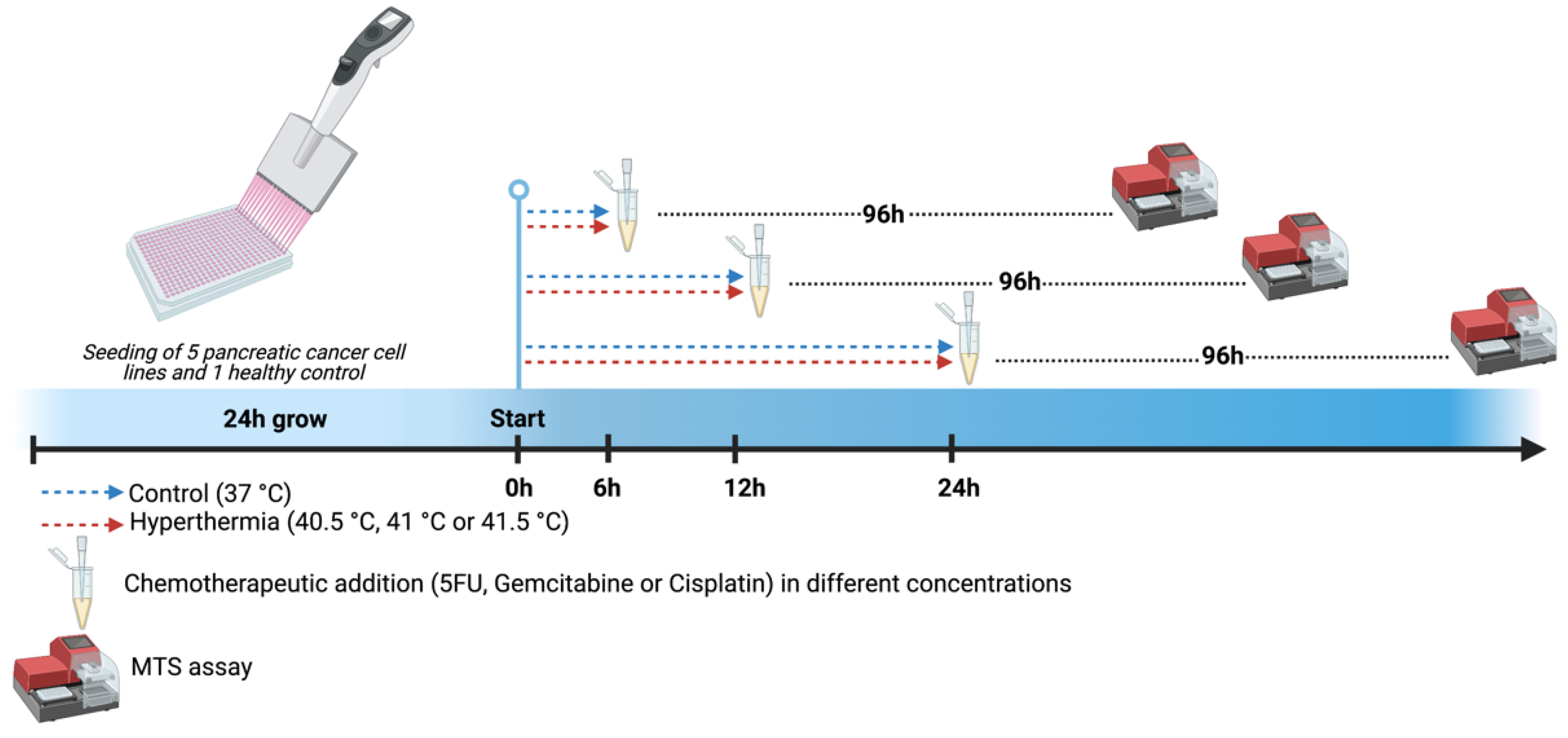
2.2. Chemotherapeutics and Hyperthermia Schedule
2.3. MTS Assay
2.4. Quantitative Reverse-Transcriptase Polymerase-Chain-Reaction (RT-qPCR)
2.5. Apoptosis and Necrosis Assay
2.6. IC50 and Combination Index Calculation
2.7. Statistical Analysis
3. Results
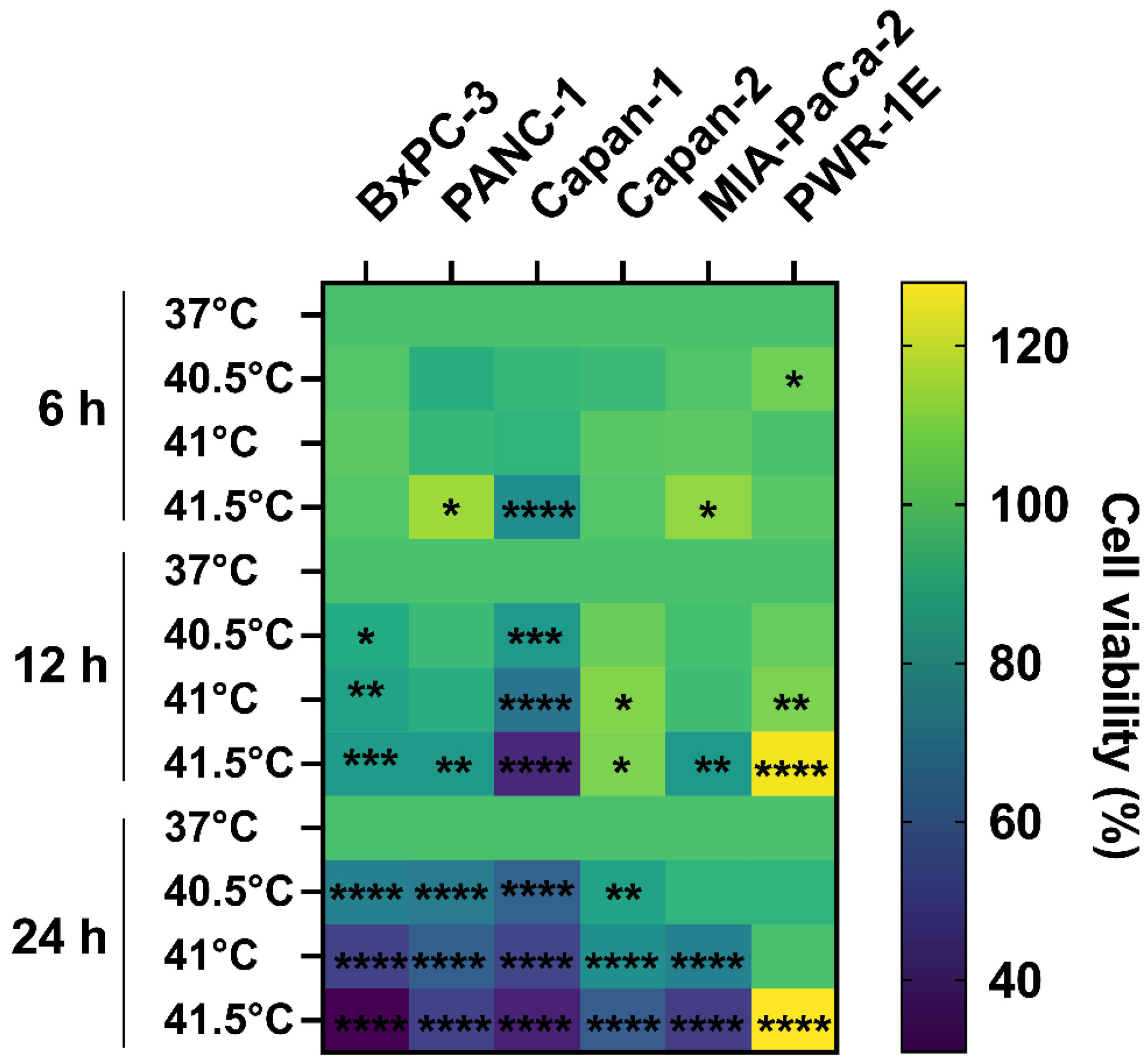
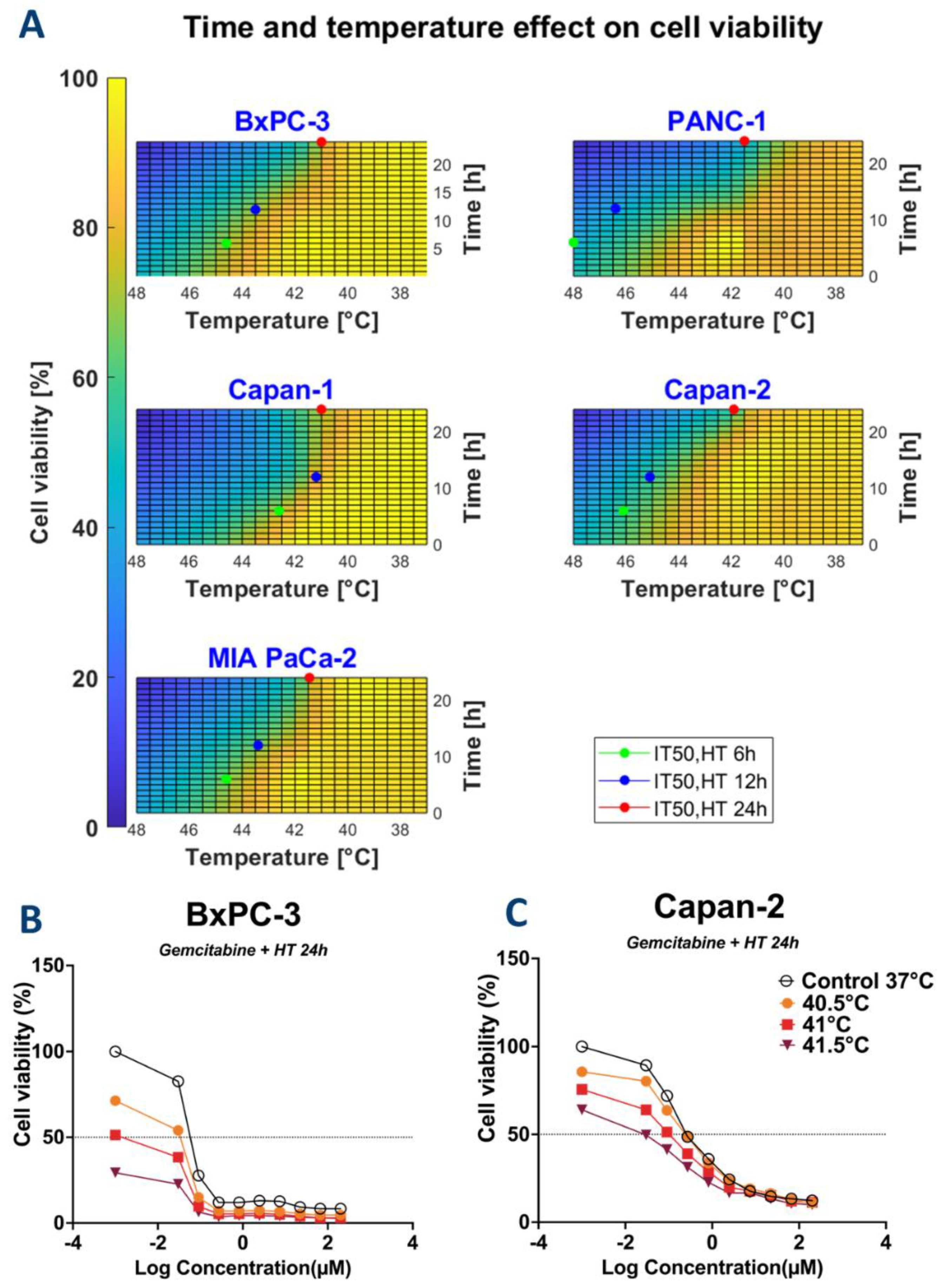
3.1. Cytotoxic Effect of HT Is Cell Type-, Temperature-, and Time-Dependent
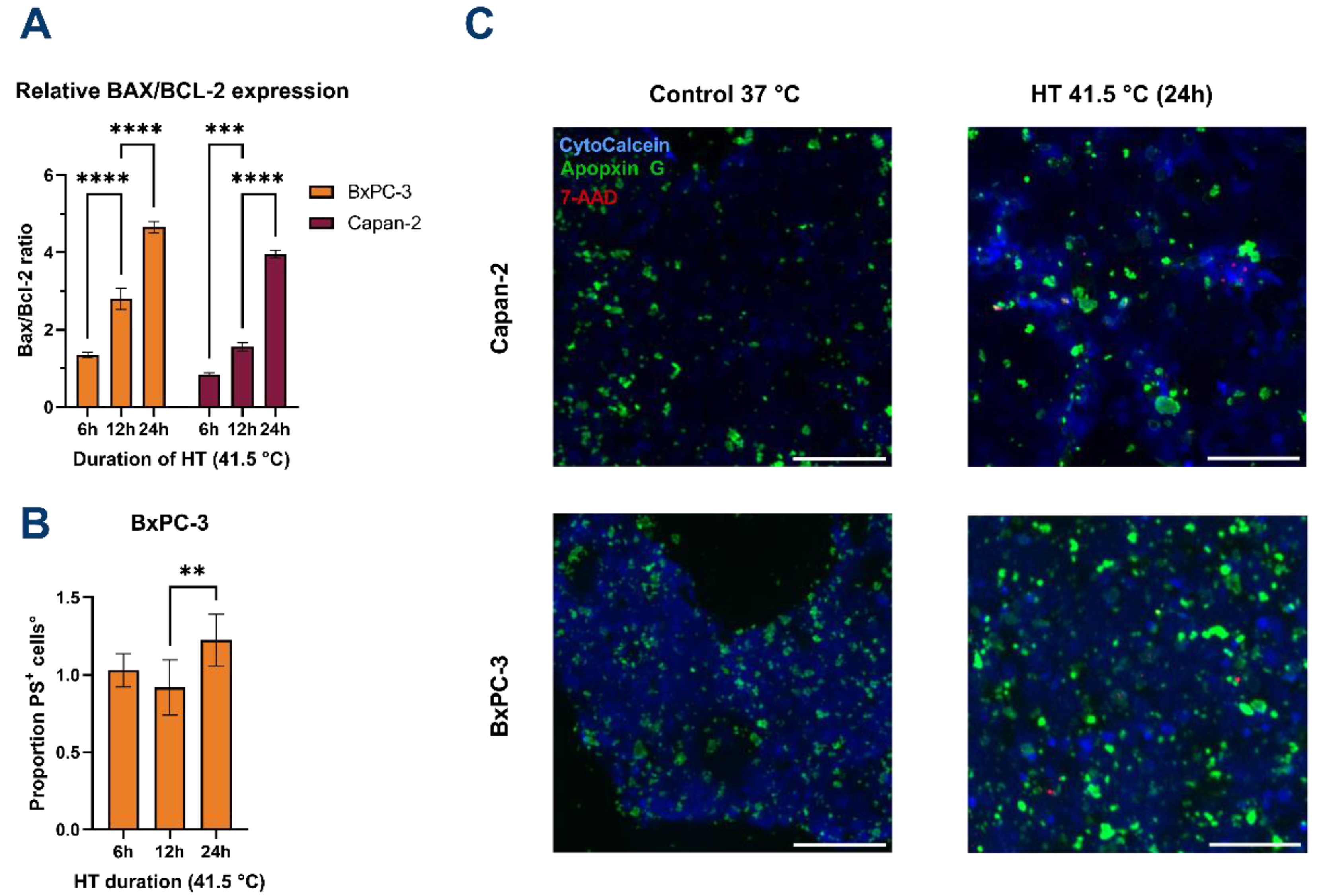
3.2. HT Induces Apoptosis of PDAC
3.3. HT May Reduce the Required Dose of Chemotherapy
3.4. HT Has an Additive/Synergistic Anticancer Effect in Some Pancreatic Cancer Cell Lines
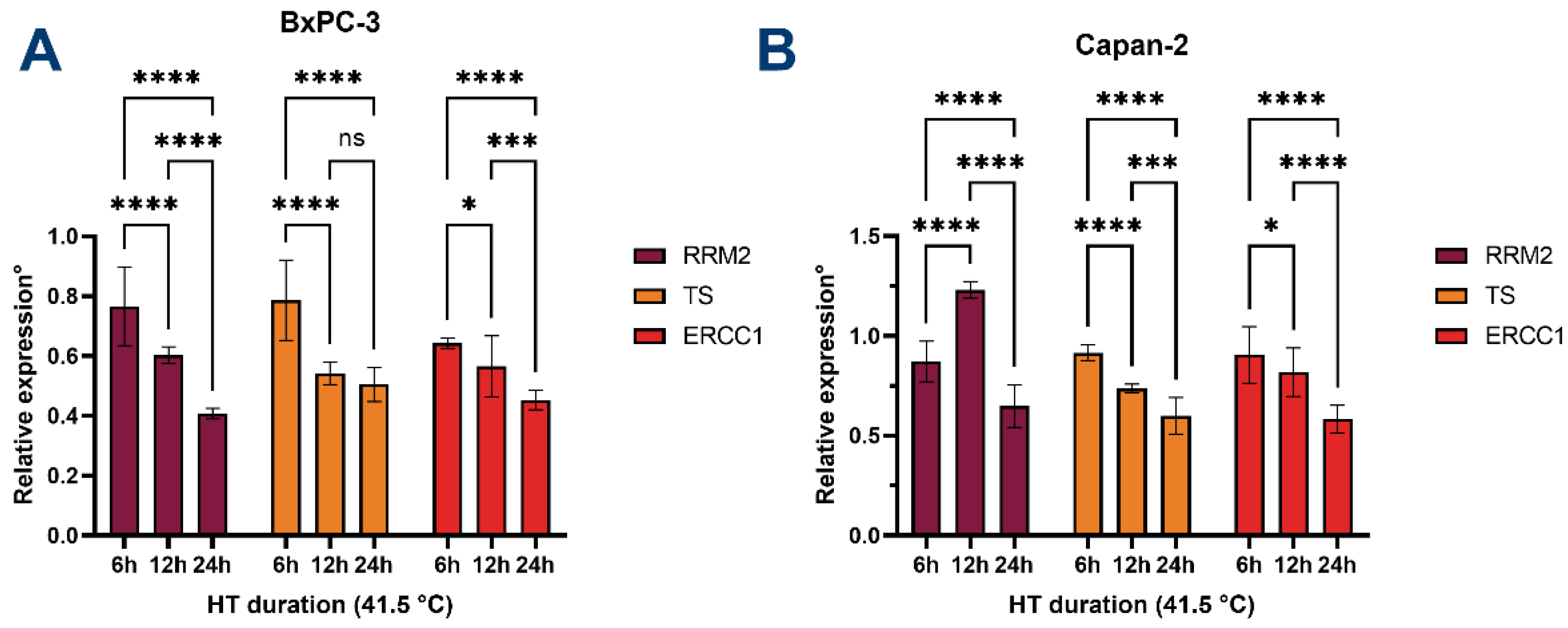
3.5. HT Downregulates the Expression of Chemoresistance-Associated Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 603–605. [Google Scholar] [CrossRef]
- Milella, M.; Bassi, C.; Boggi, U.; Brunetti, O.; Cavaliere, A.; Crippa, S.; De Vita, F.; Falconi, M.; Frassineti, G.L.; Giommoni, E.; et al. Evolving pancreatic cancer treatment: From diagnosis to healthcare management. Crit. Rev. Oncol. 2021, 169, 103571. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; Werba, G.; Lyssiotis, C.A.; Simeone, D.M. The biological underpinnings of therapeutic resistance in pancreatic cancer. Genes Dev. 2021, 35, 940–962. [Google Scholar] [CrossRef] [PubMed]
- Quintiliani, M. Review the Oxygen Effect in Radiation Inactivation of DNA and Enzymest. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 50, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Ba, M.-C.; Long, H.; Cui, S.-Z.; Gong, Y.-F.; Yan, Z.-F.; Wang, S.; Wu, Y.-B. Mild hyperthermia enhances sensitivity of gastric cancer cells to chemotherapy through reactive oxygen species-induced autophagic death. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Baumann, K.W.; Baust, J.M.; Snyder, K.K.; Baust, J.G.; Van Buskirk, R.G. Characterization of Pancreatic Cancer Cell Thermal Response to Heat Ablation or Cryoablation. Technol. Cancer Res. Treat. 2016, 16, 393–405. [Google Scholar] [CrossRef]
- Cihoric, N.; Tsikkinis, A.; Van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int. J. Hyperth. 2015, 31, 609–614. [Google Scholar] [CrossRef]
- van Rhoon, G.C.; Franckena, M.; ten Hagen, T.L.M. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv. Drug Deliv. Rev. 2020, 163–164, 145–156. [Google Scholar] [CrossRef]
- van den Tempel, N.; Horsman, M.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Huang, C.-Q.; Suo, T.; Mei, L.-J.; Yang, G.-L.; Cheng, F.-L.; Zhou, Y.-F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Improves Survival of Patients with Peritoneal Carcinomatosis from Gastric Cancer: Final Results of a Phase III Randomized Clinical Trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, H.; Vanseymortier, M.; Hudry, D.; Bogart, E.; Abdeddaim, C.; Leblanc, E.; Le Deley, M.; Narducci, F. Rationale and study design of the CHIPPI-1808 trial: A phase III randomized clinical trial evaluating hyperthermic intraperitoneal chemotherapy (HIPEC) for stage III ovarian cancer patients treated with primary or interval cytoreductive surgery☆. ESMO Open 2021, 6, 100098. [Google Scholar] [CrossRef]
- Koole, S.N.; Van Lieshout, C.; Van Driel, W.J.; Van Schagen, E.; Sikorska, K.; Kieffer, J.M.; Van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; et al. Cost Effectiveness of Interval Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in Stage III Ovarian Cancer on the Basis of a Randomized Phase III Trial. J. Clin. Oncol. 2019, 37, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- van der Horst, A.; Versteijne, E.; Besselink, M.G.H.; Daams, J.G.; Bulle, E.B.; Bijlsma, M.F.; Wilmink, J.W.; Van Delden, O.M.; Van Hooft, J.E.; Franken, N.A.P.; et al. The clinical benefit of hyperthermia in pancreatic cancer: A systematic review. Int. J. Hyperth. 2018, 34, 969–979. [Google Scholar] [CrossRef]
- Imashiro, C.; Takeshita, H.; Morikura, T.; Miyata, S.; Takemura, K.; Komotori, J. Development of accurate temperature regulation culture system with metallic culture vessel demonstrates different thermal cytotoxicity in cancer and normal cells. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Katschinski, D.M.; Boos, K.; Schindler, S.G.; Fandrey, J. Pivotal Role of Reactive Oxygen Species as Intracellular Mediators of Hyperthermia-induced Apoptosis. J. Biol. Chem. 2000, 275, 21094–21098. [Google Scholar] [CrossRef]
- Fukumura, H.; Sato, M.; Kezuka, K.; Sato, I.; Feng, X.; Okumura, S.; Fujita, T.; Yokoyama, U.; Eguchi, H.; Ishikawa, Y.; et al. Effect of ascorbic acid on reactive oxygen species production in chemotherapy and hyperthermia in prostate cancer cells. J. Physiol. Sci. 2012, 62, 251–257. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, F.; Chen, X.; Luo, M.; Hu, L.; Lu, Y. The Role of Mitochondria-Derived Reactive Oxygen Species in Hyperthermia-Induced Platelet Apoptosis. PLoS ONE 2013, 8, e75044. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Chang, W.-T.; Lin, I.-L.; Chen, Y.-F.; Padalwar, N.B.; Cheng, K.-C.; Teng, Y.-N.; Wang, C.-H.; Chiu, C.-C. The Role of Necroptosis in ROS-Mediated Cancer Therapies and Its Promising Applications. Cancers 2020, 12, 2185. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Singh, V.; Johansson, P.; Torchinsky, D.; Lin, Y.-L.; Öz, R.; Ebenstein, Y.; Hammarsten, O.; Westerlund, F. Quantifying DNA damage induced by ionizing radiation and hyperthermia using single DNA molecule imaging. Transl. Oncol. 2020, 13, 100822. [Google Scholar] [CrossRef]
- Fu, Q.; Huang, T.; Wang, X.; Lu, C.; Liu, F.; Yang, G.; Wang, Y.; Wang, B. Association of elevated reactive oxygen species and hyperthermia induced radiosensitivity in cancer stem-like cells. Oncotarget 2017, 8, 101560–101571. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Akutsu, Y.; Suganami, A.; Qin, W.; Hanari, N.; Murakam, K.; Kano, M.; Usui, A.; Suito, H.; Takahashi, M.; et al. Low-dose hyperthermia enhances the antitumor effects of chemotherapy in squamous cell carcinoma. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Sharma, A.; Özayral, S.; Caserto, J.S.; Cate, R.T.; Anders, N.M.; Barnett, J.D.; Kandala, S.K.; Henderson, E.; Stewart, J.; Liapi, E.; et al. Increased uptake of doxorubicin by cells undergoing heat stress does not explain its synergistic cytotoxicity with hyperthermia. Int. J. Hyperth. 2019, 36, 711–719. [Google Scholar] [CrossRef]
- Ko, S.H.; Ueno, T.; Yoshimoto, Y.; Yoo, J.S.; Abdel-Wahab, O.I.; Abdel-Wahab, Z.; Chu, E.; Pruitt, S.K.; Friedman, H.S.; Dewhirst, M.W.; et al. Optimizing a Novel Regional Chemotherapeutic Agent against Melanoma: Hyperthermia-Induced Enhancement of Temozolomide Cytotoxicity. Clin. Cancer Res. 2006, 12, 289–297. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, Y.; Yang, J.; Zhang, X.; Ma, S. Hyperthermia enhances the sensitivity of pancreatic cancer SW1990 cells to gemcitabine through ROS/JNK signaling. Oncol. Lett. 2018, 16, 6742–6748. [Google Scholar] [CrossRef]
- Oršolić, N.; Odeh, D.; Jembrek, M.J.; Knežević, J.; Kučan, D. Interactions between Cisplatin and Quercetin at Physiological and Hyperthermic Conditions on Cancer Cells In Vitro and In Vivo. Molecules 2020, 25, 3271. [Google Scholar] [CrossRef] [PubMed]
- Bakshandeh-Bath, A.; Stoltz, A.S.; Homann, N.; Wagner, T.; Stoelting, S.; Peters, S.O. Peters Preclinical and Clinical Aspects of Carboplatinand Gemcitabine Combined with Whole-Body Hyperthermia for Pancreatic Adenocarcinoma. Anticancer Res. 2009, 29, 3069–3078. [Google Scholar] [PubMed]
- Dewhirst, M.; Viglianti, B.L.; Lora-Michiels, M.; Hoopes, P.J.; Hanson, M.A. Thermal dose requirement for tissue effect: Experimental and clinical findings. SPIE Proc. 2003, 4954, 37–57. [Google Scholar] [CrossRef]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Hegewisch-Becker, S.; Kerner, T.; Nierhaus, A.; Bakhshandeh-Bath, A.; Janni, W.; Zumschlinge, R.; Sommer, H.; Riess, H.; Wust, P. Current status of radiant whole-body hyperthermia at temperatures >41.5 °C and practical guidelines for the treatment of adults. The German ‘Interdisciplinary Working Group on Hyperthermia’. Int. J. Hyperth. 2005, 21, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The Cellular and Molecular Basis of Hyperthermia. Crit. Rev. Oncol./Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Carneiro, M.W.; Brancato, L.; Wylleman, B.; van Zwol, E.; Conings, L.; Vueghs, P.; Gorbaslieva, I.; Bossche, J.V.D.; Rudenko, O.; Janicot, M.; et al. Safety evaluation of long-term temperature controlled whole-body thermal treatment in female Aachen minipig. Int. J. Hyperth. 2021, 38, 165–175. [Google Scholar] [CrossRef]
- Wylleman, B.; Brancato, L.; Gorbaslieva, I.; van Zwol, E.; da Cunha, M.G.M.C.; Benoit, J.; Tierny, D.; Vueghs, P.; Bossche, J.V.D.; Rudenko, O.; et al. Tolerability of long-term temperature controlled whole-body thermal treatment in advanced cancer-bearing dogs. Int. J. Hyperth. 2021, 39, 48–56. [Google Scholar] [CrossRef]
- Zhu, X.; Trueman, S.; Straubinger, R.M.; Jusko, W.J. Physiologically-based pharmacokinetic and pharmacodynamic models for gemcitabine and birinapant in pancreatic cancer xenografts. J. Pharmacokinet. Pharmacodyn. 2018, 45, 733–746. [Google Scholar] [CrossRef]
- Massihnia, D.; Avan, A.; Funel, N.; Maftouh, M.; Van Krieken, A.; Granchi, C.; Raktoe, R.; Boggi, U.; Aicher, B.; Minutolo, F.; et al. Phospho-Akt overexpression is prognostic and can be used to tailor the synergistic interaction of Akt inhibitors with gemcitabine in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 9. [Google Scholar] [CrossRef]
- Toffalorio, F.; Giovannetti, E.; De Pas, T.; Radice, D.; Pelosi, G.; Manzotti, M.; Minocci, D.; Spaggiari, L.; Spitaleri, G.; Noberasco, C.; et al. Expression of gemcitabine- and cisplatin-related genes in non-small-cell lung cancer. Pharmacogenom. J. 2009, 10, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.T.; Sousa, D.; Paiva, A.M.; Palmeira, A.; Barbosa, J.; Pedro, M.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. Modulation of Autophagy by a Thioxanthone Decreases the Viability of Melanoma Cells. Molecules 2016, 21, 1343. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- van der Heijden, A.G.; Verhaegh, G.; Jansen, C.F.; Schalken, J.A.; Witjes, J. Effect of Hyperthermia on the Cytotoxicity of 4 Chemotherapeutic Agents Currently Used for the Treatment of Transitional Cell Carcinoma of the Bladder: An In Vitro Study. J. Urol. 2005, 173, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Maftouh, M.; Avan, A.; Funel, N.; Frampton, A.E.; Fiuji, H.; Pelliccioni, S.; Castellano, L.; Galla, V.; Peters, G.J.; Giovannetti, E. miR-211 Modulates Gemcitabine Activity Through Downregulation of Ribonucleotide Reductase and Inhibits the Invasive Behavior of Pancreatic Cancer Cells. Nucleosides Nucleotides Nucleic Acids 2014, 33, 384–393. [Google Scholar] [CrossRef]
- Sarin, N.; Engel, F.; Kalayda, G.V.; Mannewitz, M.; Cinatl, J.; Rothweiler, F.; Michaelis, M.; Saafan, H.; Ritter, C.A.; Jaehde, U.; et al. Cisplatin resistance in non-small cell lung cancer cells is associated with an abrogation of cisplatin-induced G2/M cell cycle arrest. PLoS ONE 2017, 12, e0181081. [Google Scholar] [CrossRef]
- Ceppi, P.; Volante, M.; Novello, S.; Rapa, I.; Danenberg, K.; Danenberg, P.; Cambieri, A.; Selvaggi, G.; Saviozzi, S.; Calogero, R.; et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann. Oncol. 2006, 17, 1818–1825. [Google Scholar] [CrossRef]
- Peters, G.; Backus, H.; Freemantle, S.; van Triest, B.; Codacci-Pisanelli, G.; van der Wilt, C.; Smid, K.; Lunec, J.; Calvert, A.; Marsh, S.; et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2002, 1587, 194–205. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.D.; Blanco, V.M.; Sulaiman, M.K.; Lakshmi Vallabhapurapu, S.; Chu, Z.; Franco, R.S.; Qi, X. Variation in Human Cancer Cell External Phosphatidylserine Is Regulated by Flippase Activity and Intracellular Calcium. Oncotarget 2015, 6, 343756. [Google Scholar] [CrossRef]
- Bull, J.M.C.; Scott, G.L.; Strebel, F.R.; Nagle, V.L.; Oliver, D.; Redwine, M.; Rowe, R.W.; Ahn, C.W.; Koch, S.M. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-α?: A description of a phase I-II protocol. Int. J. Hyperth. 2008, 24, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Tempel, N.V.D.; Zelensky, A.N.; Odijk, H.; Laffeber, C.; Schmidt, C.K.; Brandsma, I.; Demmers, J.; Krawczyk, P.M.; Kanaar, R. on the Mechanism of Hyperthermia-Induced BRCA2 Protein Degradation. Cancers 2019, 11, 97. [Google Scholar] [CrossRef]
- Ahmed, K.; Zaidi, S.F.; Rehman, R.; Kondo, T. Hyperthermia and protein homeostasis: Cytoprotection and cell death. J. Therm. Biol. 2020, 91, 102615. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C. Role of the Heat Shock Response and Molecular Chaperones in Oncogenesis and Cell Death. JNCI J. Natl. Cancer Inst. 2000, 92, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Neznanov, N.; Komarov, A.P.; Neznanova, L.; Stanhope-Baker, P.; Gudkov, A.V. Proteotoxic stress targeted therapy (PSTT): Induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget 2011, 2, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, M.; Szymańska, Z.; Lasota, S.; Gambin, A. Modelling the efficacy of hyperthermia treatment. J. R. Soc. Interface 2013, 10, 20130527. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, P.E.; Olive, K.P. Pancreatic cancer: Why is it so hard to treat? Ther. Adv. Gastroenterol. 2013, 6, 321–337. [Google Scholar] [CrossRef]
- Grasso, C.; Jansen, G.; Giovannetti, E. Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Crit. Rev. Oncol. 2017, 114, 139–152. [Google Scholar] [CrossRef] [PubMed]
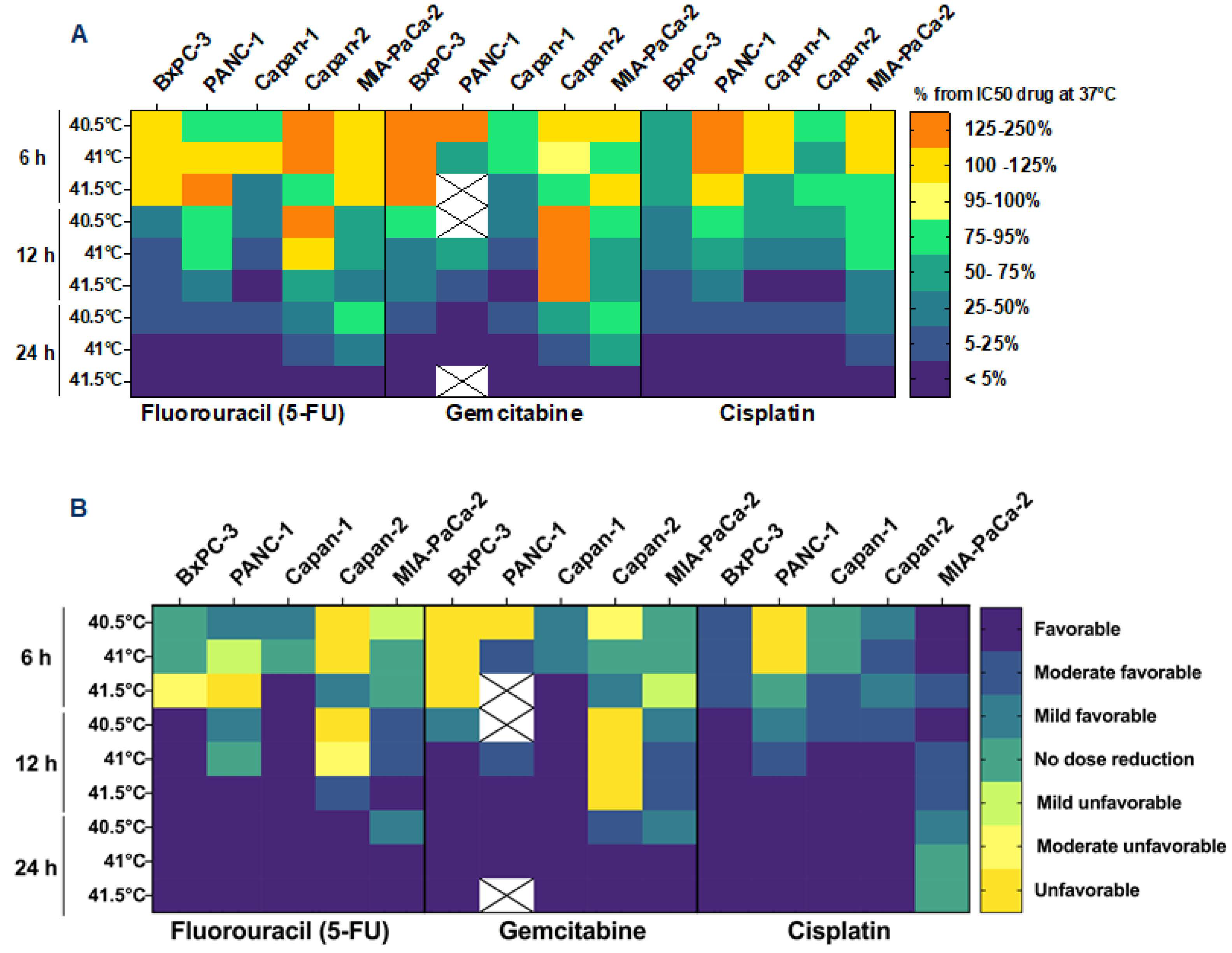
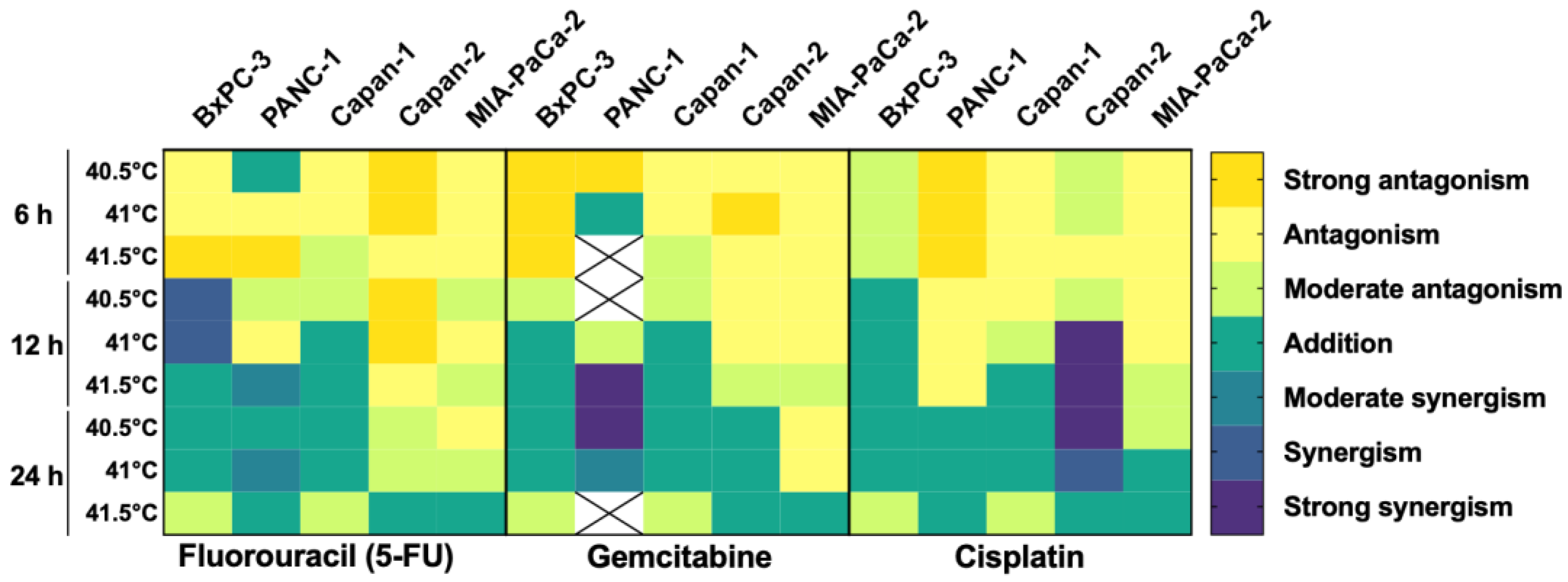
| BxPC-3 | PANC-1 | Capan-1 | Capan-2 | MIA-PaCa-2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Time | ||||||||||||||||
| 5-Fluorouracil | 24 h | 40.5 | 41 | 5.04 | 0.90 | 41.5 | 9.11 | 1.63 | 41 | 0.26 | 0.018 | 41.9 | 41.36 | 13.57 | 41.45 | 5.9 | 4.70 |
| 41 | 0.04 | 0.12 | 0.0036 | 6.03 | 2.37 | ||||||||||||
| 41.5 | 0.00015 | 0.13 | 0.0002 | 1.18 | 0.03 | ||||||||||||
| Gemcitabine | 24 h | 40.5 | 41 | 0.06 | 0.01 | 41.5 | 19.34 | 0.20 | 41 | 0.06 | 0.0055 | 41.9 | 0.40 | 0.28 | 41.45 | 0.12 | 0.10 |
| 41 | 0.0016 | 0.05 | 0.0013 | 0.08 | 0.06 | ||||||||||||
| 41.5 | 0.00006 | - | 0.0002 | 0.015 | 0.0008 | ||||||||||||
| Cisplatin | 24 h | 40.5 | 41 | 1.40 | 0.12 | 41.5 | 12.11 | 1.03 | 41 | 0.27 | 0.017 | 41.9 | 0.27 | 0.017 | 41.45 | 5.59 | 2.72 |
| 41 | 0.0016 | 0.06 | 0.005 | 0.0053 | 0.33 | ||||||||||||
| 41.5 | 0.00012 | 0.014 | 0.0007 | 0.0007 | 0.0007 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurici, C.E.; Colenbier, R.; Wylleman, B.; Brancato, L.; van Zwol, E.; Van den Bossche, J.; Timmermans, J.-P.; Giovannetti, E.; Mori da Cunha, M.G.M.C.; Bogers, J. Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines. Biomolecules 2022, 12, 651. https://doi.org/10.3390/biom12050651
Maurici CE, Colenbier R, Wylleman B, Brancato L, van Zwol E, Van den Bossche J, Timmermans J-P, Giovannetti E, Mori da Cunha MGMC, Bogers J. Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines. Biomolecules. 2022; 12(5):651. https://doi.org/10.3390/biom12050651
Chicago/Turabian StyleMaurici, Costanza E., Robin Colenbier, Britta Wylleman, Luigi Brancato, Eke van Zwol, Johan Van den Bossche, Jean-Pierre Timmermans, Elisa Giovannetti, Marina G. M. C. Mori da Cunha, and Johannes Bogers. 2022. "Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines" Biomolecules 12, no. 5: 651. https://doi.org/10.3390/biom12050651
APA StyleMaurici, C. E., Colenbier, R., Wylleman, B., Brancato, L., van Zwol, E., Van den Bossche, J., Timmermans, J.-P., Giovannetti, E., Mori da Cunha, M. G. M. C., & Bogers, J. (2022). Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines. Biomolecules, 12(5), 651. https://doi.org/10.3390/biom12050651








