A Systematic Review and Meta-Analysis of Serum Concentrations of Ischaemia-Modified Albumin in Acute Ischaemic Stroke, Intracerebral Haemorrhage, and Subarachnoid Haemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Statistical Analysis
3. Results
3.1. Systematic Research
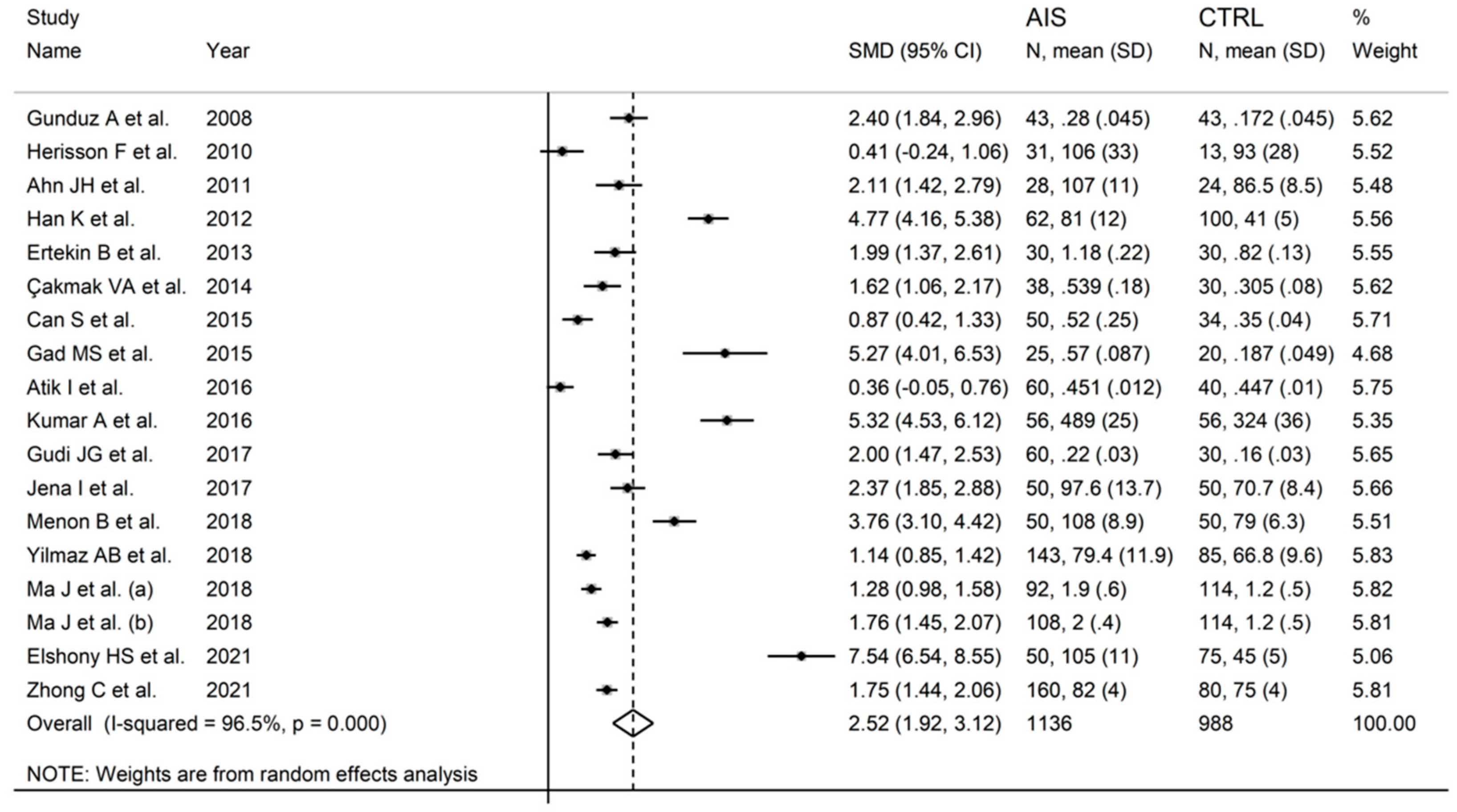
3.2. Acute Ischaemic Stroke
3.2.1. Studies Selected
3.2.2. Risk of Bias
3.2.3. Results of Individual Studies and Syntheses
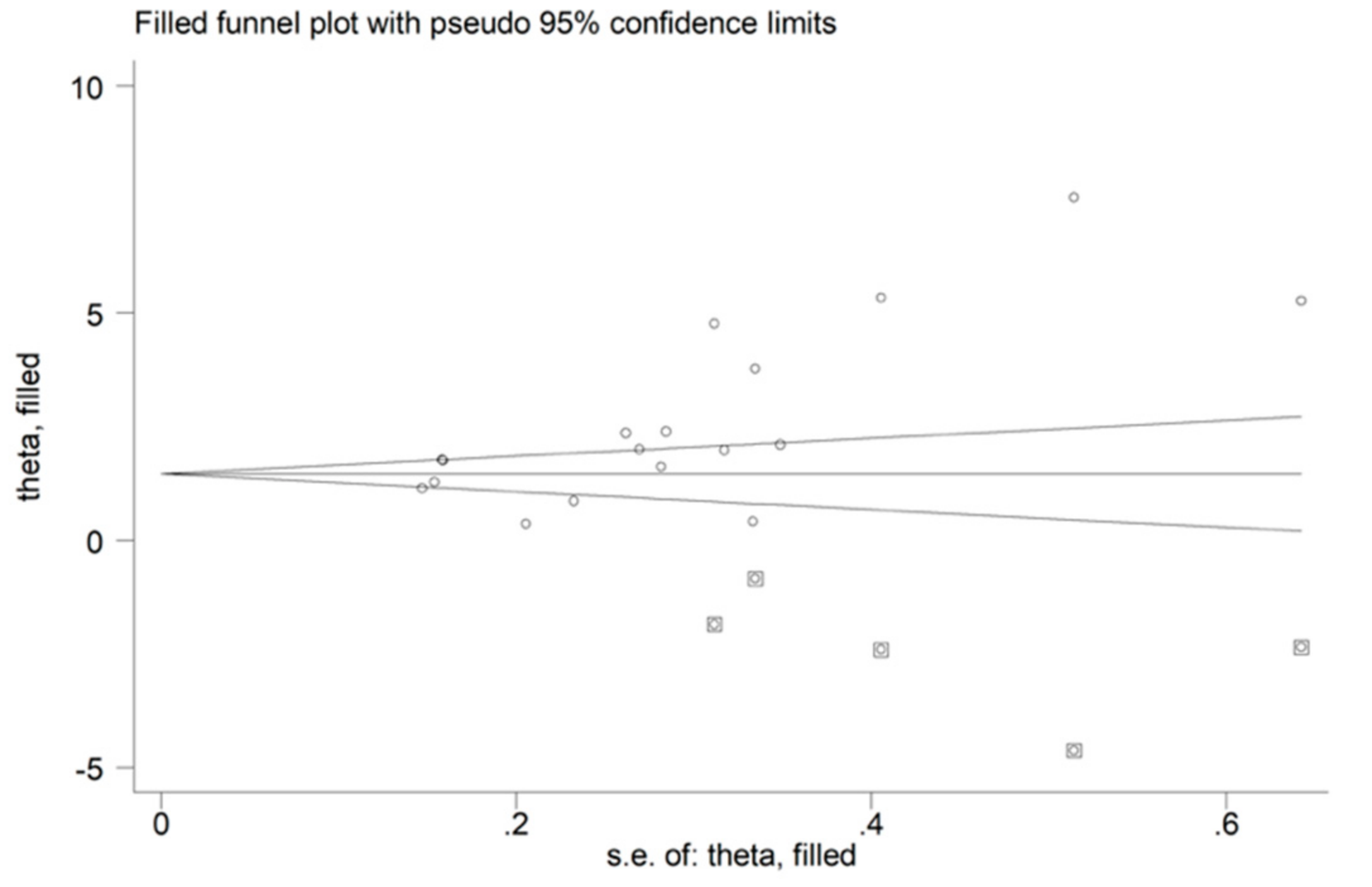
3.2.4. Publication Bias
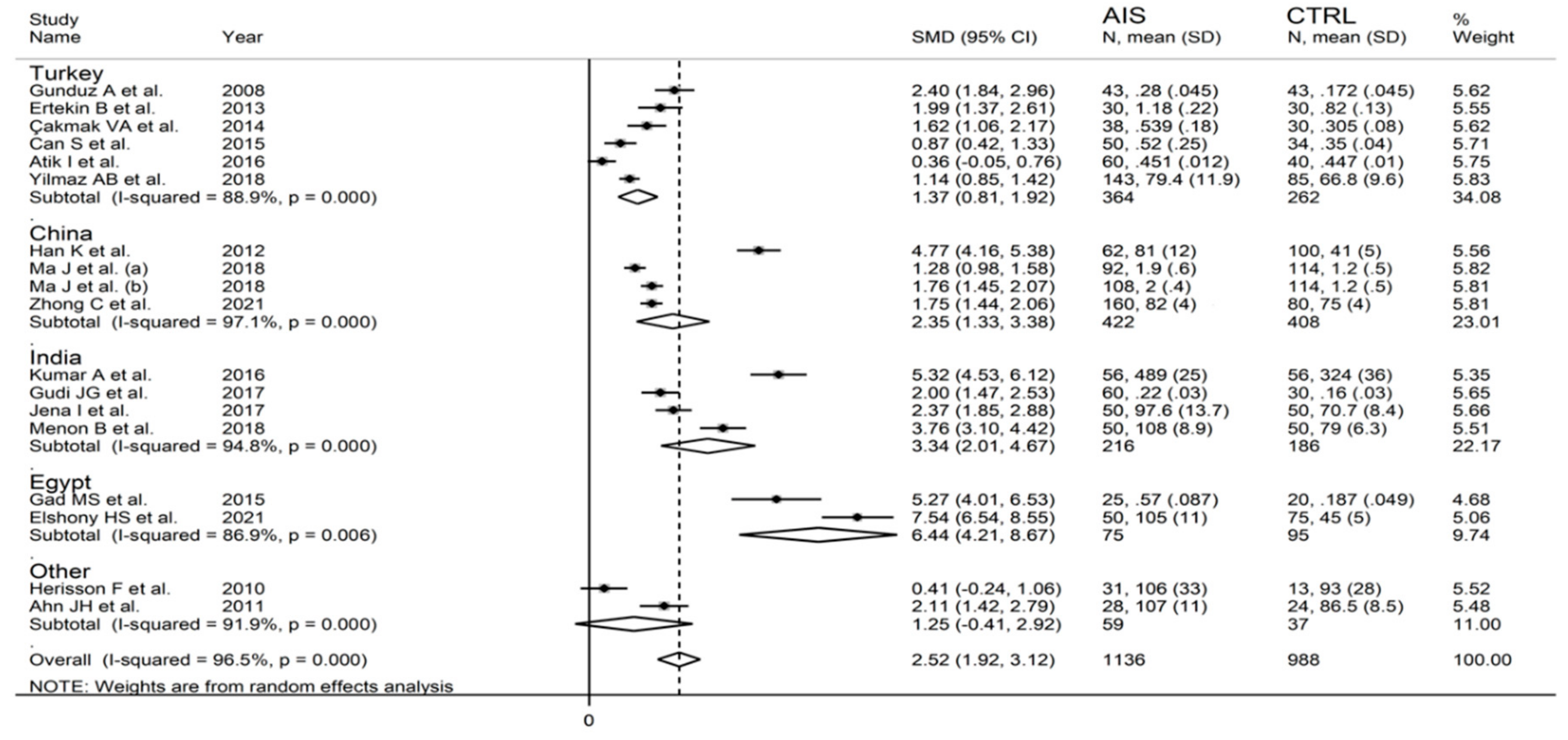
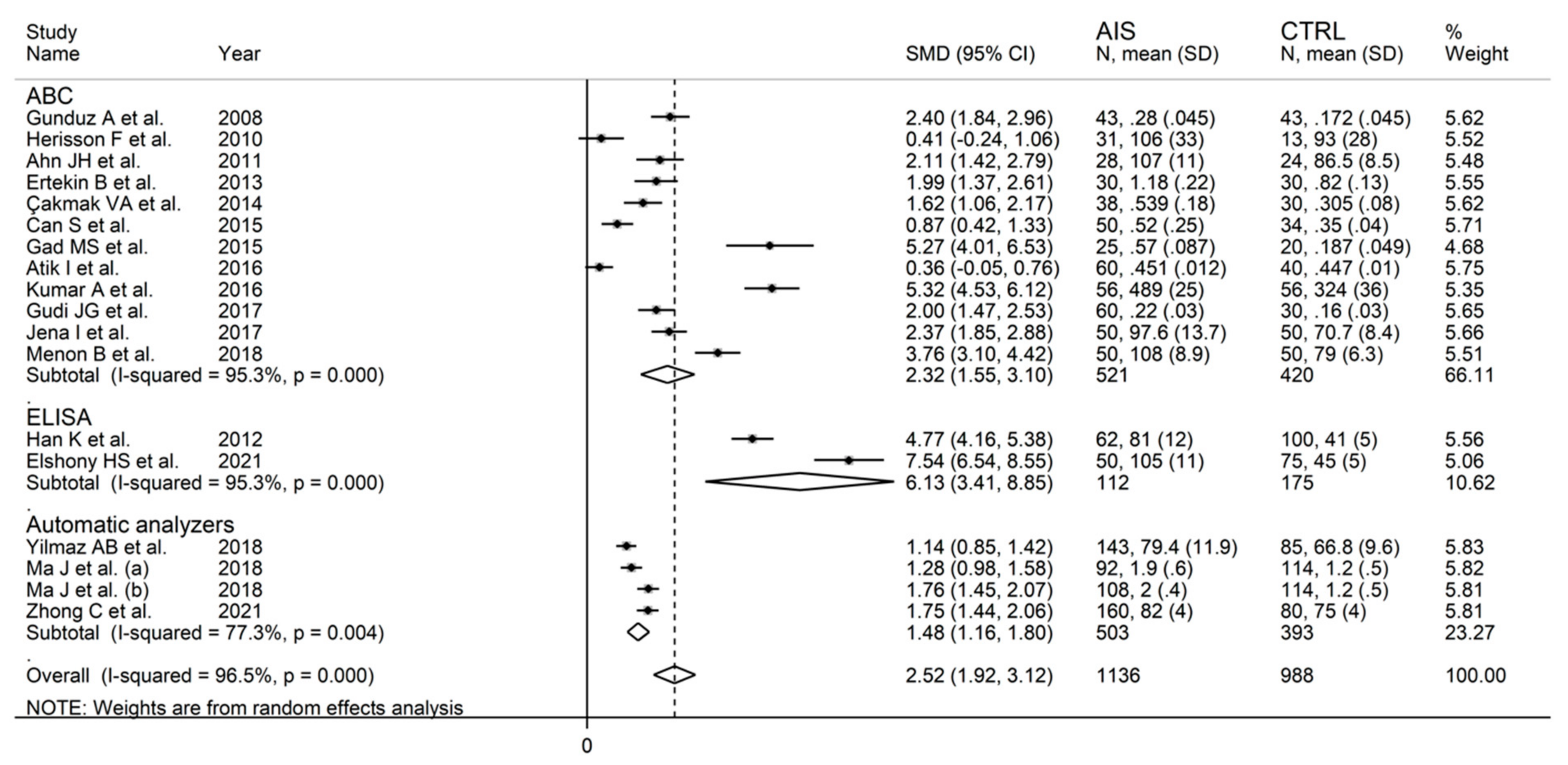
3.2.5. Meta-Regression and Subgroup Analysis
3.2.6. Certainty of Evidence
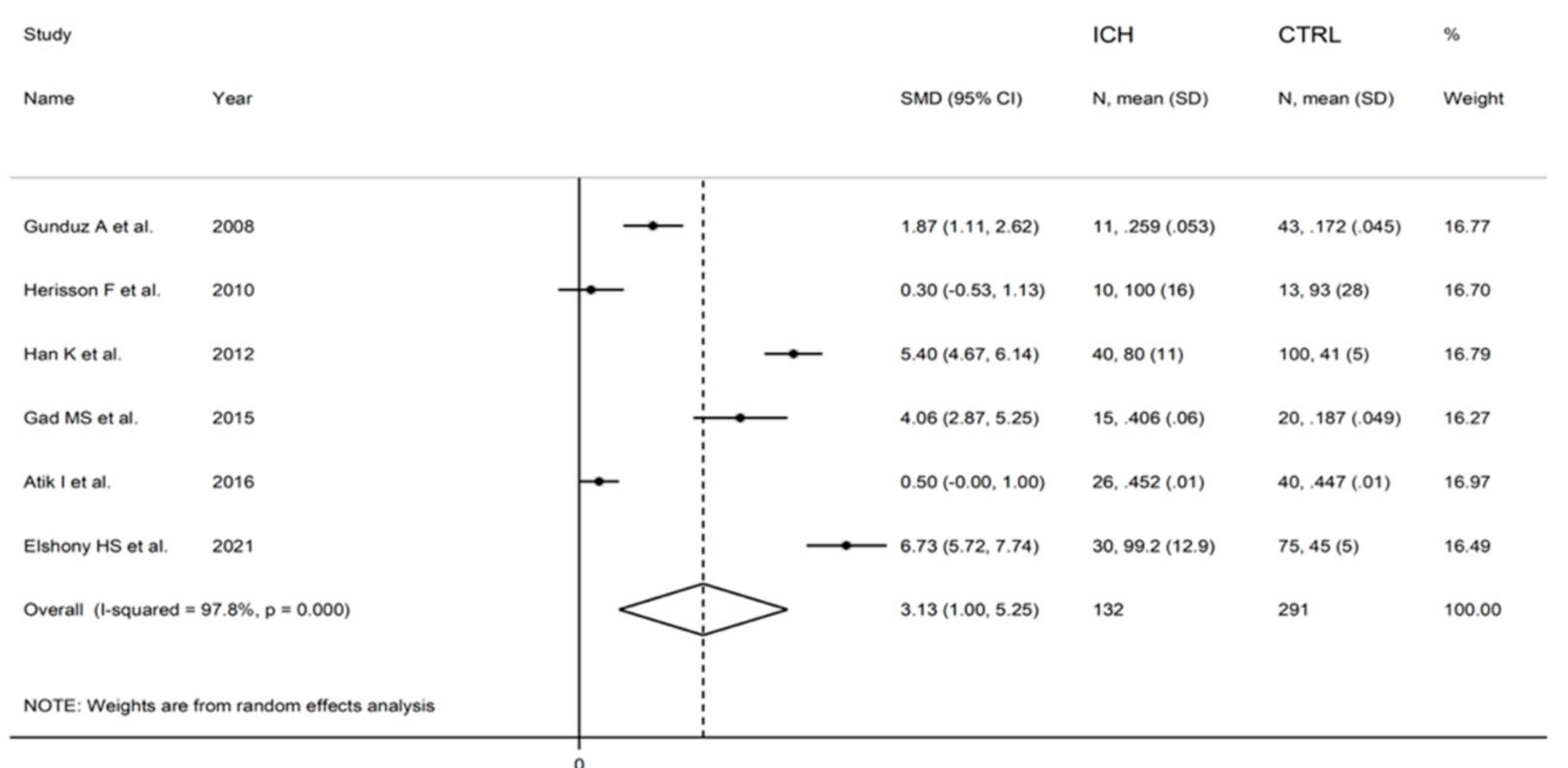
3.3. Intracerebral Haemorrhage
3.3.1. Studies Selected
3.3.2. Risk of Bias
3.3.3. Results of Individual Studies and Syntheses
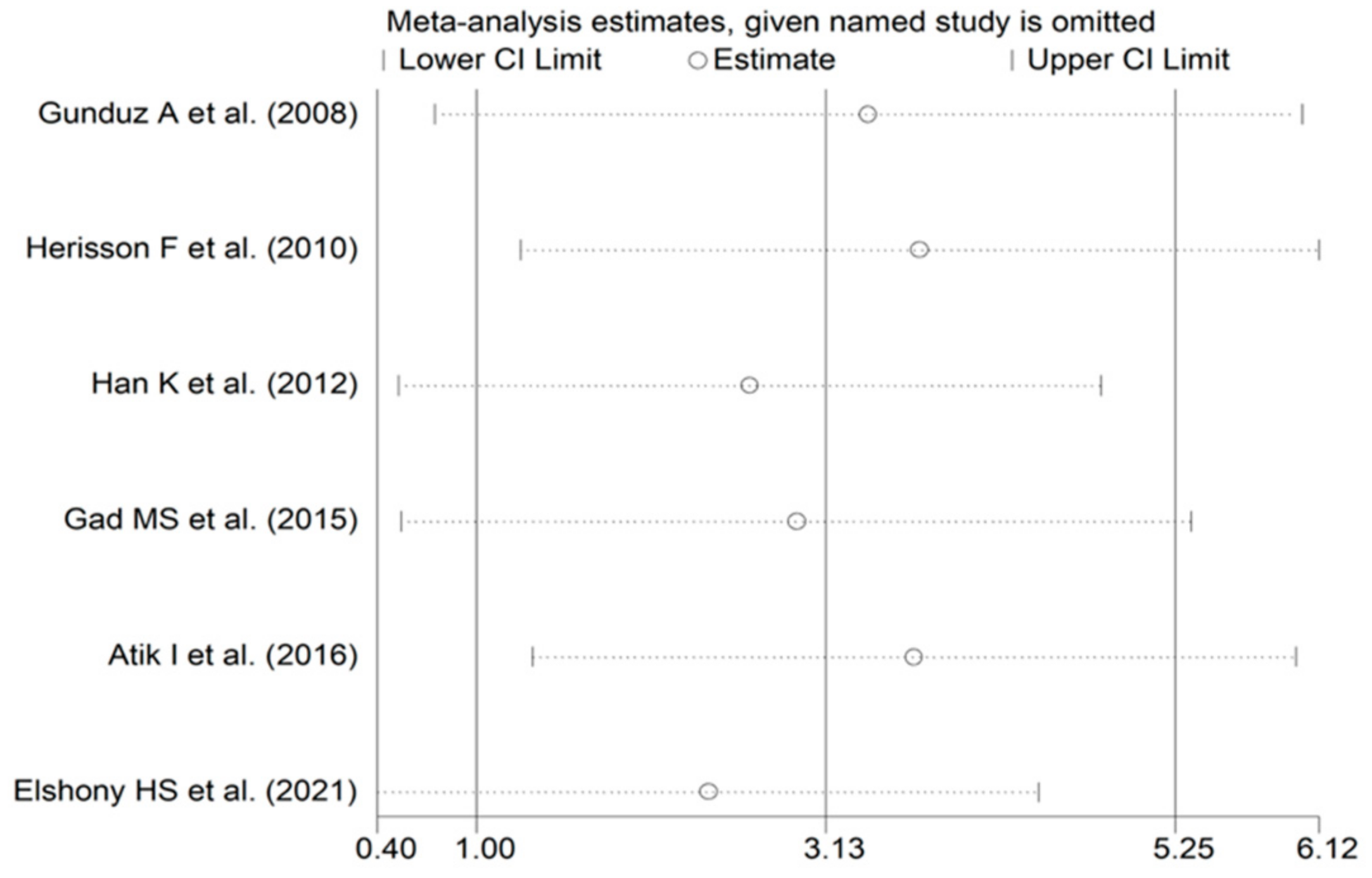
3.3.4. Publication Bias
3.3.5. Meta-Regression and Sub-Group Analysis
3.3.6. Certainty of Evidence
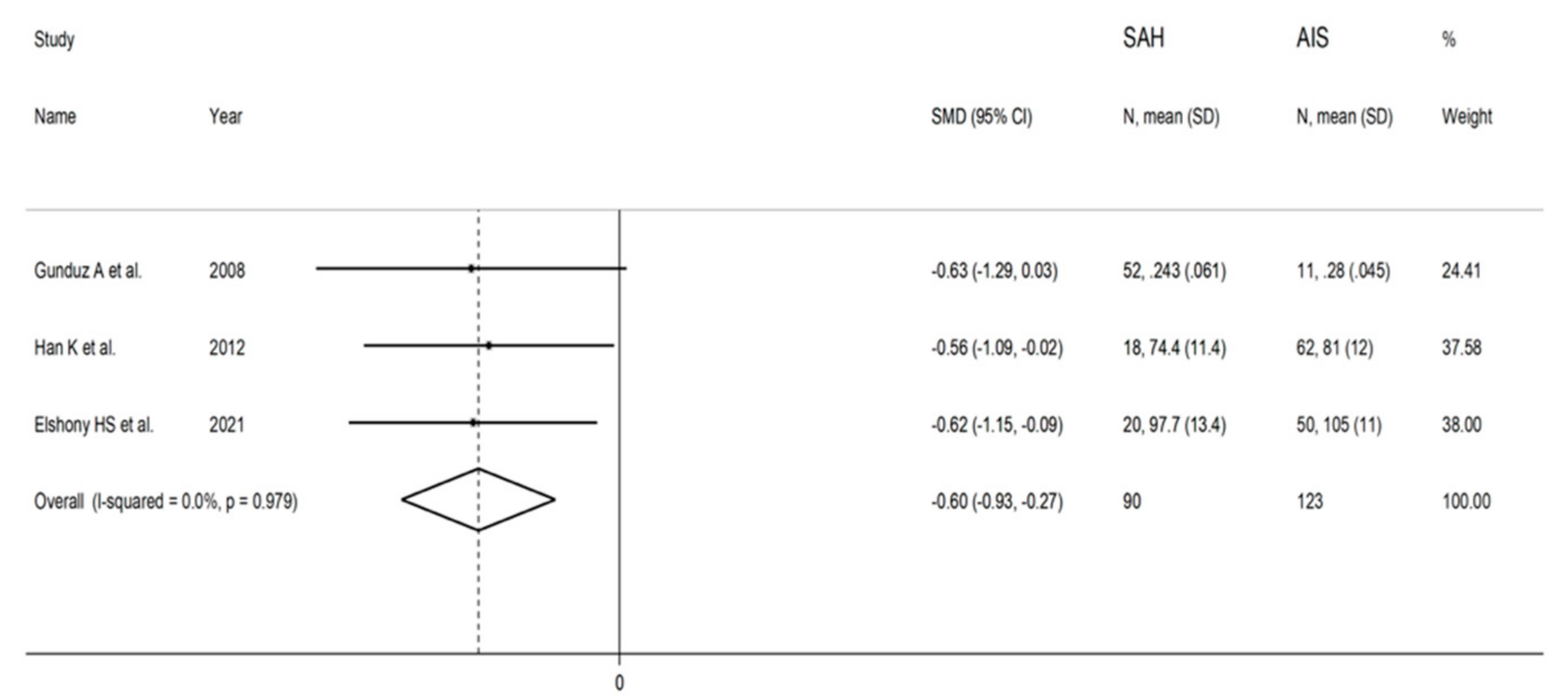
3.4. Subarachonid Haemorrhage
3.4.1. Studies Selected
3.4.2. Risk of Bias
3.4.3. Results of Individual Studies and Syntheses
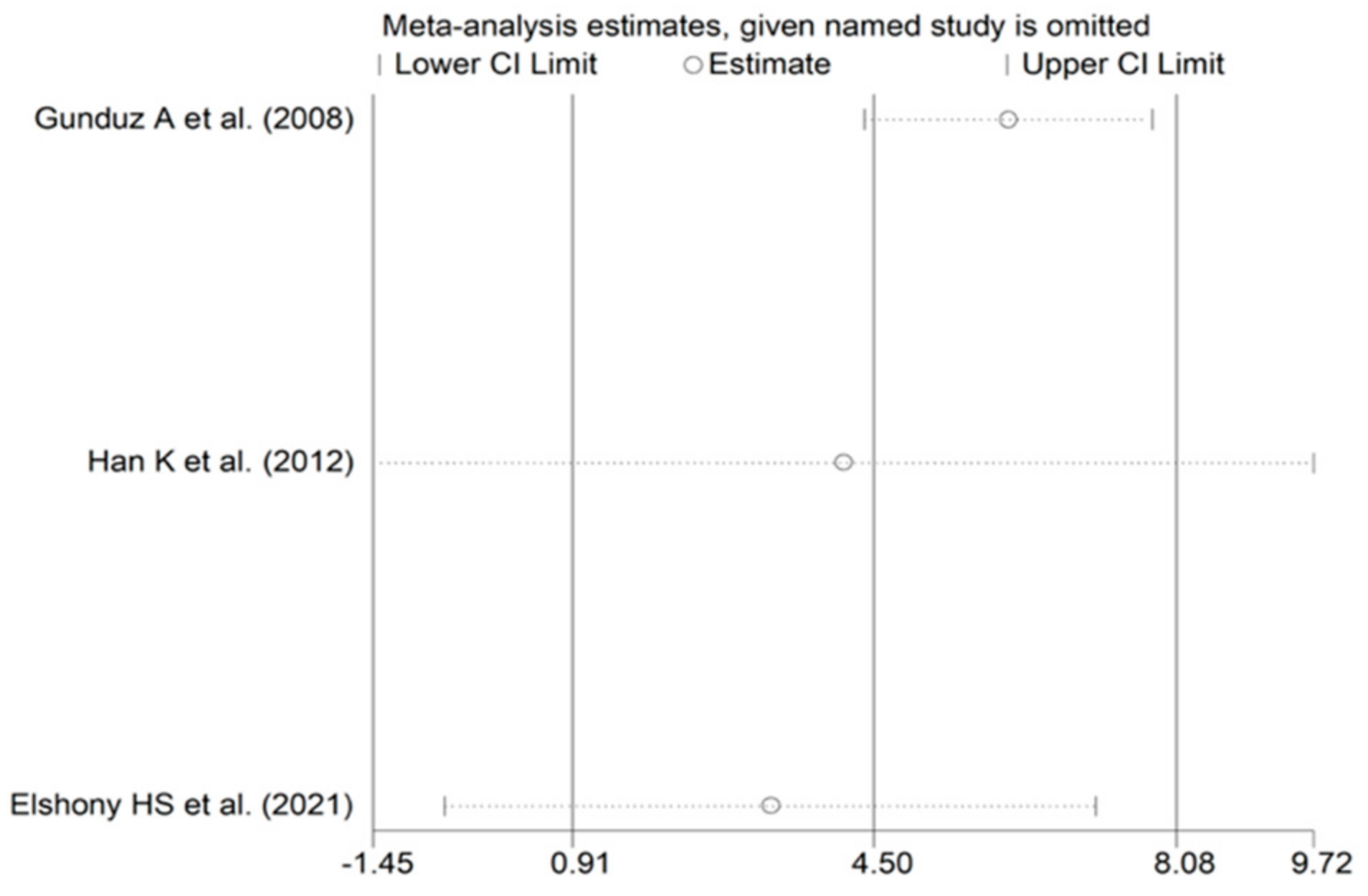
3.4.4. Publication Bias
3.4.5. Meta-Regression and Subgroup Analysis
3.4.6. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Demaerschalk, B.M.; Kleindorfer, D.O.; Adeoye, O.M.; Demchuk, A.M.; Fugate, J.E.; Grotta, J.C.; Khalessi, A.A.; Levy, E.I.; Palesch, Y.Y.; Prabhakaran, S.; et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, 581–641. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Lawton, M.T.; Vates, G.E. Subarachnoid Hemorrhage. N. Engl. J. Med. 2017, 377, 257–266. [Google Scholar] [CrossRef]
- Vasan, R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006, 113, 2335–2362. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Jickling, G.C. Blood Biomarkers for Stroke Diagnosis and Management. Neuromolecular. Med. 2019, 21, 344–368. [Google Scholar] [CrossRef]
- Makris, K.; Haliassos, A.; Chondrogianni, M.; Tsivgoulis, G. Blood biomarkers in ischemic stroke: Potential role and challenges in clinical practice and research. Crit. Rev. Clin. Lab. Sci. 2018, 55, 294–328. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.J.L.; Quek, A.M.L.; Cheung, C.; Arumugam, T.V.; Seet, R.C.S. Stroke biomarkers in clinical practice: A critical appraisal. Neurochem. Int. 2017, 107, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H.; Labreuche, J.; Meseguer, E.; Lavallee, P.C.; Simon, O.; Olivot, J.M.; Mazighi, M.; Dehoux, M.; Benessiano, J.; Steg, P.G.; et al. Ischemia-modified albumin in acute stroke. Cerebrovasc. Dis. 2007, 23, 216–220. [Google Scholar] [CrossRef]
- Menon, B.; Ramalingam, K.; Krishna, V. Study of Ischemia Modified Albumin as a Biomarker in Acute Ischaemic Stroke. Ann. Neurosci. 2018, 25, 187–190. [Google Scholar] [CrossRef]
- Elshony, H.S.; Okda, M.A.; El-Kabany, R.A. Ischemia-modified albumin and fibulin-5 as diagnostic and prognostic markers for acute cerebrovascular disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 12. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; Johanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane handbook for systematic reviews of interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, A.; Turedi, S.; Mentese, A.; Altunayoglu, V.; Turan, I.; Karahan, S.C.; Topbas, M.; Aydin, M.; Eraydin, I.; Akcan, B. Ischemia-modified albumin levels in cerebrovascular accidents. Am. J. Emerg. Med. 2008, 26, 874–878. [Google Scholar] [CrossRef]
- Herisson, F.; Delaroche, O.; Auffray-Calvier, E.; Duport, B.D.; Guillon, B. Ischemia-modified albumin and heart fatty acid-binding protein: Could early ischemic cardiac biomarkers be used in acute stroke management? J Stroke Cerebrovasc. Dis. 2010, 19, 279–282. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, S.C.; Lee, W.G.; Jung, Y.S. The usefulness of albumin-adjusted ischemia-modified albumin index as early detecting marker for ischemic stroke. Neurol. Sci. 2011, 32, 133–138. [Google Scholar] [CrossRef]
- Han, K.; Jia, N.; Yang, L.; Min, L.Q. Correlation between ischemia-modified albumin and lipid levels in patients with acute cerebrovascular disease. Mol. Med. Rep. 2012, 6, 621–624. [Google Scholar] [CrossRef][Green Version]
- Ertekin, B.; Koçak, S.; Dündar, Z.D.; Girişgin, S.; Cander, B.; Gül, M.; Döşeyici, S.; Mehmetoğlu, İ.; Şahin, T.K. Diagnostic value of ischemia-modified albumin in acute coronary syndrome and acute ischemic stroke. Pak. J. Med. Sci. 2013, 29, 1003–1007. [Google Scholar] [CrossRef]
- Altunayoglu Cakmak, V.; Gunduz, A.; Karaca, Y.; Alioglu, Z.; Mentese, A.; Topbas, M. Diagnostic Significance of Ischemia-Modified Albumin, S100b, and Neuron-Specific Enolase in Acute Ischemic Stroke. J. Acad. Emerg. Med. 2014, 13, 112–117. [Google Scholar] [CrossRef]
- Can, S.; Akdur, O.; Yildirim, A.; Adam, G.; Cakir, D.U.; Karaman, H.I. Myelin basic protein and ischemia modified albumin levels in acute ischemic stroke cases. Pak. J. Med. Sci. 2015, 31, 1110–1114. [Google Scholar] [CrossRef]
- Gad, M.S.; Zakaria, N.H.E.-D.; Elgayar, N.H.A.A.-M. Evaluation of the role of ischemia modified albumin as a new biochemical marker for differentiation between ischemic and hemorrhagic stroke. Alex. J. Med. 2019, 51, 213–217. [Google Scholar] [CrossRef][Green Version]
- Atik, I.; Kozaci, N.; Beydilli, I.; Avci, M.; Ellidag, H.; Kesapli, M. Investigation of oxidant and antioxidant levels in patients with acute stroke in the emergency service. Am. J. Emerg. Med. 2016, 34, 2379–2383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kalasker, V.; Pratibha, K.; Rao, K.S.; Bhat, H. Prognostic significance of Ischemia Modified Albumin and role of high sensitive Creactive protein levels in acute ischemic stroke patients. Int. J. Clin. Biochem. Res. 2016, 3, 262–266. [Google Scholar] [CrossRef]
- Gudi, J.; Malleshappa, A.; Naik, K. Correlation of serum albumin with ischemia modified albumin in acute stroke patients with or without diabetes mellitus. Indian J. Health Sci. Biomed. Res. (KLEU) 2017, 10, 29–53. [Google Scholar] [CrossRef]
- Jena, I.; Nayak, S.R.; Behera, S.; Singh, B.; Ray, S.; Jena, D.; Singh, S.; Sahoo, S.K. Evaluation of ischemia-modified albumin, oxidative stress, and antioxidant status in acute ischemic stroke patients. J. Nat. Sci. Biol. Med. 2017, 8, 110–113. [Google Scholar] [CrossRef]
- Yilmaz, A.B.; Gokhan, S.; Sener, A.; Erel, O. Analysis of Neutrophil/Lymphocyte ratio and Thiol/Disulfide homeostasis parameters in patients admitted to the emergency department with ischemic stroke. Pak. J. Med. Sci. 2018, 34, 1418–1423. [Google Scholar] [CrossRef]
- Ma, J.; Shen, L.; Bao, L.; Yuan, H.; Wang, Y.; Liu, H.; Wang, Q. A novel prognosis prediction model, including cytotoxic T lymphocyte-associated antigen-4, ischemia-modified albumin, lipoprotein-associated phospholipase A2, glial fibrillary acidic protein, and homocysteine, for ischemic stroke in the Chinese hypertensive population. J. Clin. Lab. Anal. 2021, 35, e23756. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, T.; Shen, Y.; Zhang, Y.; Liu, Y.; Ning, L. The effects of serum ischemia modified albumin on diagnosis of cerebral infarction and vertebral basilar artery stenosis. Brain INJ 2021, 35, 1457–1461. [Google Scholar] [CrossRef]
- Lee, P.; Wu, X. Review: Modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef]
- Levine, R.L. Ischemia: From acidosis to oxidation. FASEB J. 1993, 7, 1242–1246. [Google Scholar] [CrossRef]
- Roy, D.; Quiles, J.; Gaze, D.C.; Collinson, P.; Kaski, J.C.; Baxter, G.F. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart 2006, 92, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Vazquez, J.M.; Calvino, R.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Effects of balloon occlusion during percutaneous coronary intervention on circulating Ischemia Modified Albumin and transmyocardial lactate extraction. Heart 2006, 92, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Ellidag, H.Y.; Eren, E.; Yilmaz, N.; Cekin, Y. Oxidative stress and ischemia-modified albumin in chronic ischemic heart failure. Redox Rep. 2014, 19, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Altunoglu, E.; Guntas, G.; Erdenen, F.; Akkaya, E.; Topac, I.; Irmak, H.; Derici, H.; Yavuzer, H.; Gelisgen, R.; Uzun, H. Ischemia-modified albumin and advanced oxidation protein products as potential biomarkers of protein oxidation in Alzheimer’s disease. Geriatr. Gerontol. Int. 2015, 15, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Muddeshwar, M.G.; Ghosh, K. Ischemia Modified Albumin Test to Detect Early Diabetic Complications. Am. J. Med. Sci. 2017, 354, 467–470. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Prefumo, F.; Leslie, K.; Gaze, D.C.; Collinson, P.O.; Thilaganathan, B. Defective endovascular trophoblast invasion in the first trimester is associated with increased maternal serum ischemia-modified albumin. Hum. Reprod. 2008, 23, 803–806. [Google Scholar] [CrossRef]
- Ozdemir, S.; Kiyici, A.; Balci, O.; Goktepe, H.; Cicekler, H.; Celik, C. Assessment of ischemia-modified albumin level in patients with recurrent pregnancy loss during the first trimester. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 209–212. [Google Scholar] [CrossRef]
- Rossi, A.; Bortolotti, N.; Vescovo, S.; Romanello, I.; Forzano, L.; Londero, A.P.; Ambrosini, G.; Marchesoni, D.; Curcio, F. Ischemia-modified albumin in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 348–351. [Google Scholar] [CrossRef]
- Kundaktepe, B.P.; Sozer, V.; Durmus, S.; Kocael, P.C.; Kundaktepe, F.O.; Papila, C.; Gelisgen, R.; Uzun, H. The evaluation of oxidative stress parameters in breast and colon cancer. Medicine 2021, 100, e25104. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Khan, S.; Liu, Y.; Wu, G.; Yong, V.W.; Xue, M. Oxidative Stress Following Intracerebral Hemorrhage: From Molecular Mechanisms to Therapeutic Targets. Front. Immunol. 2022, 13, 847246. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Z.; Li, G.; Zhou, L.; Huang, K.; Wu, Z.; Zhan, R.; Shen, J. Inflammation and Oxidative Stress: Potential Targets for Improving Prognosis After Subarachnoid Hemorrhage. Front. Cell. Neurosci. 2021, 15, 739506. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K.; Ostrowski, R. The Role of Oxidative Stress in Early Brain Injury after Subarachnoid Hemorrhage. Oxidative Med. Cell. Longev. 2020, 2020, 8877116. [Google Scholar] [CrossRef]
- Shi, W.; Tang, G.; Zhou, X.; Ye, Y. Appraising the Accuracy of Ischaemia-Modified Albumin in Diagnosing Stroke: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2021, 50, 365–370. [Google Scholar] [CrossRef]
- Stea, S.; Pasquale, D.; Beraudi, A.; Catalani, S.; Amabile, M.; Bracci, G.; Toni, A. Ischemia-Modified Albumin Expression: Is there a Difference between Male and Female Subjects? Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Miller, A.A.; De Silva, T.M.; Jackman, K.A.; Sobey, C.G. Effect of gender and sex hormones on vascular oxidative stress. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1037–1043. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Salvagno, G.L.; Guidi, G.C. Reference ranges and diagnostic thresholds of laboratory markers of cardiac damage and dysfunction in a population of apparently healthy black Africans. Clin. Chem. Lab. Med. 2008, 46, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; De Greef, J.; Delport, R.; Becker, P.J.; Vermaak, W.J. Biological variation of ischaemia-modified albumin in healthy subjects. Cardiovasc. J. Afr. 2008, 19, 141–144. [Google Scholar] [PubMed]
- Morris, A.A.; Zhao, L.; Patel, R.S.; Jones, D.P.; Ahmed, Y.; Stoyanova, N.; Gibbons, G.H.; Vaccarino, V.; Din-Dzietham, R.; Quyyumi, A.A. Differences in systemic oxidative stress based on race and the metabolic syndrome: The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab. Syndr. Relat. Disord. 2012, 10, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Feairheller, D.L.; Park, J.Y.; Sturgeon, K.M.; Williamson, S.T.; Diaz, K.M.; Veerabhadrappa, P.; Brown, M.D. Racial differences in oxidative stress and inflammation: In vitro and in vivo. Clin. Transl. Sci. 2011, 4, 32–37. [Google Scholar] [CrossRef]






| Controls | Patients with Stroke | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AIS | ICH | SAH | ||||||||
| First Author and Year, Country [Ref] | N | Age * (Years) | M/F | IMA Mean ± SD | N | Age * (Years) | M/F | IMA Mean ± SD | IMA Mean ± SD | IMA Mean ± SD |
| Gunduz A et al. 2008, Turkey [27] | 43 | 57 | NR | 0.172 ± 0.045 (ABSU) | 43 | 71 | 23/20 | 0.280 ± 0.045 (ABSU) | 0.259 ± 0.053 (ABSU) | 0.243 ± 0.061 (ABSU) |
| Herisson F et al. 2010, France [28] | 13 | 49 | 5/8 | 93 ± 28 (U/mL) | 31 | 59 | 20/11 | 106 ± 33 (U/mL) | 100 ± 16 (U/mL) | - |
| Ahn JH et al. 2011, South Korea [29] | 24 | NR | NR | 86.5 ± 8.5 (U/mL) | 28 | NR | NR | 107.4 ± 11.0 (U/mL) | - | - |
| Han K et al. 2012, China [30] | 100 | 61 | 53/47 | 41 ± 5 (U/mL) | 62 | 59 | 38/24 | 81 ± 12 (U/mL) | 80 ± 11 (U/mL) | 74.4 ± 11.4 (U/mL) |
| Ertekin B et al. 2013, Turkey [31] | 30 | 52 | 14/16 | 0.82 ± 0.13 (ABSU) | 30 | 66 | 12/18 | 1.18 ± 0.22 (ABSU) | - | - |
| Çakmak VA et al. 2014, Turkey [32] | 30 | 65 | 20/10 | 0.305 ± 0.08 (ABSU) | 38 | 66 | 20/18 | 0.539 ± 0.18 (ABSU) | - | - |
| Can S et al. 2015, Turkey [33] | 34 | 58 | 18/16 | 0.35 ± 0.04 (ABSU) | 50 | 68 | 26/24 | 0.52 ± 0.25 (ABSU) | - | - |
| Gad MS et al. 2015, Egypt [34] | 20 | NR | NR | 0.187 ± 0.049 (ABSU) | 25 | NR | NR | 0.57 ± 0.087 (ABSU) | 0.406 ± 0.060 (ABSU) | - |
| Atik I et al. 2016, Turkey [35] | 40 | NR | NR | 0.447 ± 0.010 (ABSU) | 60 | NR | 30/30 | 0.451 ± 0.012 (ABSU) | 0.452 ± 0.010 (ABSU) | - |
| Kumar A et al. 2016, India [36] | 56 | NR | NR | 324 ± 36 (ABSU) | 56 | NR | 46/10 | 489 ± 25 (ABSU) | - | - |
| Gudi JG et al. 2017, India [37] | 30 | NR | NR | 0.16 ± 0.03 (ABSU) | 60 | NR | NR | 0.22 ± 0.03 (ABSU) | - | - |
| Jena I et al. 2017, India [38] | 50 | 55 | 28/22 | 70.7 ± 8.4 (U/mL) | 50 | 60 | 26/24 | 97.6 ± 13.6 (U/mL) | - | - |
| Menon B et al. 2018, India [15] | 50 | 56 | 30/20 | 79 ± 6.3 (IU/mL) | 50 | 58 | 39/11 | 108 ± 8.9 (IU/mL) | - | - |
| Yilmaz AB et al. 2017, Turkey [39] | 85 | 70 | 44/41 | 66.8 ± 9.6 (g/dL) | 143 | 72 | 76/67 | 79.4 ± 11.9 (g/dL) | - | - |
| Ma J et al. (a) 2018, China [40] | 114 | 54 | 54/60 | 1.2 ± 0.5 (pg/mL) | 92 | 54 | 48/44 | 1.9 ± 0.6 (pg/mL) | - | - |
| Ma J et al. (b) 2018, China [40] | 114 | 54 | 54/60 | 1.2 ± 0.5 (pg/mL) | 108 | 54 | 55/53 | 2.0 ± 0.4 (pg/mL) | - | - |
| Elshony HS et al. 2021, Egypt [16] | 75 | NR | NR | 45 ± 5 (U/mL) | 50 | NR | NR | 105 ± 11 (U/mL) | 99.2 ± 12.9 (U/mL) | 97.7 ± 13.7 (U/mL) |
| Zhong C et al. 2021, China [41] | 80 | 62 | 48/32 | 75 ± 4 (U/mL) | 160 | 62 | 86/74 | 82 ± 4 (U/mL) | - | - |
| Study | Were the Criteria for Inclusion Clearly Defined? | Were the Subjects and the Setting Described in Detail? | Was the Exposure Measured in a Valid and Reliable Way? | Were Objective, Standard Criteria Used for Measurement of the Condition? | Were Confounding Factors Identified? | Were Strategies to Deal with Confounding Factors Stated? | Were the Outcomes Measured in a Valid and Reliable Way? | Was Appropriate Statistical Analysis Used? | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Gunduz A [27] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Herisson F [28] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Ahn JH [29] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Han K [30] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Ertekin B [31] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Çakmak VA [32] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Can S [33] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Gad MS [34] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Atik I [35] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Kumar A [36] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Gudi JG [37] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Jena I [38] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Menon B [15] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Yilmaz AB [39] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Ma J [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Elshony HS [16] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Zhong C [41] | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangoni, A.A.; Zinellu, A. A Systematic Review and Meta-Analysis of Serum Concentrations of Ischaemia-Modified Albumin in Acute Ischaemic Stroke, Intracerebral Haemorrhage, and Subarachnoid Haemorrhage. Biomolecules 2022, 12, 653. https://doi.org/10.3390/biom12050653
Mangoni AA, Zinellu A. A Systematic Review and Meta-Analysis of Serum Concentrations of Ischaemia-Modified Albumin in Acute Ischaemic Stroke, Intracerebral Haemorrhage, and Subarachnoid Haemorrhage. Biomolecules. 2022; 12(5):653. https://doi.org/10.3390/biom12050653
Chicago/Turabian StyleMangoni, Arduino A., and Angelo Zinellu. 2022. "A Systematic Review and Meta-Analysis of Serum Concentrations of Ischaemia-Modified Albumin in Acute Ischaemic Stroke, Intracerebral Haemorrhage, and Subarachnoid Haemorrhage" Biomolecules 12, no. 5: 653. https://doi.org/10.3390/biom12050653
APA StyleMangoni, A. A., & Zinellu, A. (2022). A Systematic Review and Meta-Analysis of Serum Concentrations of Ischaemia-Modified Albumin in Acute Ischaemic Stroke, Intracerebral Haemorrhage, and Subarachnoid Haemorrhage. Biomolecules, 12(5), 653. https://doi.org/10.3390/biom12050653







