Expression of MMP-2, MMP-9, and NGAL in Tissue and Serum of Patients with Vascular Aneurysms and Their Modulation by Statin Treatment: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Experimental Study
2.4. Endpoints
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Protein Extraction and Immunoblot Analysis
2.7. Safety Evaluation
2.8. Statistical Analysis
3. Results
3.1. Patients
3.2. Elisa Test of MMP-2, MMP-9, and NGAL Plasma Levels
3.3. Protein Expression Levels of MMP-2, MMP-9, and NGAL in Aneurysmal Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davies, M.J. Aortic aneurysm formation: Lessons from human studies and experimental models. Circulation 1998, 98, 193–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontaine, V.; Jacob, M.P.; Houard, X.; Rossignol, P.; Plissonnier, D.; Angles-Cano, E.; Michel, J.B. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am. J. Pathol. 2002, 161, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Petersen, E.; Wagberg, F.; Angquist, K.A. Proteolysis of the abdominal aortic aneurysm wall and the association with rupture. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakalihasan, N.; Delvenne, P.; Nusgens, B.V.; Limet, R.; Lapiere, C.M. Activated forms of mmp2 and mmp9 in abdominal aortic aneurysms. J. Vasc. Surg. 1996, 24, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Katsuda, S.; Okada, Y.; Imai, K.; Nakanishi, I. Matrix metalloproteinase-9 (92-kd gelatinase/type iv collagenase equals gelatinase b) can degrade arterial elastin. Am. J. Pathol. 1994, 145, 1208–1218. [Google Scholar]
- Goodall, S.; Crowther, M.; Hemingway, D.M.; Bell, P.R.; Thompson, M.M. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 2001, 104, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Grande, R.; Montemurro, R.; Butrico, L.; Calio, F.G.; Mastrangelo, D.; Scarcello, E.; Gallelli, L.; Buffone, G.; de Franciscis, S. The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015, 157, 155–162. [Google Scholar] [CrossRef]
- Ziora, D.; Dworniczak, S.; Kozielski, J. Induced sputum metalloproteinases and their inhibitors in relation to exhaled nitrogen oxide and sputum nitric oxides and other inflammatory cytokines in patients with chronic obstructive pulmonary disease. J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 809–817. [Google Scholar]
- De Franciscis, S.; Gallelli, L.; Amato, B.; Butrico, L.; Rossi, A.; Buffone, G.; Calio, F.G.; De Caridi, G.; Grande, R.; Serra, R. Plasma mmp and timp evaluation in patients with deep venous thrombosis: Could they have a predictive role in the development of post-thrombotic syndrome? Int. Wound J. 2016, 13, 1237–1245. [Google Scholar] [CrossRef]
- Wilson, W.R.; Anderton, M.; Schwalbe, E.C.; Jones, J.L.; Furness, P.N.; Bell, P.R.; Thompson, M.M. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation 2006, 113, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Volpentesta, G.; Gallelli, L.; Grande, R.; Buffone, G.; Lavano, A.; de Franciscis, S. Metalloproteinase-9 and neutrophil gelatinase-associated lipocalin plasma and tissue levels evaluation in middle cerebral artery aneurysms. Br. J. Neurosurg. 2014. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Gallelli, L.; Conti, A.; De Caridi, G.; Massara, M.; Spinelli, F.; Buffone, G.; Calio, F.G.; Amato, B.; Ceglia, S.; et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des. Dev. Ther. 2014, 8, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, R.; Gallelli, L.; Buffone, G.; Molinari, V.; Stillitano, D.M.; Palmieri, C.; de Franciscis, S. Doxycycline speeds up healing of chronic venous ulcers. Int. Wound J. 2015, 12, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Goraca, A.; Demyanets, S.; Mittlboeck, M.; Domenig, C.; Neumayer, C.; Wojta, J.; Nanobachvili, J.; Huk, I.; Klinger, M. Simvastatin decreases free radicals formation in the human abdominal aortic aneurysm wall via nf-kappab. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Piechota-Polanczyk, A.; Demyanets, S.; Nykonenko, O.; Huk, I.; Mittlboeck, M.; Domenig, C.M.; Neumayer, C.; Wojta, J.; Nanobachvili, J.; Klinger, M. Decreased tissue levels of cyclophilin a, a cyclosporine a target and phospho-erk1/2 in simvastatin patients with abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.K. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am. J. Cardiol. 2005, 96, 24F–33F. [Google Scholar] [CrossRef] [Green Version]
- Gallelli, L.; Falcone, D.; Scaramuzzino, M.; Pelaia, G.; D’Agostino, B.; Mesuraca, M.; Terracciano, R.; Spaziano, G.; Maselli, R.; Navarra, M.; et al. Effects of simvastatin on cell viability and proinflammatory pathways in lung adenocarcinoma cells exposed to hydrogen peroxide. BMC Pharmacol. Toxicol. 2014, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Falcone, D.; Gallelli, L.; Di Virgilio, A.; Tucci, L.; Scaramuzzino, M.; Terracciano, R.; Pelaia, G.; Savino, R. Effects of simvastatin and rosuvastatin on ras protein, matrix metalloproteinases and nf-kappab in lung cancer and in normal pulmonary tissues. Cell Prolif. 2013, 46, 172–182. [Google Scholar] [CrossRef]
- Pelaia, G.; Cuda, G.; Vatrella, A.; Gallelli, L.; Fratto, D.; Gioffre, V.; D’Agostino, B.; Caputi, M.; Maselli, R.; Rossi, F.; et al. Effects of hydrogen peroxide on mapk activation, il-8 production and cell viability in primary cultures of human bronchial epithelial cells. J. Cell. Biochem. 2004, 93, 142–152. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Buffone, G.; Scarcello, E.; Tripodi, F.; Rende, P.; Gallelli, L.; de Franciscis, S. Effects of glucocorticoids and tumor necrosis factor-alpha inhibitors on both clinical and molecular parameters in patients with takayasu arteritis. J. Pharmacol. Pharmacother. 2014, 5, 193–196. [Google Scholar]
- Singh, S.R.; Sullo, N.; Matteis, M.; Spaziano, G.; McDonald, J.; Saunders, R.; Woodman, L.; Urbanek, K.; De Angelis, A.; De Palma, R.; et al. Nociceptin/orphanin FQ (N/OFQ) modulates immunopathology and airway hyperresponsiveness representing a novel target for the treatment of asthma. Br. J. Pharmacol. 2016, 8, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Gallelli, L.; Grande, R.; Amato, B.; De Caridi, G.; Sammarco, G.; Ferrari, F.; Butrico, L.; Gallo, G.; Rizzuto, A.; et al. Hemorrhoids and matrix metalloproteinases: A multicenter study on the predictive role of biomarkers. Surgery 2016, 159, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Gallelli, L.; Renda, T.; Fratto, D.; Falcone, D.; Caraglia, M.; Busceti, M.T.; Terracciano, R.; Vatrella, A.; Maselli, R.; et al. Effects of statins and farnesyl transferase inhibitors on erk phosphorylation, apoptosis and cell viability in non-small lung cancer cells. Cell Prolif. 2012, 45, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Pelaia, G.; Fratto, D.; Muto, V.; Falcone, D.; Vatrella, A.; Curto, L.S.; Renda, T.; Busceti, M.T.; Liberto, M.C.; et al. Effects of budesonide on p38 mapk activation, apoptosis and il-8 secretion, induced by tnf-alpha and haemophilus influenzae in human bronchial epithelial cells. Int. J. Immunopathol. Pharmacol. 2010, 23, 471–479. [Google Scholar] [CrossRef]
- Rende, P.; Paletta, L.; Gallelli, G.; Raffaele, G.; Natale, V.; Brissa, N.; Costa, C.; Gratteri, S.; Giofre, C.; Gallelli, L. Retrospective evaluation of adverse drug reactions induced by antihypertensive treatment. J. Pharmacol. Pharmacother. 2013, 4, S47–S50. [Google Scholar]
- Gallelli, L.; Galasso, O.; Urzino, A.; Sacca, S.; Falcone, D.; Palleria, C.; Longo, P.; Corigliano, A.; Terracciano, R.; Savino, R.; et al. Characteristics and clinical implications of the pharmacokinetic profile of ibuprofen in patients with knee osteoarthritis. Clin. Drug Investig. 2012, 32, 827–833. [Google Scholar] [CrossRef]
- Gallelli, L.; Colosimo, M.; Tolotta, G.A.; Falcone, D.; Luberto, L.; Curto, L.S.; Rende, P.; Mazzei, F.; Marigliano, N.M.; De Sarro, G.; et al. Prospective randomized double-blind trial of racecadotril compared with loperamide in elderly people with gastroenteritis living in nursing homes. Eur. J. Clin. Pharmacol. 2010, 66, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Ciliberto, D.; Ierardi, A.; Caroleo, B.; Scalise, L.; Cimellaro, A.; Colangelo, L.; Spaziano, G.; Gallelli, L. Panitumumab induced forearm panniculitis in two women with metastatic colon cancer. Curr. Drug Saf. 2019, 14, 233–237. [Google Scholar]
- Krueger, F.; Kappert, K.; Foryst-Ludwig, A.; Kramer, F.; Clemenz, M.; Grzesiak, A.; Sommerfeld, M.; Paul Frese, J.; Greiner, A.; Kintscher, U.; et al. At1-receptor blockade attenuates outward aortic remodeling associated with diet-induced obesity in mice. Clin. Sci. (Lond) 2017, 131, 1989–2005. [Google Scholar] [CrossRef]
- Dorman, G.; Cseh, S.; Hajdu, I.; Barna, L.; Konya, D.; Kupai, K.; Kovacs, L.; Ferdinandy, P. Matrix metalloproteinase inhibitors: A critical appraisal of design principles and proposed therapeutic utility. Drugs 2010, 70, 949–964. [Google Scholar] [CrossRef]
- Hu, J.; Van den Steen, P.E.; Sang, Q.X.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Design Dev. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, R.; Butrico, L.; Grande, R.; Placida, G.D.; Rubino, P.; Settimio, U.F.; Quarto, G.; Amato, M.; Furino, E.; Compagna, R.; et al. Venous aneurysm complicating arteriovenous fistula access and matrix metalloproteinases. Open Med. (Wars) 2015, 10, 519–522. [Google Scholar]
- Yuwen, L.; Ciqiu, Y.; Yi, S.; Ruilei, L.; Yuanhui, L.; Bo, L.; Songqi, L.; Weiming, L.; Jie, L. A pilot study of protein microarray for simultaneous analysis of 274 cytokines between abdominal aortic aneurysm and normal aorta. Angiology 2019, 70, 830–837. [Google Scholar] [CrossRef]
- De Franciscis, S.; Mastroroberto, P.; Gallelli, L.; Buffone, G.; Montemurro, R.; Serra, R. Increased plasma levels of metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in a rare case of multiple artery aneurysm. Ann. Vasc. Surg. 2013, 27, 1185.e5–1185.e7. [Google Scholar] [CrossRef]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (mmp) activity is a complex of gelatinase b/mmp-9 and neutrophil gelatinase-associated lipocalin (ngal). Modulation of mmp-9 activity by ngal. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef] [Green Version]
- De Franciscis, S.; De Caridi, G.; Massara, M.; Spinelli, F.; Gallelli, L.; Buffone, G.; Calio, F.G.; Butrico, L.; Grande, R.; Serra, R. Biomarkers in post-reperfusion syndrome after acute lower limb ischaemia. Int. Wound J. 2016, 13, 854–859. [Google Scholar] [CrossRef]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, Y.H.; LeMaire, S.A. Thoracic aortic dissection: Are matrix metalloproteinases involved? Vascular 2009, 17, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.A.; Zavadzkas, J.A.; Chang, E.I.; Sheats, N.; Koval, C.; Stroud, R.E.; Spinale, F.G.; Ikonomidis, J.S. Cellular phenotype transformation occurs during thoracic aortic aneurysm development. J. Thorac. Cardiovasc. Surg. 2010, 140, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.A.; Barbour, J.R.; Lowry, A.S.; Bouges, S.; Beck, C.; McClister, D.M., Jr.; Mukherjee, R.; Ikonomidis, J.S. Spatiotemporal expression and localization of matrix metalloproteinas-9 in a murine model of thoracic aortic aneurysm. J. Vasc. Surg. 2006, 44, 1314–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Mozo, P.; Madrigal-Matute, J.; Vega de Ceniga, M.; Blanco-Colio, L.M.; Meilhac, O.; Feldman, L.; Michel, J.B.; Clancy, P.; Golledge, J.; Norman, P.E.; et al. Increased plasma levels of ngal, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis 2012, 220, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Swedenborg, J.; Eriksson, P. The intraluminal thrombus as a source of proteolytic activity. Ann. N. Y. Acad. Sci. 2006, 1085, 133–138. [Google Scholar] [CrossRef]
- Luan, Z.; Chase, A.J.; Newby, A.C. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Via, D.; Canavesi, M.; Pfister, P.; Fumagalli, R.; Paoletti, R.; Bernini, F. Hmg-coa reductase inhibitors reduce mmp-9 secretion by macrophages. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1671–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.; Lumma, W.C.; Smith, A.M.; Sisko, J.T.; Wright, S.D.; Cai, T.Q. Statins suppress thp-1 cell migration and secretion of matrix metalloproteinase 9 by inhibiting geranylgeranylation. J. Leukoc. Biol. 2001, 69, 959–962. [Google Scholar]
- Kawamukai, M. Biosynthesis of coenzyme q in eukaryotes. Biosci. Biotechnol. Biochem. 2016, 80, 23–33. [Google Scholar] [CrossRef]
- Zlatohlavek, L.; Vrablik, M.; Grauova, B.; Motykova, E.; Ceska, R. The effect of coenzyme q10 in statin myopathy. Neuro Endocrinol. Lett. 2012, 33 (Suppl. 2), 98–101. [Google Scholar]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of coenzyme q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef] [Green Version]
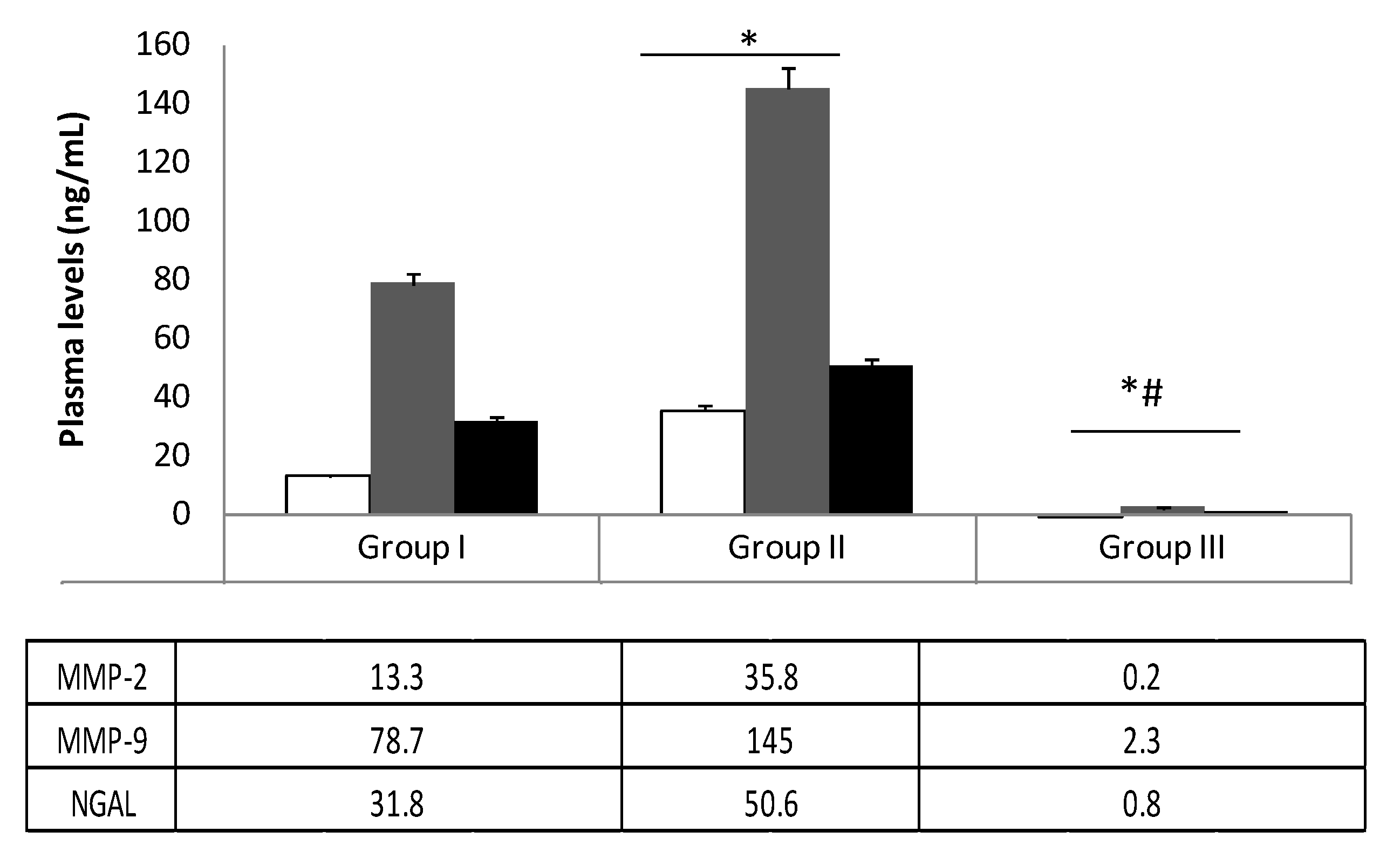
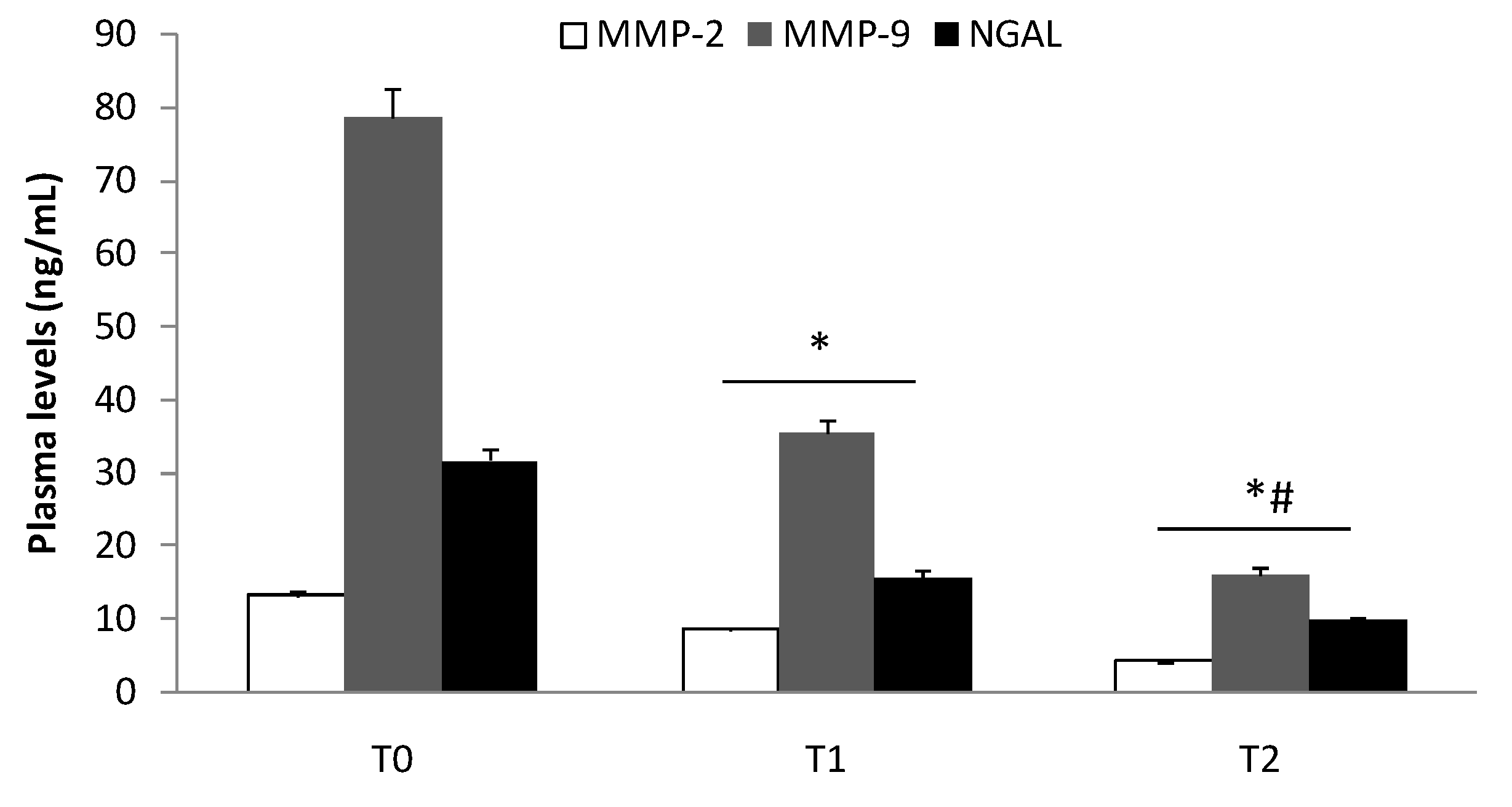
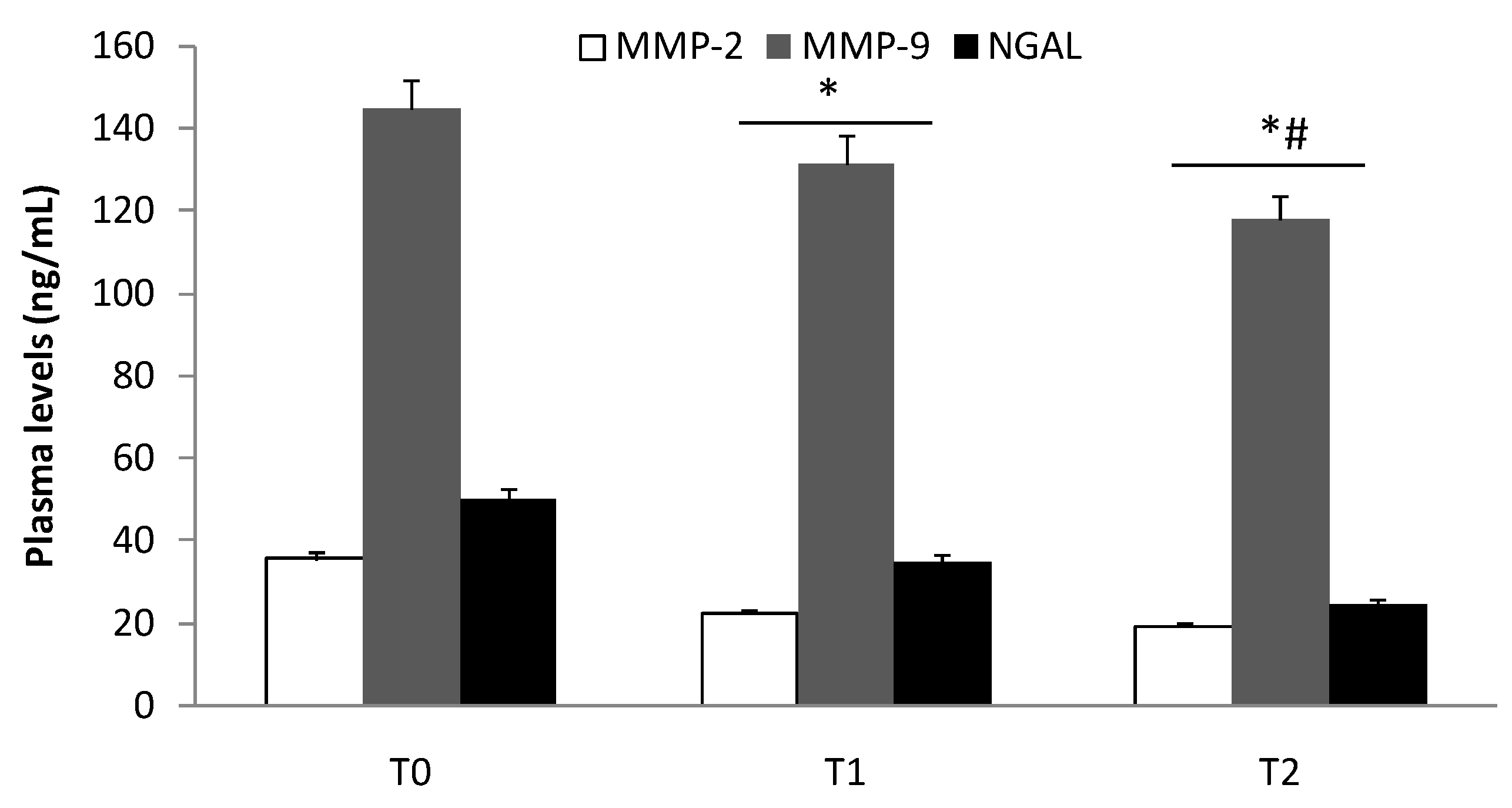

| Characteristics | Group I (n = 95) | Group II (n = 119) | Group III (n = 122) |
|---|---|---|---|
| Mean age (years) | 71.5 ± 6.3 | 70.6 ± 6.4 | 69.3 ± 6.3 |
| Male | 65 | 78 | 68 |
| Female | 30 | 41 | 54 |
| Aneurysm diameter | 54.2 ± 15.2 | 49.3 ± 17.9 | ---- |
| Cigarette smokers | 42 | 63 | 59 |
| Body mass index | |||
| >30 | 12 | 10 | 22 |
| 25–29.9 | 70 | 94 | 83 |
| 18.5–24.9 | 13 | 15 | 17 |
| <18.5 | 0 | 0 | 0 |
| Glutamic oxaloacetic transaminase | 33.3 ± 3.2 | 28.1 ± 4.9 | 26.7 ± 4.1 |
| Glutamic pyruvic transaminase | 32.5 ± 4.3 | 31.1 ± 3.6 | 29.3 ± 5.5 |
| Platelet count | 290.630 ± 21.315/µL | 312.250 ± 28.530/µL | 279.870 ± 53.210/µL |
| Comorbidity | |||
| Blood hypertension | 86 | 112 | 108 |
| COPD | 45 | 60 | 36 |
| Hypercholesterolemia | 95 | ---- | 68 |
| Acute coronary syndrome | 55 | ---- | 15 |
| Diabetes type II | 42 | 21 | 35 |
| Diabetes type I | 8 | 10 | 9 |
| Pharmacological therapy | |||
| Antihypertensive | 86 | 112 | 108 |
| Antiarrhythmic | 32 | ---- | 2 |
| Bronchodilators | 45 | 60 | 36 |
| Statins | 95 | ---- | 68 |
| Hypoglycemic drugs | 39 | 16 | 35 |
| Insulin | 8 | 10 | 9 |
| ADRs related to statins | |||
| Myalgia | 38 | 39 | |
| Gastrointestinal disturbance | 12 | 16 | |
| Increase in transaminases | 8 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cione, E.; Piegari, E.; Gallelli, G.; Caroleo, M.C.; Lamirata, E.; Curcio, F.; Colosimo, F.; Cannataro, R.; Ielapi, N.; Colosimo, M.; et al. Expression of MMP-2, MMP-9, and NGAL in Tissue and Serum of Patients with Vascular Aneurysms and Their Modulation by Statin Treatment: A Pilot Study. Biomolecules 2020, 10, 359. https://doi.org/10.3390/biom10030359
Cione E, Piegari E, Gallelli G, Caroleo MC, Lamirata E, Curcio F, Colosimo F, Cannataro R, Ielapi N, Colosimo M, et al. Expression of MMP-2, MMP-9, and NGAL in Tissue and Serum of Patients with Vascular Aneurysms and Their Modulation by Statin Treatment: A Pilot Study. Biomolecules. 2020; 10(3):359. https://doi.org/10.3390/biom10030359
Chicago/Turabian StyleCione, Erika, Elena Piegari, Giuseppe Gallelli, Maria Cristina Caroleo, Elena Lamirata, Francesca Curcio, Federica Colosimo, Roberto Cannataro, Nicola Ielapi, Manuela Colosimo, and et al. 2020. "Expression of MMP-2, MMP-9, and NGAL in Tissue and Serum of Patients with Vascular Aneurysms and Their Modulation by Statin Treatment: A Pilot Study" Biomolecules 10, no. 3: 359. https://doi.org/10.3390/biom10030359







