Prevalence and Management of Alkyl-Methoxypyrazines in a Changing Climate: Viticultural and Oenological Considerations
Abstract
:1. Introduction
2. Endogenous Methoxypyrazines
2.1. Distribution of MPs
2.2. Accumulation and Degradation of MPs
2.3. Impact of Climate Change
2.4. Influence of Viticultural Practices
3. Exogenous Methoxypyrazines
3.1. Coccinellidae
3.2. Climate Change and Coccinellidae
3.3. Managing Coccinellidae in the Vineyard
3.3.1. Semiochemical Push–Pull Approaches
3.3.2. Spraying
3.3.3. Removing Beetles after Harvest
4. Remediating Methoxypyrazines in the Winery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, B.P.; Brown, W.V.; Rothschild, M. Methylalkylpyrazines in Aposematic Insects, Their Hostplants and Mimics. Chemoecology 1990, 1, 43–51. [Google Scholar] [CrossRef]
- Murray, K.E.; Whitfield, F.B. The Occurrence of 3-Alkyl-2-Methoxypyrazines in Raw Vegetables. J. Sci. Food Agric. 1975, 26, 973–986. [Google Scholar] [CrossRef]
- Zhao, X.; Ju, Y.; Wei, X.; Dong, S.; Sun, X.; Fang, Y. Significance and Transformation of 3-Alkyl-2-Methoxypyrazines through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP–MP Cycle. Molecules 2019, 24, 4598. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W.V. Contribution of Methoxypyrazines to Sauvignon Blanc Wine Aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Coetzee, C.; du Toit, W.J. A Comprehensive Review on Sauvignon Blanc Aroma with a Focus on Certain Positive Volatile Thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Allen, M.S.; Lacey, M.J.; Boyd, S. Determination of Methoxypyrazines in Red Wines by Stable Isotope Dilution Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 1994, 42, 1734–1738. [Google Scholar] [CrossRef]
- Roujou de Boubée, D.; Van Leeuwen, C.; Dubourdieu, D. Organoleptic Impact of 2-Methoxy-3-Isobutylpyrazine on Red Bordeaux and Loire Wines. Effect of Environmental Conditions on Concentrations in Grapes during Ripening. J. Agric. Food Chem. 2000, 48, 4830–4834. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Mestres, M.; Martí, M.P.; Busto, O.; Guasch, J. Headspace Solid-Phase Microextraction Method for Determining 3-Alkyl-2-Methoxypyrazines in Musts by Means of Polydimethylsiloxane–Divinylbenzene Fibres. J. Chromatogr. A 2000, 880, 93–99. [Google Scholar] [CrossRef]
- Belancic, A.; Agosin, E. Methoxypyrazines in Grapes and Wines of Vitis Vinifera Cv. Carmenere. Am. J. Enol. Vitic. 2007, 58, 462–469. [Google Scholar]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Inglis, D.; Brindle, I.D.; Sears, M.; Beh, A.-L. Yeast Strain Affects 3-Isopropyl-2-Methoxypyrazine Concentration and Sensory Profile in Cabernet Sauvignon Wine. Aust. J. Grape Wine Res. 2008, 14, 230–237. [Google Scholar] [CrossRef]
- Pickering, G.J.; Botezatu, A. A Review of Ladybug Taint in Wine: Origins, Prevention, and Remediation. Molecules 2021, 26, 4341. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Beloqui, A.A.; Bertrand, A.; Doazan, J.P. An Analytical Method for Studying the Volatile Compounds of Merlot Noir Clone Wines. Am. J. Enol. Vitic. 1998, 49, 44–48. [Google Scholar]
- Sala, C.; Busto, O.; Guasch, J.; Zamora, F. Influence of Vine Training and Sunlight Exposure on the 3-Alkyl-2-Methoxypyrazines Content in Musts and Wines from the Vitis Vinifera Variety Cabernet Sauvignon. J. Agric. Food Chem. 2004, 52, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Armario, M.; Fontaine, A.; Fourure, M.; Pasquier, G.; Semadeni, T.; Chervin, C. 3-Isobutyl-2-Methoxypyrazine Is Neutrally Perceived by Consumers at Usual Concentrations in French Sauvignon and Fer Wines from the Gaillac Area. OENO One 2020, 54, 1133–1142. [Google Scholar] [CrossRef]
- Maga, J. Sensory and stability properties of added methoxypyrazines to model and authentic wines. In Proceedings of the 6th International Flavor Conference: Flavors and Off-Flavors ’89, Rethymnon, Greece, 5–7 July 1989; Charalambous, G., Ed.; Elsevier Science Limited: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Pickering, G.J.; Karthik, A.; Inglis, D.; Sears, M.; Ker, K. Determination of Ortho- and Retronasal Detection Thresholds for 2-Isopropyl-3-Methoxypyrazine in Wine. J. Food Sci. 2007, 72, S468–S472. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Pickering, G.J. Determination of Ortho- and Retronasal Detection Thresholds and Odor Impact of 2,5-Dimethyl-3-Methoxypyrazine in Wine. J. Food Sci. 2012, 77, S394–S398. [Google Scholar] [CrossRef]
- Allen, M.; Lacey, M. Methoxypyrazine Grape Flavour: Influence of Climate, Cultivar and Viticulture. Wein-Wissenschaft 1993, 48, 211–213. [Google Scholar]
- Ryona, I.; Leclerc, S.; Sacks, G.L. Correlation of 3-Isobutyl-2-Methoxypyrazine to 3-Isobutyl-2-Hydroxypyrazine during Maturation of Bell Pepper (Capsicum Annuum) and Wine Grapes (Vitis Vinifera). J. Agric. Food Chem. 2010, 58, 9723–9730. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N. Effect of Winter Rainfall on Yield Components and Fruit Green Aromas of V Itis Vinifera L. Cv. Merlot in California. Aust. J. Grape Wine Res. 2014, 20, 100–110. [Google Scholar] [CrossRef]
- Roy, H.E.; Brown, P.M.J.; Adriaens, T.; Berkvens, N.; Borges, I.; Clusella-Trullas, S.; Comont, R.F.; De Clercq, P.; Eschen, R.; Estoup, A.; et al. The Harlequin Ladybird, Harmonia Axyridis: Global Perspectives on Invasion History and Ecology. Biol. Invasions 2016, 18, 997–1044. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Boss, P.K. Two O-Methyltransferases Involved in the Biosynthesis of Methoxypyrazines: Grape-Derived Aroma Compounds Important to Wine Flavour. Plant. Mol. Biol. 2010, 74, 77–89. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines Biosynthesis and Metabolism in Grape: A Review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef]
- Roujou de Boubée, D.; Cumsille, A.M.; Pons, M.; Dubourdieu, D. Location of 2-Methoxy-3-Isobutylpyrazine in Cabernet Sauvignon Grape Bunches and Its Extractability during Vinification. Am. J. Enol. Vitic. 2002, 53, 1–5. [Google Scholar]
- Roujou de Boubée, D. Research on 2-Methoxy-3-Isobutylpyrazine in Grapes and Wine; Academie Amorim: Paris, France, 2003; pp. 1–21. [Google Scholar]
- Guerrini, L.; Masella, P.; Angeloni, G.; Calamai, L.; Spinelli, S.; Blasi, S.D.; Parenti, A. Harvest of Sangiovese Grapes: The Influence of Material Other than Grape and Unripe Berries on Wine Quality. Eur. Food Res. Technol. 2018, 244, 1487–1496. [Google Scholar] [CrossRef]
- Hendrickson, D.A.; Oberholster, A. Review of the Impact of Mechanical Harvesting and Optical Berry Sorting on Grape and Wine Composition. Catal. Discov. Into Pract. 2017, 1, 21–26. [Google Scholar] [CrossRef]
- Capone, D.L.; Barker, A.; Pearson, W.; Francis, I.L. Influence of Inclusion of Grapevine Leaves, Rachis and Peduncles during Fermentation on the Flavour and Volatile Composition of Vitis Vinifera Cv. Shiraz Wine. Aust. J. Grape Wine Res. 2021, 27, 348–359. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Nicholson, E.L.; Maffei, S.M.; Boss, P.K. Determining the Methoxypyrazine Biosynthesis Variables Affected by Light Exposure and Crop Level in Cabernet Sauvignon. Am. J. Enol. Vitic. 2013, 64, 450–458. [Google Scholar] [CrossRef]
- Guillaumie, S.; Ilg, A.; Réty, S.; Brette, M.; Trossat-Magnin, C.; Decroocq, S.; Léon, C.; Keime, C.; Ye, T.; Baltenweck-Guyot, R.; et al. Genetic Analysis of the Biosynthesis of 2-Methoxy-3-Isobutylpyrazine, a Major Grape-Derived Sroma Compound Impacting Wine Quality. Plant. Physiol. 2013, 162, 604–615. [Google Scholar] [CrossRef] [Green Version]
- Vallarino, J.G.; López-Cortés, X.A.; Dunlevy, J.D.; Boss, P.K.; González-Nilo, F.D.; Moreno, Y.M. Biosynthesis of Methoxypyrazines: Elucidating the Structural/Functional Relationship of Two Vitis Vinifera O-Methyltransferases Capable of Catalyzing the Putative Final Step of the Biosynthesis of 3-Alkyl-2-Methoxypyrazine. J. Agric. Food Chem. 2011, 59, 7310–7316. [Google Scholar] [CrossRef]
- Ryona, I.; Pan, B.S.; Intrigliolo, D.S.; Lakso, A.N.; Sacks, G.L. Effects of Cluster Light Exposure on 3-Isobutyl-2-Methoxypyrazine Accumulation and Degradation Patterns in Red Wine Grapes (Vitis Vinifera L. Cv. Cabernet Franc). J. Agric. Food Chem. 2008, 56, 10838–10846. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, S.; Chen, H.; Guan, X.; Zhang, Z. Behavior of 3-Isobutyl-2-Methoxypyrazine Biosynthesis Related to Proposed Precursor and Intermediate in Wine Grape. Food Chem. 2019, 277, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, K.; Samuta, T. Grape Maturity and Light Exposure Affect Berry Methoxypyrazine Concentration. Am. J. Enol. Vitic. 1999, 50, 194–198. [Google Scholar]
- Heymann, H.; Noble, A.C.; Boulton, R.B. Analysis of Methoxypyrazines in Wines. 1. Development of a Quantitative Procedure. J. Agric. Food Chem. 1986, 34, 268–271. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and Management Practices Affecting Grape Composition and Wine Quality—A Review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between Wine Composition and Temperature: Impact on Bordeaux Wine Typicity in the Context of Global Warming—Review. Crit Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef]
- Harris, S.A.; Ryona, I.; Sacks, G.L. Behavior of 3-Isobutyl-2-Hydroxypyrazine (IBHP), a Key Intermediate in 3-Isobutyl-2-Methoxypyrazine (IBMP) Metabolism, in Ripening Wine Grapes. J. Agric. Food Chem. 2012, 60, 11901–11908. [Google Scholar] [CrossRef] [PubMed]
- Marais, J.; Hunter, J.J.; Haasbroek, P.D. Effect of Canopy Microclimate, Season and Region on Sauvignon Blanc Grape Composition and Wine Quality. S. Afr. J. Enol. Vitic. 1999, 20, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.M.; Matthews, M.A.; Guinard, J.-X. Sensory Attributes of Cabernet Sauvignon Wines Made from Vines with Different Crop Yields. Am. J. Enol. Vitic. 2004, 55, 325–334. [Google Scholar]
- Hewer, M.J.; Gough, W.A. Assessing the Impact of Projected Climate Change on the Future of Grape Growth and Wine Production in the Niagara Peninsula (Canada). J. Wine Res. 2020, 31, 6–34. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate Influences on Grapevine Phenology, Grape Composition, and Wine Production and Quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- Jones, G.V.; Webb, L.B. Climate Change, Viticulture, and Wine: Challenges and Opportunities. J. Wine Res. 2010, 21, 103–106. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; Rességuier, L.; de Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, C.V.; Friant, P.; Chroné, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of Climate, Soil, and Cultivar on Terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar]
- Willwerth, J.; Reynolds, A.; Lesschaeve, I. Sensory Analysis of Ontario Riesling Wines from Various Water Status Zones. OENO One 2018, 52, 145–171. [Google Scholar] [CrossRef] [Green Version]
- Field, C.; Barros, V.; Dokken, D.; Mach, K.; Mastrandrea, M.; Bilir, T.; Chatterjee, M.; Ebi, K.; Estrada, Y.; Genova, R.; et al. (Eds.) IPCC Climate Change 2014: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Suter, B.; Triolo, R.; Pernet, D.; Dai, Z.; Van Leeuwen, C. Modeling Stem Water Potential by Separating the Effects of Soil Water Availability and Climatic Conditions on Water Status in Grapevine (Vitis Vinifera L.). Front. Plant. Sci. 2019, 10, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.; Willwerth, J.J. Spatial Variability in Ontario Riesling Vineyards: I. Soil, Vine Water Status and Vine Performance. OENO One 2020, 54, 311–333. [Google Scholar] [CrossRef]
- Sala, C.; Busto, O.; Guasch, J.; Zamora, F. Contents of 3-Alkyl-2-Methoxypyrazines in Musts and Wines from Vitis Vinifera Variety Cabernet Sauvignon: Influence of Irrigation and Plantation Density. J. Sci. Food Agric. 2005, 85, 1131–1136. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N.K. Effect of Increased Irrigation and Additional Nitrogen Fertilisation on the Concentration of Green Aroma Compounds in V Itis Vinifera L. Merlot Fruit and Wine. Aust. J. Grape Wine Res. 2014, 20, 80–90. [Google Scholar] [CrossRef]
- Smart, R.E. Aspects of Water Relations of the Grapevine (Vitis Vinifera). Am. J. Enol. Vitic. 1974, 25, 84–91. [Google Scholar]
- Scheiner, J.J.; Sacks, G.L.; Pan, B.; Ennahli, S.; Tarlton, L.; Wise, A.; Lerch, S.D.; Heuvel, J.E.V. Impact of Severity and Timing of Basal Leaf Removal on 3-Isobutyl-2-Methoxypyrazine Concentrations in Red Winegrapes. Am. J. Enol. Vitic. 2010, 61, 358–364. [Google Scholar]
- Parr, W.V.; Schlich, P.; Theobald, J.C.; Harsch, M.J. Association of Selected Viniviticultural Factors with Sensory and Chemical Characteristics of New Zealand Sauvignon Blanc Wines. Food Res. Int. 2013, 53, 464–475. [Google Scholar] [CrossRef]
- Gregan, S.M.; Jordan, B. Methoxypyrazine Accumulation and O-Methyltransferase Gene Expression in Sauvignon Blanc Grapes: The Role of Leaf Removal, Light Exposure, and Berry Development. J. Agric. Food Chem. 2016, 64, 2200–2208. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; McCarthy, M.; Ford, C.M.; Dokoozlian, N. Seasonal and Regional Variation of Green Aroma Compounds in Commercial Vineyards of Vitis Vinifera L. Merlot in California. Am. J. Enol. Vitic. 2013, 64, 430–436. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of Grapevine Training Systems on Vine Growth and Fruit Composition: A Review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Reynolds, A.G.; Wardle, D.A. Impact of Training System and Vine Spacing on Vine Performance and Berry Composition of Seyval Blanc. Am. J. Enol. Vitic. 1994, 45, 444–451. [Google Scholar]
- Smart, R.E. Shoot Spacing and Canopy Light Microclimate. Am. J. Enol. Vitic. 1988, 39, 325–333. [Google Scholar]
- Reynolds, A.G.; Wardle, D.A.; Naylor, A.P. Impact of Training System, Vine Spacing, and Basal Leaf Removal on Riesling. Vine Performance, Berry Composition, Canopy Microclimate, and Vineyard Labor Requirements. Am. J. Enol. Vitic. 1996, 47, 63–76. [Google Scholar]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Keller, M.; Romero, P.; Gohil, H.; Smithyman, R.P.; Riley, W.R.; Casassa, L.F.; Harbertson, J.F. Deficit Irrigation Alters Grapevine Growth, Physiology, and Fruit Microclimate. Am. J. Enol. Vitic. 2016, 67, 426–435. [Google Scholar] [CrossRef]
- Shellie, K.C. Water Productivity, Yield, and Berry Composition in Sustained versus Regulated Deficit Irrigation of Merlot Grapevines. Am. J. Enol. Vitic. 2014, 65, 197–205. [Google Scholar] [CrossRef]
- Strub, L.; Kurth, A.; Loose, S.M. Effects of Viticultural Mechanization on Working Time Requirements and Production Costs. Am. J. Enol. Vitic. 2021, 72, 46–55. [Google Scholar] [CrossRef]
- Bellvert, J.; Mata, M.; Vallverdú, X.; Paris, C.; Marsal, J. Optimizing Precision Irrigation of a Vineyard to Improve Water Use Efficiency and Profitability by Using a Decision-Oriented Vine Water Consumption Model. Precis. Agric. 2021, 22, 319–341. [Google Scholar] [CrossRef]
- Sanchez, L.A.; Sams, B.; Alsina, M.M.; Hinds, N.; Klein, L.J.; Dokoozlian, N. Improving Vineyard Water Use Efficiency and Yield with Variable Rate Irrigation in California. Adv. Anim. Biosci. 2017, 8, 574–577. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Cai, L.; Koziel, J.A.; O’Neal, M.E. Determination of Characteristic Odorants from Harmonia Axyridis Beetles Using in vivo Solid-Phase Microextraction and Multidimensional Gas Chromatography–Mass Spectrometry–Olfactometry. J. Chromatogr. A 2007, 1147, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Galvan, T.L.; Burkness, E.C.; Vickers, Z.; Stenberg, P.; Mansfield, A.K.; Hutchison, W.D. Sensory-Based Action Threshold for Multicolored Asian Lady Beetle-Related Taint in Winegrapes. Am. J. Enol. Vitic. 2007, 58, 518–522. [Google Scholar]
- Ross, C.; Ferguson, H.; Keller, M.; Walsh, D.; Weller, K.; Spayd, S. Determination of Ortho-Nasal Aroma Threshold for Multicolored Asian Lady Beetle in Concord Grape Juice. J. Food Qual. 2007, 30, 855–863. [Google Scholar] [CrossRef]
- Pickering, G.J.; Ker, K.; Soleas, G.J. Determination of the Critical Stages of Processing and Tolerance Limits for Harmonia Axyridis for “ladybug Taint” in Wine. Vitis 2007, 46, 85–90. [Google Scholar]
- Pickering, G.; Lin, J.; Riesen, R.; Reynolds, A.; Brindle, I.; Soleas, G. Influence of Harmonia Axyridis on the Sensory Properties of White and Red Wine. Am. J. Enol. Vitic. 2004, 55, 153–159. [Google Scholar]
- Ross, C.F.; Weller, K. Sensory Evaluation of Suspected Harmonia Axyridis—Tainted Red Wine Using Untrained Panelists. J. Wine Res. 2007, 18, 187–193. [Google Scholar] [CrossRef]
- Botezatu, A.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. A Survey of Methoxypyrazines in Wine. J. Food Agric. Environ. 2016, 14, 24–29. [Google Scholar] [CrossRef]
- Ker, K.W.; Pickering, G.J. Biology and control of the novel grapevine pest—The Multicolored Asian lady beetle harmonia axyridis. In Crops: Growth, Quality and Biotechnology; Dris, R., Ed.; WFL Publisher: Helsinki, Finland, 2005; pp. 991–997. ISBN 952-91-8601-0. [Google Scholar]
- Vincent, C.; Pickering, G. Multicolored Asian ladybeetle, Harmonia axyridis (Coleoptera: Coccinellidae). In Biological Control Programmes in Canada, 2001–2012; Mason, P.G., Gillespie, D.R., Eds.; CABI: Wallingford, UK, 2013; ISBN 978-1-78064-257-4. [Google Scholar]
- Koch, R.L. The Multicolored Asian Lady Beetle, Harmonia Axyridis: A Review of Its Biology, Uses in Biological Control, and Non-Target Impacts. J. Insect Sci. 2003, 3, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godelmann, R.; Limmert, S.; Kuballa, T. Implementation of Headspace Solid-Phase-Microextraction–GC–MS/MS Methodology for Determination of 3-Alkyl-2-Methoxypyrazines in Wine. Eur. Food Res. Technol. 2007, 227, 449. [Google Scholar] [CrossRef]
- Kögel, S.; Botezatu, A.; Hoffmann, C.; Pickering, G. Methoxypyrazine Composition of Coccinellidae-Tainted Riesling and Pinot Noir Wine from Germany. J. Sci. Food Agric. 2015, 95, 509–514. [Google Scholar] [CrossRef]
- Pickering, G.J.; Lin, Y.; Reynolds, A.; Soleas, G.; Riesen, R.; Brindle, I. The Influence of Harmonia Axyridis on Wine Composition and Aging. J. Food Sci. 2005, 70, S128–S135. [Google Scholar] [CrossRef]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Brindle, I.D.; Sears, M.; Inglis, D. Morbidity of Harmonia Axyridis Mediates Ladybug Taint in Red Wine. J. Food Agric. Environ. 2008, 6, 133–138. [Google Scholar] [CrossRef]
- Botezatu, A.I.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. Occurrence and Contribution of Alkyl Methoxypyrazines in Wine Tainted by Harmonia Axyridis and Coccinella Septempunctata. J. Sci. Food Agric. 2013, 93, 803–810. [Google Scholar] [CrossRef]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Brindle, I.D.; Sears, M.; Inglis, D. The Influence of Harmonia Axyridis Morbidity on 2-Isopropyl-3-Methoxypyrazine in “Cabernet Sauvignon” Wine. Vitis 2008, 47, 227–230. [Google Scholar] [CrossRef]
- Cudjoe, E.; Wiederkehr, T.B.; Brindle, I.D. Headspace Gas Chromatography-Mass Spectrometry: A Fast Approach to the Identification and Determination of 2-Alkyl-3- Methoxypyrazine Pheromones in Ladybugs. Analyst 2005, 130, 152. [Google Scholar] [CrossRef]
- Kögel, S.; Gross, J.; Hoffmann, C.; Ulrich, D. Diversity and Frequencies of Methoxypyrazines in Hemolymph of Harmonia Axyridis and Coccinella Septempunctata and Their Influence on the Taste of Wine. Eur. Food Res. Technol. 2012, 234, 399–404. [Google Scholar] [CrossRef]
- Raak-van den Berg, C.L.; Hemerik, L.; van der Werf, W.; de Jong, P.W.; van Lenteren, J.C. Life History of the Harlequin Ladybird, Harmonia Axyridis: A Global Meta-Analysis. BioControl 2017, 62, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Hiller, T.; Haelewaters, D. A Case of Silent Invasion: Citizen Science Confirms the Presence of Harmonia Axyridis (Coleoptera, Coccinellidae) in Central America. PLoS ONE 2019, 14, e0220082. [Google Scholar] [CrossRef] [Green Version]
- Biranvand, A.; Nedvěd, O.; Tomaszewska, W.; Al Ansi, A.N.; Fekrat, L.; Haghghadam, Z.M.; Khormizi, M.Z.; Noorinahad, S.; Şenal, D.; Shakarami, J.; et al. The Genus Harmonia (Coleoptera, Coccinellidae) in the Middle East Region. Acta Entomol. Musei Natl. Pragae 2019, 59, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Pons, X.; Roca, M.; Lumbierres, B.; Lucas, É. Characterization of a Newly Established Aggregation of the Invasive Ladybeetle Harmonia Axyridis and Current Status of the Invader in Spain. Span. J. Agric. Res. 2015, 13, e1006. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.M.J.; Thomas, C.E.; Lombaert, E.; Jeffries, D.L.; Estoup, A.; Lawson Handley, L.-J. The Global Spread of Harmonia Axyridis (Coleoptera: Coccinellidae): Distribution, Dispersal and Routes of Invasion. BioControl 2011, 56, 623–641. [Google Scholar] [CrossRef]
- Al Ansi, A.; Alkhalaf, A.A.; Fadl, H.; Rasool, I.; Al Dhafer, H. An Annotated Checklist of Coccinellidae (Insecta, Coleoptera) with Eight New Records from the Kingdom of Saudi Arabia. ZooKeys 2020, 1006, 35–89. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Heredia, D.F.; Peñaherrera-Romero, E. Invasion History of Harmonia Axyridis (Pallas, 1773) (Coleoptera: Coccinellidae) in Ecuador. PeerJ 2020, 8, e10461. [Google Scholar] [CrossRef]
- Lakhal, M.A.; Ghezali, D.; Nedvěd, O.; Doumandji, S. Checklist of Ladybirds of Algeria with Two New Recorded Species (Coleoptera, Coccinellidae). ZooKeys 2018, 774, 41–52. [Google Scholar] [CrossRef]
- Poutsma, J.; Loomans, A.J.M.; Aukema, B.; Heijerman, T. Predicting the Potential Geographical Distribution of the Harlequin Ladybird, Harmonia Axyridis, Using the CLIMEX Model. BioControl 2008, 53, 103–125. [Google Scholar] [CrossRef]
- Bidinger, K.; Lötters, S.; Rödder, D.; Veith, M. Species Distribution Models for the Alien Invasive Asian Harlequin Ladybird (Harmonia Axyridis). J. Appl. Entomol. 2012, 136, 109–123. [Google Scholar] [CrossRef]
- Evans, K.; Simpson, B. Predicting the potential geographical distribution of the harlequin ladybird, harmonia axyridis, using the CLIMEX model. In What Makes an Alien Invasive? Risk and Policy Responses: Aspects of Applied Biology (Volume 104); Evans, A., Ed.; Association of Applied Biologists (AAB): Warwick, UK, 2010; pp. 29–35. [Google Scholar]
- Bazzocchi, G.G.; Lanzoni, A.; Accinelli, G.; Burgio, G. Overwintering, Phenology and Fecundity of Harmonia Axyridis in Comparison with Native Coccinellid Species in Italy. BioControl 2004, 49, 245–260. [Google Scholar] [CrossRef]
- Katsoyannos, P.; Kontodimas, D.C.; Stathas, G.J.; Tsartsalis, C.T. Establishment of Harmonia Axyridis on Citrus and Some Data on Its Phenology in Greece. Phytoparasitica 1997, 25, 183–191. [Google Scholar] [CrossRef]
- Brown, P.M.J.; Roy, H.E.; Rothery, P.; Roy, D.B.; Ware, R.L.; Majerus, M.E.N. Harmonia axyridis in Great Britain: Analysis of the spread and distribution of a non-native coccinellid. In From Biological Control to Invasion: The Ladybird Harmonia Axyridis as a Model Species; Roy, H.E., Wajnberg, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 55–67. [Google Scholar]
- Steenberg, T.; Harding, S. The Harlequin Ladybird (Harmonia Axyridis Pallas) in Denmark: Spread and Phenology during the Initial Phase of Invasion. Entomol. Medd. 2009, 77, 27–39. [Google Scholar]
- Brown, P.M.J.; Frost, R.; Doberski, J.; Sparks, T.; Harrington, R.; Roy, H.E. Decline in Native Ladybirds in Response to the Arrival of Harmonia Axyridis: Early Evidence from England. Ecol. Entomol. 2011, 36, 231–240. [Google Scholar] [CrossRef]
- Honek, A.; Dixon, A.F.; Soares, A.O.; Skuhrovec, J.; Martinkova, Z. Spatial and Temporal Changes in the Abundance and Compostion of Ladybird (Coleoptera: Coccinellidae) Communities. Curr. Opin. Insect Sci. 2017, 20, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, A.J.; Soares, A.O.; Vergara, P.M.; Azevedo, E.B.; Grez, A.A. The Failed Invasion of Harmonia Axyridis in the Azores, Portugal: Climatic Restriction or Wrong Population Origin? Insect Sci. 2021, 28, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Galvan, T.L.; Burkness, E.C.; Hutchison, W.D. Enumerative and Binomial Sequential Sampling Plans for the Multicolored Asian Lady Beetle (Coleoptera: Coccinellidae) in Wine Grapes. J. Econ. Entomol. 2007, 100, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- The Society of American Foresters “Definition of Semiochemical” in the Dictionary of Forestry. Available online: http://dictionaryofforestry.org/dict/term/semiochemical (accessed on 27 July 2021).
- Glemser, E.J.; Dowling, L.; Inglis, D.; Pickering, G.J.; Mcfadden-Smith, W.; Sears, M.K.; Hallett, R.H. A Novel Method for Controlling Multicolored Asian Lady Beetle (Coleoptera: Coccinellidae) in Vineyards. Environ. Entomol. 2012, 41, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Leroy, P.D.; Schillings, T.; Farmakidis, J.; Heuskin, S.; Lognay, G.; Verheggen, F.J.; Brostaux, Y.; Haubruge, E.; Francis, F. Testing Semiochemicals from Aphid, Plant and Conspecific: Attraction of Harmonia Axyridis. Insect Sci. 2012, 19, 372–382. [Google Scholar] [CrossRef]
- Pickering, G.J.; Glemser, E.J.; Hallett, R.; Inglis, D.; McFadden-Smith, W.; Ker, K. Good Bugs Gone Bad: Coccinellidae, Sustainability and Wine. WIT Trans. Ecol. Environ. 2011, 167, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Glemser, E.; McFadden-Smith, W.; Parent, J.-P. Evaluation of Compounds for Repellency of the Multicoloured Asian Lady Beetle (Coleoptera: Coccinellidae) in Vineyards. Can. Entomol. 2021, 1–12. [Google Scholar] [CrossRef]
- Botezatu, A.; Pickering, G. Ladybug (Coccinellidae) taint in wine. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; Volume 2, pp. 418–429. ISBN 978-1-84569-484-5. [Google Scholar]
- Kotseridis, Y.S.; Spink, M.; Brindle, I.D.; Blake, A.J.; Sears, M.; Chen, X.; Soleas, G.; Inglis, D.; Pickering, G.J. Quantitative Analysis of 3-Alkyl-2-Methoxypyrazines in Juice and Wine Using Stable Isotope Labelled Internal Standard Assay. J. Chromatogr. A 2008, 1190, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Roujou de Boubée, D. Research on the Vegetal Green Pepper Character in Grapes and Wines. Rev. Oenol. 2004, 31, 6–10. [Google Scholar]
- Baggio, P. Flash Extraction—What Can It Do for You? In Proceedings of the ASVO Proceedings: Managing the Best Out of Difficult Vintages; 2017; pp. 29–31. Available online: https://www.dtpacific.com/dev/wp-content/uploads/2017/03/Art-ASVO-Flash-Bio-Thermo-Extraction-What-can-it-do-for-you.pdf (accessed on 1 April 2021).
- Cejudo-Bastante, M.J.; Pérez-Coello, M.S.; Hermosín-Gutiérrez, I. Effect of Wine Micro-Oxygenation Treatment and Storage Period on Colour-Related Phenolics, Volatile Composition and Sensory Characteristics. LWT 2011, 44, 866–874. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Improvement of Cencibel Red Wines by Oxygen Addition after Malolactic Fermentation: Study on Color-Related Phenolics, Volatile Composition, and Sensory Characteristics. J. Agric. Food Chem. 2012, 60, 5962–5973. [Google Scholar] [CrossRef]
- Oberholster, A.; Elmendorf, B.L.; Lerno, L.A.; King, E.S.; Heymann, H.; Brenneman, C.E.; Boulton, R.B. Barrel Maturation, Oak Alternatives and Micro-Oxygenation: Influence on Red Wine Aging and Quality. Food Chem. 2015, 173, 1250–1258. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Henschen, C.; Cantu, A.; Watrelot, A.A.; Waterhouse, A.L. Understanding Microoxygenation: Effect of Viable Yeasts and Sulfur Dioxide Levels on the Sensory Properties of a Merlot Red Wine. Food Res. Int. 2018, 108, 505–515. [Google Scholar] [CrossRef]
- Blake, A.; Kotseridis, Y.; Brindle, I.D.; Inglis, D.; Sears, M.; Pickering, G.J. Effect of Closure and Packaging Type on 3-Alkyl-2-Methoxypyrazines and Other Impact Odorants of Riesling and Cabernet Franc Wines. J. Agric. Food Chem. 2009, 57, 4680–4690. [Google Scholar] [CrossRef]
- Pickering, G.; Lin, J.; Reynolds, A.; Soleas, G.; Riesen, R. The Evaluation of Remedial Treatments for Wine Affected by Harmonia Axyridis. Int. J. Food Sci. Technol. 2006, 41, 77–86. [Google Scholar] [CrossRef]
- Wilson, K. Applications of Radiation within the Wine Industry, Can. Bachelor’s Thesis, McMaster University, Hamilton, ON, Canada, 2003. [Google Scholar]
- Wilson, K.J.; Moran, G.; Boreham, D. Application of Radiation within the Wine Industry. In Proceedings of the 12th Quadrennial Congress of the International Association for Radiation Research Incorporating the 50th Annual Meeting of Radiation Research Society, RANZCR Radiation Oncology Annual Scientific Meeting and AINSE Radiation Science Conference, Brisbane, Australia, 17 August 2003. [Google Scholar]
- Inglis, D.; Beh, A.L.; Brindle, I.D.; Pickering, G.; Humes, E.F. Method for Reducing Methoxypyrazines in Grapes and Grape Products. US Patent No. 8859026B2, 14 October 2014. [Google Scholar]
- Pickering, G.; Inglis, D.; Botezatu, A.; Beh, A.; Humes, E.; Brindle, I. New approaches to removing alkyl-methoxypyrazines from grape juice and wine. In Scientific Bulletin. Series F. Biotechnologies, Vol. XVIII; The University of Agronomic Sciences and Veterinary Medicine: Bucharest, Romania, 2014; pp. 130–134. [Google Scholar]
- Pickering, G.J.; Blake, A.J.; Soleas, G.J.; Inglis, D.L. Remediation of Wine with Elevated Concentrations of 3-Alkyl-2-Methoxypyrazines Using Cork and Synthetic Closures. J. Food Agric. Environ. 2010, 8, 97–101. [Google Scholar]
- Ryona, I.; Reinhardt, J.; Sacks, G.L. Treatment of Grape Juice or Must with Silicone Reduces 3-Alkyl-2-Methoxypyrazine Concentrations in Resulting Wines without Altering Fermentation Volatiles. Food Res. Int. 2012, 47, 70–79. [Google Scholar] [CrossRef]
- Botezatu, A.; Pickering, G.J. Application of Plastic Polymers in Remediating Wine with Elevated Alkyl-Methoxypyrazine Levels. Food Addit. Contam. A 2015, 32, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Kemp, B.; Pickering, G. Chemical and Sensory Evaluation of Silicone and Polylactic Acid-Based Remedial Treatments for Elevated Methoxypyrazine Levels in Wine. Molecules 2016, 21, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Jeffery, D.W.; Taylor, D.K. Preparation of Magnetic Polymers for the Elimination of 3-Isobutyl-2-Methoxypyrazine from Wine. Molecules 2018, 23, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Ristic, R.; Stevenson, R.; Jiranek, V.; Jeffrey, D. Green Characters: Using Magnetic Polymers to Remove Overpowering Green Capsicum Flavour from Cabernet Sauvignon Wine. Wine Vitic. J. 2019, 34, 24–26. [Google Scholar]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine Analysis and Influence of Viticultural and Enological Procedures on Their Levels in Grapes, Musts, and Wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Vinsonneau, E.; Escaffre, P.; Crachereau, J.C.; Praud, S. Evaluation Du Procédé de Vinification Par “Flash Détente” Dans Le Bordelais; ITV France Bordeaux: Blanquefort, France, 2006. [Google Scholar]
- Logan, S. Sniffing out the case for flash détente. Aust. N. Z. Grapegrow. Winemaker 2020, 680, 89–95. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
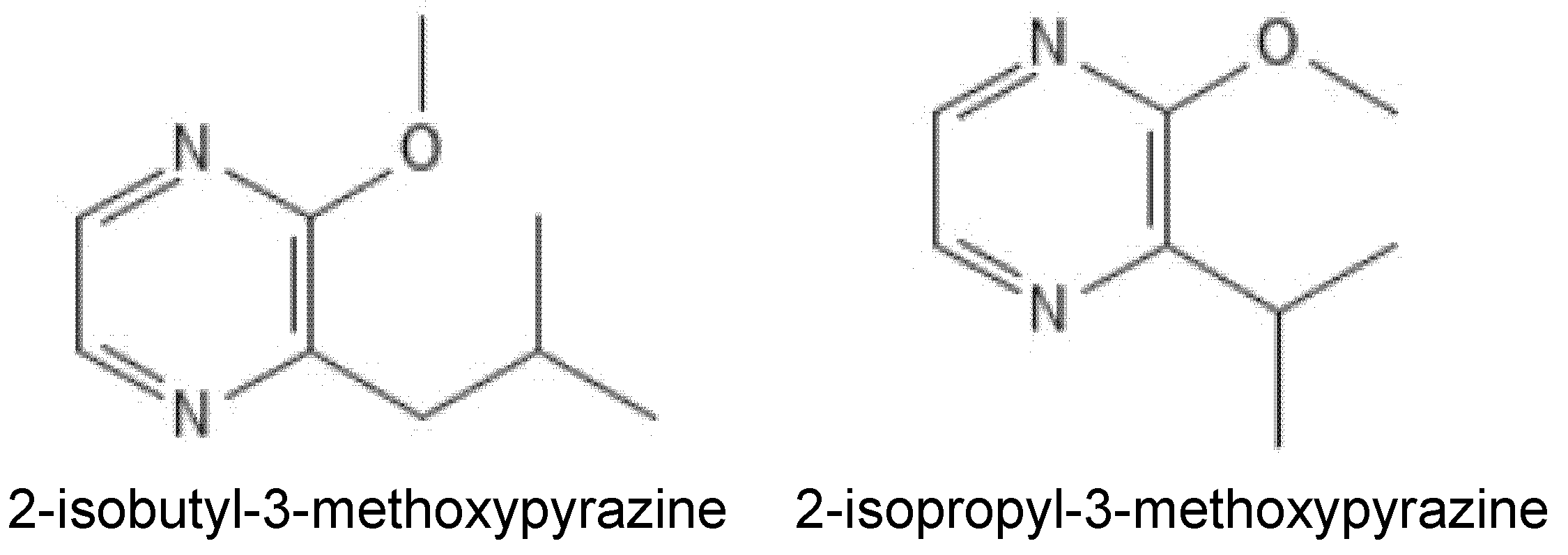
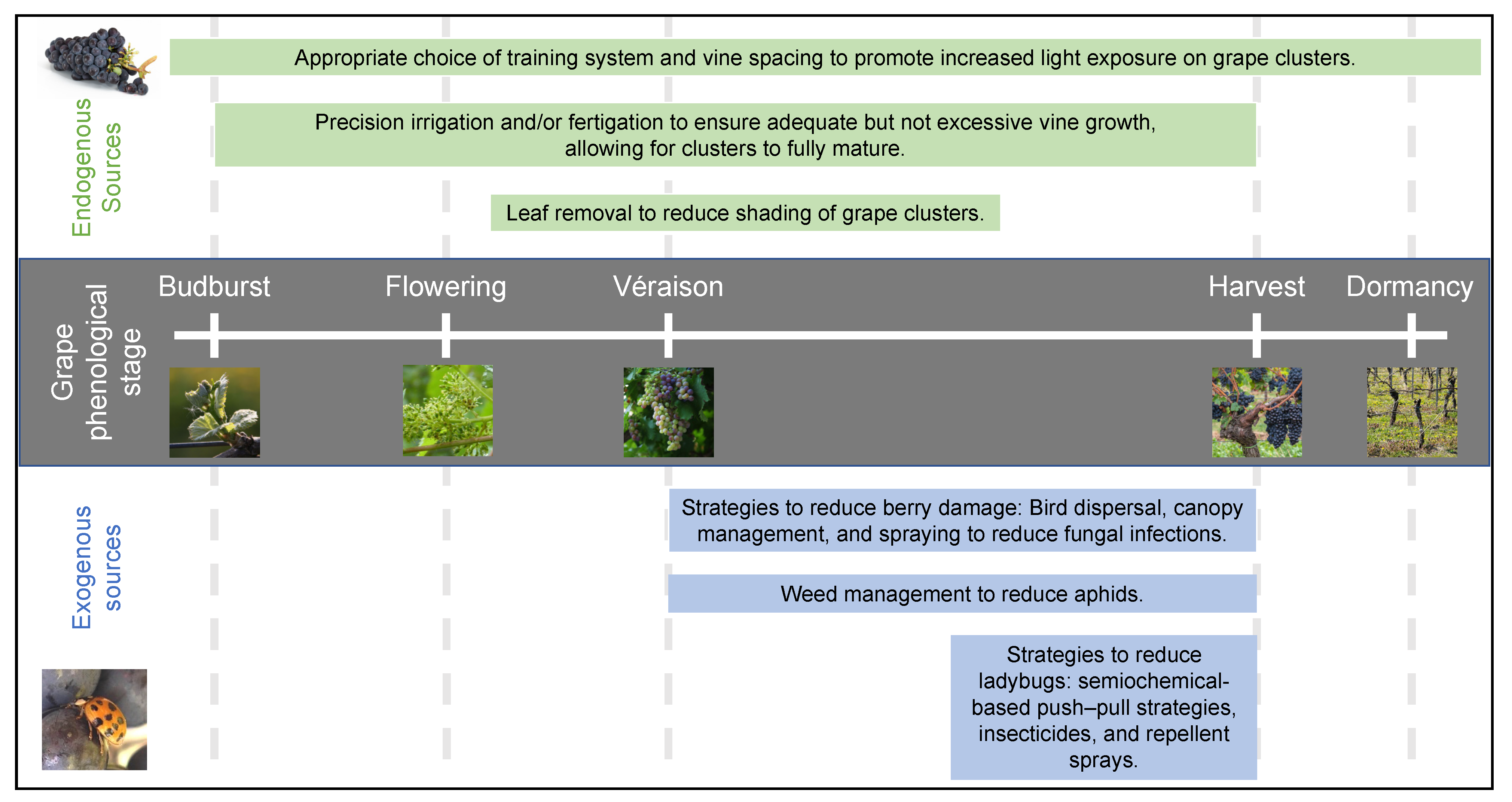
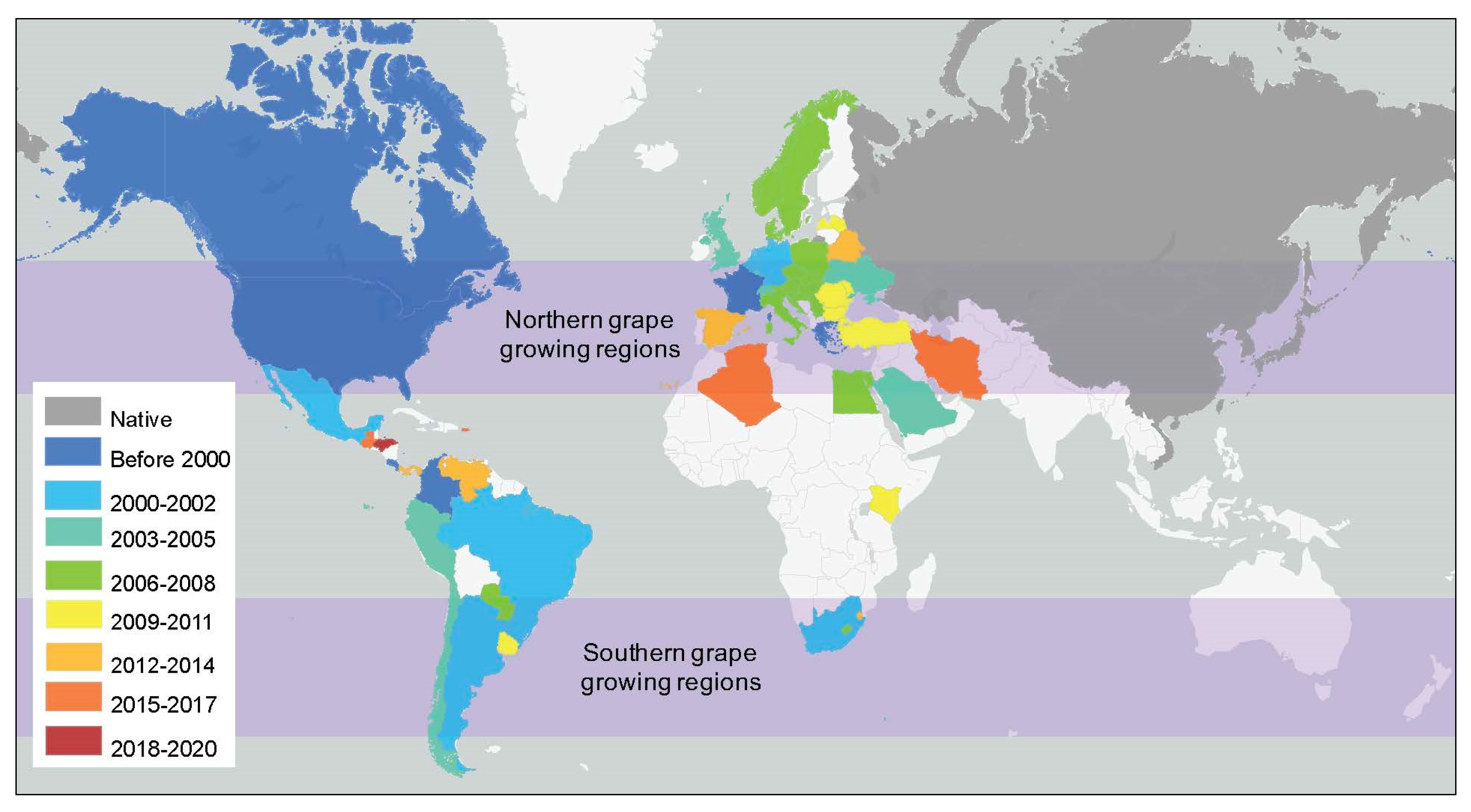
| Type of Intervention | Treatment or Intervention | Matrix | Major Compounds Targeted or Measured | Main Findings/ Limitations | Efficacy/ Potential | Citation(s) |
|---|---|---|---|---|---|---|
| Clarification | Clarification with bentonite or natural settling | Juice | IBMP | -Up to 50% reduction after 24hrs settling -Cannot be applied to wines requiring skin contact | Mod. | [24,112] |
| Heat and oxygen | Thermovinification | Juice | IBMP | -A 29–67% reduction -Leads to cooked aromas and flavors | Mod. | [80,113] |
| ThermoFlash/ Flash Detente | Juice, must—various varietals | IBMP | -Reductions of up to 95% reported -Reduction in vegetal notes in wines -Limited to red wines -Data do not appear to have been peer-reviewed | High | [114] | |
| Micro- oxygenation | Wine | ND | -Reduction in MP-related notes -Some reports of increase in vegetal attributes -Not clear if effects are due to MP reduction or perceptual masking -Limited to red wines -MPs were not quantified | Fair | [115,116,117,118] | |
| Packaging | Closure and packaging type | Wine spiked with IBMP, SBMP, and IPMP | IBMP, SBMP, IPMP | -Tetra Pak was most effective at reducing all three MPs (up to 41% for IPMP) -Synthetic closures also led to reductions in MPs (up to 21% for IPMP) -Tetra Pak is not a common packaging option for wines | Mod. | [119] |
| Radiation/irradiation | Light and UV light | Wine affected by LBT | IPMP | -No effect | Poor | [120] |
| Irradiation at 100 Gy (cobalt-60 source) | Wine tainted with LBT | ND | -Improvement in MP-related sensory characteristics reported -Potential for free radicals generated to adversely impact wine quality -Data do not appear to have been peer-reviewed | Low | [121,122] | |
| Fining and additives | Selected yeast strains used for fermentation | Juice spiked with IPMP | IPMP | -Lalvin BM45 increased IPMP by 45% -Lalvin D80 produced wines with high MP-related sensory attributes -Lalvin D21 produced wines with lowest MP-related sensory attributes | Poor | [10] |
| Activated charcoal, bentonite | Wine affected by LBT | IPMP | Activated charcoal -Reduced IPMP by 34% in white wine -MP related attributes did not change in white wine. -In red wine, asparagus and bell pepper flavor reduced Bentonite -No effect on IPMP -Reduced asparagus/bell pepper flavor in red wines | Low | [120] | |
| Oak chips | Wine affected by LBT | IPMP | -Neither oak chips nor deodorised oak chips affected IPMP concentrations. -Oak chips reduced MP-related sensory attributes in both red and white wine (masking effect) | Mod. | [120] | |
| Odorant-binding proteins (OBP) | Juice | IPMP, IBMP | -mMUP2 applied to juice and, subsequently, fined with bentonite and filtered with a 10 kDa polyethersulfone membrane removed >99% of IPMP and IBMP -No reports of efficacy in wine -Not yet commercialised | High | [123,124] | |
| Polymers | Natural and synthetic closures added to wine | Wine spiked with IBMP, SBMP, and IPMP | IBMP, SBMP, IPMP | -All closures led to MP reductions -Synthetic closures were most efficient (70–89% MP reduction) -SBMP was most affected -Impact on non-target compounds not determined -Limited commercial application | Mod. | [125] |
| Silicone added to juice | Model juice, grape juice, and must | IBMP, IPMP | -IPMP reduced by 93% after 48 hrs. -IBMP reduced by 90% after 40 hrs. -IPMP and IBMP also decreased in control wines -Some non-target volatile compounds decreased with treatment | High | [126] | |
| Plastic polymers added to wine | Wine spiked with IBMP, SBMP, and IPMP | IBMP, SBMP, IPMP | -Polylactic acid reduced IPMP by 52% and IBMP by 36% after 24 hrs. -Silicone reduced IPMP by 96% and IBMP by 100% after 24 hrs. | Mod. (PLA) to High (silicone) | [127] | |
| Polylactic acid and silicone added to wine | Wine spiked with IBMP, SBMP and IPMP | IBMP, SBMP, IPMP | -Reduction of 38–44% in MPs for silicone polymer -Reduction of 75–78% for MPs for polylactic acid polymer -Minimal impact on other volatile compounds -Sensory impacts were not clear, and generally showed minimal effect from the treatments | Fair | [128] | |
| Magnetic polymers (molecularly imprinted (MIMP) and non-molecularly imprinted (N-MIMP)) | Wine spiked with IBMP | IBMP | -MIMP reduced IBMP by 45% after 30 minutes of contact -N-MIMP reduced IBMP by 38% after 30 minutes of contact -Magnetic polymers are recoverable and reusable -Not yet commercially available | High | [129] | |
| Molecularly imprinted magnetic polymers and polylactic acid (PLA) | Grape must spiked with IBMP, pre- and post-fermentation | IBMP | -Pre-fermentation MIMP led to 30–40% reduction in IBMP -Post-fermentation MIMP led to 74% reduction in IBMP -Post fermentation PLA led to 18% reduction in IBMP -MIMP led to reduction in “fresh green” aromas in wines -Not yet commercially available | Fair (PLA)–High (MIMP) | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickering, G.J.; Willwerth, J.; Botezatu, A.; Thibodeau, M. Prevalence and Management of Alkyl-Methoxypyrazines in a Changing Climate: Viticultural and Oenological Considerations. Biomolecules 2021, 11, 1521. https://doi.org/10.3390/biom11101521
Pickering GJ, Willwerth J, Botezatu A, Thibodeau M. Prevalence and Management of Alkyl-Methoxypyrazines in a Changing Climate: Viticultural and Oenological Considerations. Biomolecules. 2021; 11(10):1521. https://doi.org/10.3390/biom11101521
Chicago/Turabian StylePickering, Gary J., Jim Willwerth, Andreea Botezatu, and Margaret Thibodeau. 2021. "Prevalence and Management of Alkyl-Methoxypyrazines in a Changing Climate: Viticultural and Oenological Considerations" Biomolecules 11, no. 10: 1521. https://doi.org/10.3390/biom11101521






