Sodium Thiosulfate in the Pregnant Dahl Salt-Sensitive Rat, a Model of Preeclampsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Protocol
2.2.1. Sodium Thiosulfate Treatment
2.2.2. Renal Injury Measurements
2.2.3. Uterine Artery Resistance Index
2.2.4. Terminal Mean Arterial Pressure (MAP) Measured under Anesthesia
2.2.5. Tissue and Blood Collection
2.2.6. Plasma and Urinary Thiosulfate and Sulfate Concentration
2.2.7. Renal Histology
2.2.8. Placental Histology
2.2.9. Statistical Analyses
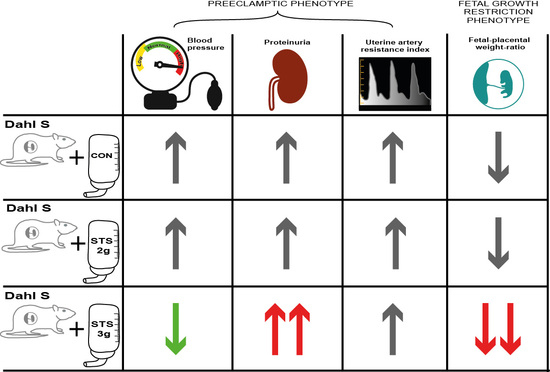
3. Results
3.1. Effect of STS Treatment on Renal Function Parameters
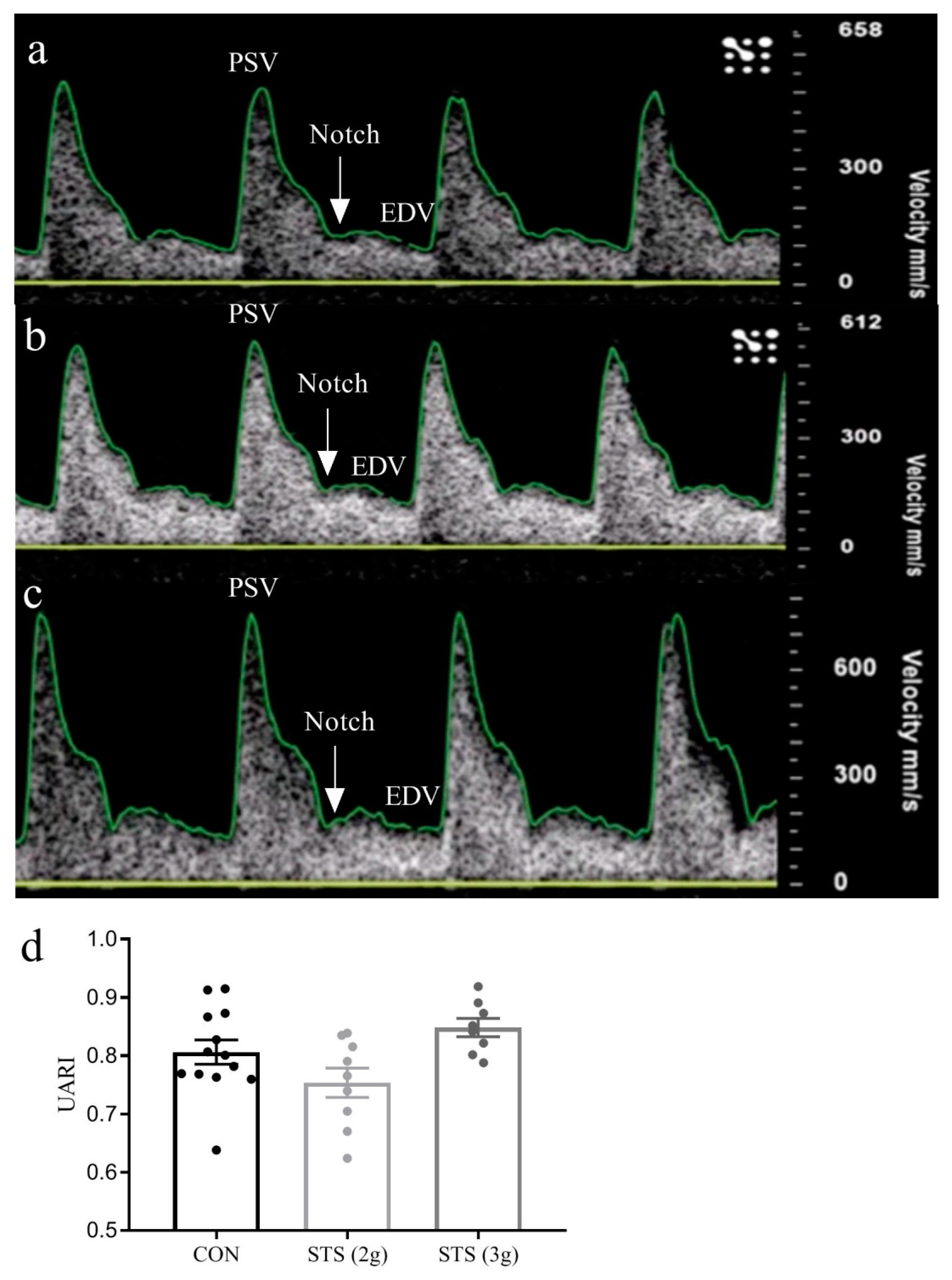
3.2. Effect of STS Treatment on Uterine Artery Resistance Index
3.3. Effect of STS Treatment on MAP
3.4. Effect of STS Treatment on Fetal and Placental Outcomes
3.5. Effect of STS Treatment on Gestational Weight Gain and Organ Weight
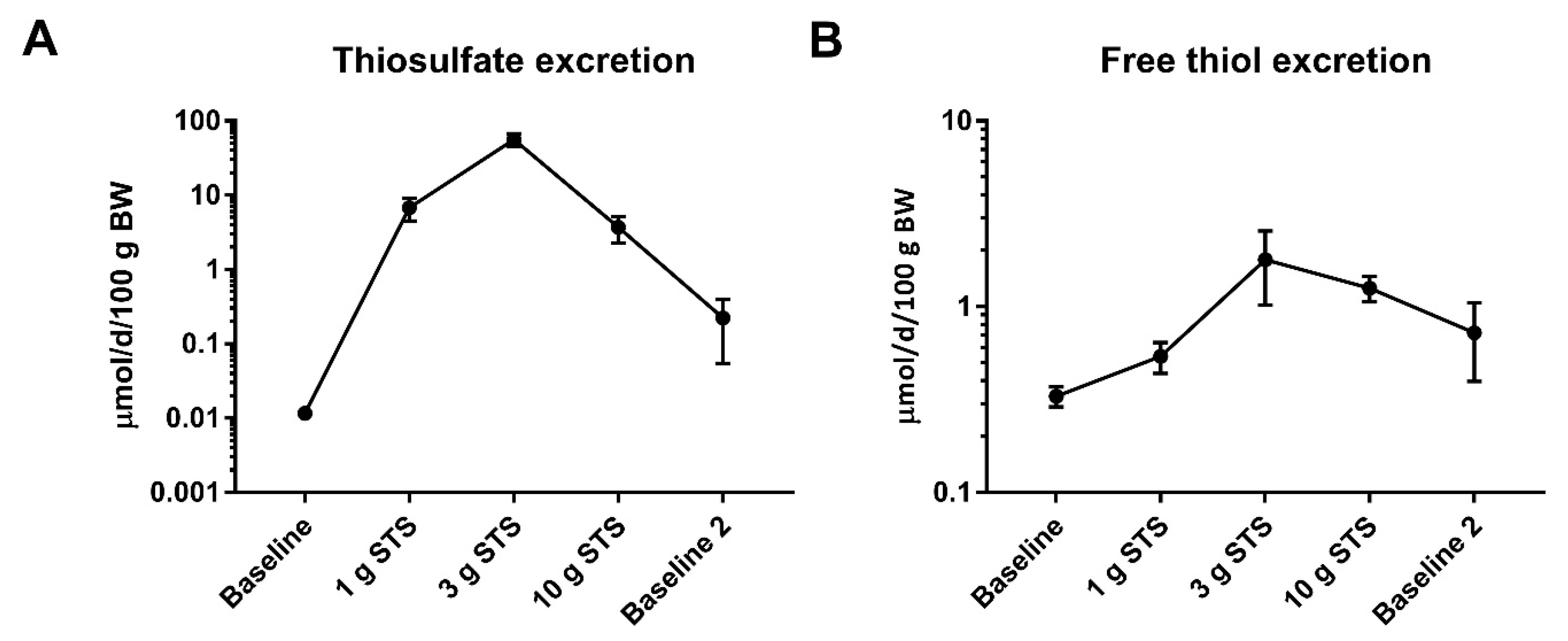
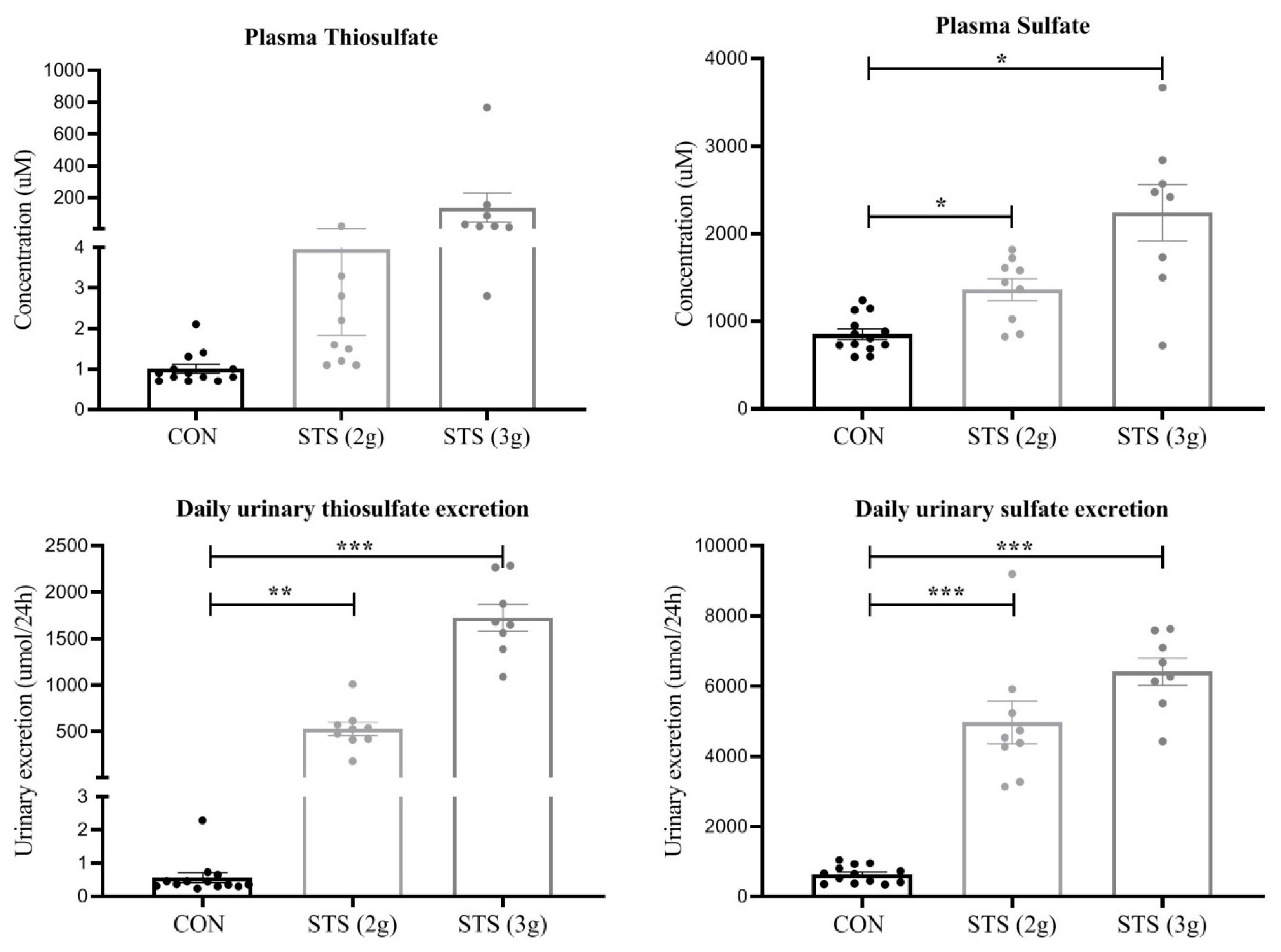
3.6. Plasma and Urinary Thiosulfate and Sulfate Concentration
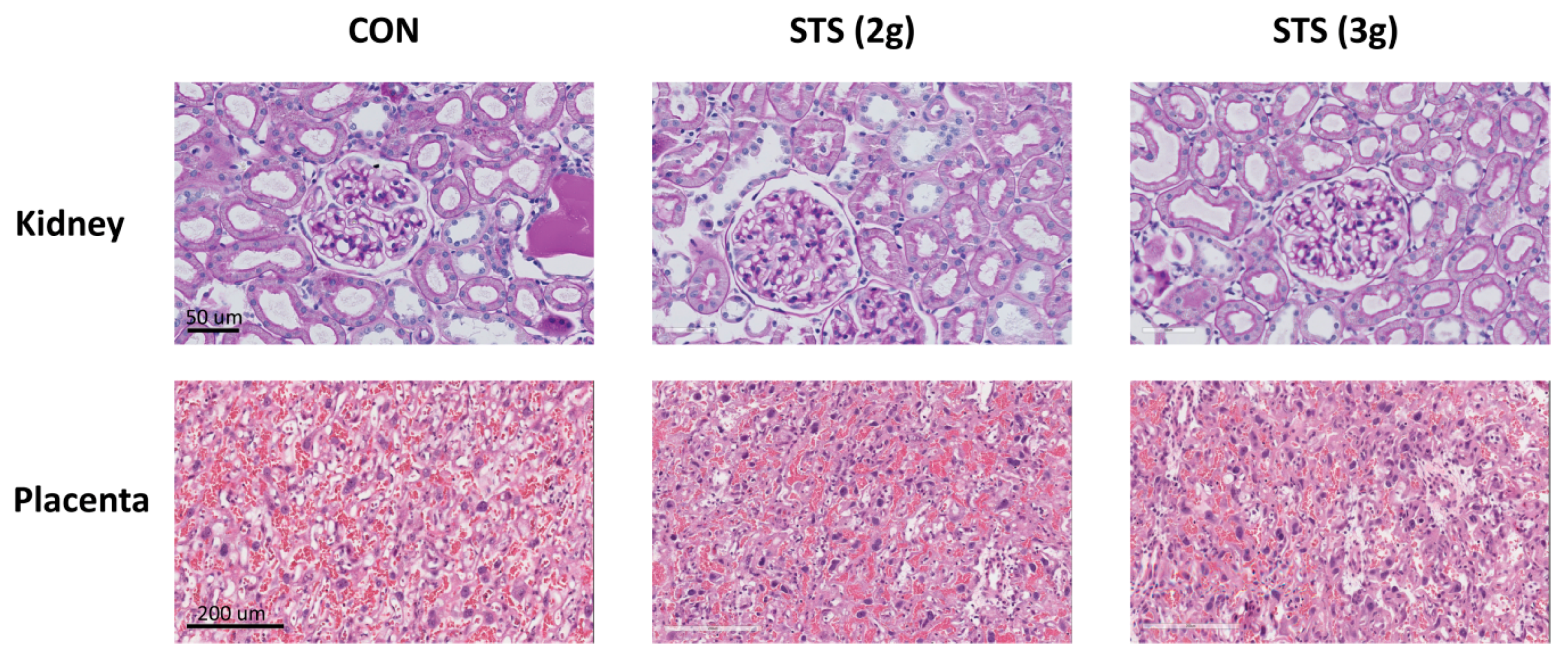
3.7. Effect of STS Treatment on Glomerular Injury
3.8. Effect of STS Treatment on Placenta Histomorphology
4. Discussion
Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Look, P.F.A. Van WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; Mccarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcdonald, S.D.; Han, Z.; Walsh, M.W.; Gerstein, H.C.; Devereaux, P.J. Kidney Disease After Preeclampsia: A Systematic Review. Am. J. Kidney Dis. 2010, 55, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M.; Way, A.S. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Dasinger, J.; Davis, G.; Newsome, A.; Alexander, B. The Developmental Programming of Hypertension: Physiological Mechanisms. Hypertension 2016, 68, 826–831. [Google Scholar] [CrossRef]
- Karumanchi, S. Angiogenic factors in pre-eclampsia: Implications for clinical practice. BJOG 2018, 125, 1396. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.Q.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.F.; et al. Dysregulation of Hydrogen Sulfide Producing Enzyme Cystathionine gamma-lyase Contributes to Maternal Hypertension and Placental Abnormalities in Preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef] [Green Version]
- Holwerda, K.M.; Karumanchi, S.A.; Lely, A.T. Hydrogen sulfide: Role in vascular physiology and pathology. Curr. Opin. Nephrol. Hypertens. 2015, 24, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Cindrova-Davies, T.; Herrera, E.A.; Niu, Y.; Kingdom, J.; Giussani, D.A.; Burton, G.J. Reduced cystathionine gamma-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am. J. Pathol. 2013, 182, 1448–1458. [Google Scholar] [CrossRef] [Green Version]
- Holwerda, K.M.; Bos, E.M.; Rajakumar, A.; Ris-stalpers, C.; Van Pampus, M.G.; Timmer, A.; Erwich, J.J.H.M.; Faas, M.M.; Van Goor, H.; Lely, A.T. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta 2012, 33, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Guo, X.; Wang, G.; Gao, L.; He, P.; Xia, Y.; Gu, H.; Ni, X.; Ph, D. MiR133b is involved in endogenous hydrogen sulfide suppression of sFlt-1 production in human placenta. Placenta 2017, 52, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, L.; Hodges, J.K.; Lechuga, T.J.; Zhang, H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol. Rep. 2017, 97, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Olson, K.R. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Possomato-Vieira, J.S.; Goncalves-Rizzi, V.H.; Graca, T.U.S.; Nascimento, R.A.; Dias-Junior, C.A. Sodium hydrosulfide prevents hypertension and increases in vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 in hypertensive pregnant rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Snijder, P.M.; Frenay, A.R.; De Boer, R.A.; Pasch, A.; Hillebrands, J.L.; Leuvenink, H.; Van Goor, H. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease. Br. J. Pharmacol. 2015, 172, 1494–1504. [Google Scholar] [CrossRef] [Green Version]
- Koj, A.; Frendo, J.; Janik, Z. Thiosulphate Oxidation by Rat Liver Mitochondria in the Presence of Glutathione. Biochem. J. 1967, 103, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Zicha, J.; Dobešová, Z.; Vokurková, M.; Rauchová, H.; Hojná, S.; Kadlecová, M.; Behuliak, M.; Vaněčková, I.; Kuneš, J. Age-Dependent Salt Hypertension in Dahl Rats: Fifty Years of Research. Physiol. Res. 2012, 61, S35–S87. [Google Scholar]
- Rapp, J.P.; Garrett, M.R. Will the Real Dahl S Rat Please Stand Up? Am. J. Physiol. Physiol. 2019. [Google Scholar] [CrossRef]
- Gillis, E.E.; Williams, J.M.; Garrett, M.R.; Mooney, J.N.; Sasser, J.M. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, 62–70. [Google Scholar] [CrossRef]
- Sasser, J.M.; Moningka, N.C.; Cunningham, M.W.; Croker, B.; Baylis, C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, H.; Fidler, V.; Weening, J.; Grond, J. Determinants of focal and segmental glomerulosclerosis in the rat after renal ablation. Evidence for involvement of macrophages and lipids. Lab Investig. 1991, 64, 754–765. [Google Scholar] [PubMed]
- Charest, P.L.; Vrolyk, V.; Herst, P.; Lessard, M.; Sloboda, D.M.; Dalvai, M.; Haruna, J.; Bailey, J.L.; Benoit-Biancamano, M.O. Histomorphologic Analysis of the Late-term Rat Fetus and Placenta. Toxicol. Pathol. 2018, 46, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Sasser, J.M.; Baylis, C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Chen, F.; Cheng, Y.; Tang, C.; Du, J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2013, 21, 1879–1885. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.H.; Islam, M.T.; Prieto, M.C.; Majid, D.S.A. Interdependency of Cystathione γ-Lyase and Cystathione β-Synthase in Hydrogen Sulfide–Induced Blood Pressure Regulation in Rats. Am. J. Hypertens. 2012, 25, 74–81w. [Google Scholar] [CrossRef] [Green Version]
- Roes, M.E.; Raijmakers, M.T.; De Boo, T.M.; Zusterzeel, P.L.; Merkus, H.M.; Peters, W.H.; Steegers, E.A. Oral N -acetylcysteine administration does not stabilise the process of established severe preeclampsia. Eur. J. Obs. Gynecol. Reprod. Biol. 2006, 127, 61–67. [Google Scholar] [CrossRef]
- Shirozu, K.; Tokuda, K.; Marutan, E.; Lefer, D.; Wang, R.; Ichinose, F. Cystathionine γ-lyase deficiency protects mice from galactosamine/lipopolysaccharide-induced acute liver failure. Antioxid. Redox Signal. 2014, 20, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, K.; Kida, K.; Marutani, E.; Crimi, E.; Bougaki, M.; Khatri, A.; Kimura, H.; Ichinose, F. Inhaled Hydrogen Sulfide Prevents Endotoxin-Induced Systemic Inflammation and Improves Survival by Altering Sulfide Metabolism in Mice. Antioxid. Redox Signal. 2012, 17, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Gillis, E.E.; Mooney, J.N.; Garrett, M.R.; Granger, J.P.; Sasser, J.M. Sildenafil Treatment Ameliorates the Maternal Syndrome of Preeclampsia and Rescues Fetal Growth in the Dahl Salt-Sensitive Rat. Hypertension 2016, 67, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Barton, J.R.; Sibai, B.M. Controversies Regarding Diagnosis and Treatment of Severe Hypertension in Pregnancy. Clin. Obstet. Gynecol. 2017, 60, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Giannubilo, S.R.; Bezzeccheri, V.; Cecchi, S.; Landi, B.; Battistoni, G.I.; Vitali, P.; Cecchi, L.; Tranquilli, A.L. Nifedipine versus labetalol in the treatment of hypertensive disorders of pregnancy. Arch. Gynecol. Obstet. 2012, 286, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Donhoffer, S.; Svezgvari, G.; Jarai, I.; Farkas, M. Thiosulphate Metabolism in the Animal Organism. Nature 1959, 184, 994–995. [Google Scholar]
- Olson, K.R.; DeLeon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 592–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | CON (n = 13) | STS (2 g) (n = 9) | STS (3 g) (n = 8) | p-Value |
|---|---|---|---|---|
| Water intake (mL) GD19 | 42.7 ± 1.9 | 48.0 ± 3.2 | 42.3 ± 3.6 | 0.30 |
| Urine production GD19 (mL/kg BW per hour) | 2.20 ± 0.2 | 2.21 ± 0.3 | 1.89 ± 0.1 | 0.59 |
| Proteinuria GD19 (mg/24h) | 56 ± 5 a | 51 ± 8 | 89 ± 15 a | 0.02 |
| Urea plasma GD20 (mmol/L) | 7.7 ± 0.2 | 8.5 ± 0.6 | 8.4 ± 0.6 | 0.57 |
| Body weight (grams) GD19 | 306 ± 4 | 316 ± 7 | 294 ± 3 | 0.02 |
| Gestational weight gain GD0-19 (%) | 32.5 ± 1.9 | 37.9 ± 1.7 | 24.3 ± 1.6 | <0.001 |
| Fetal and Placental Outcome | CON (n = 13) | STS (2 g) (n = 9) | STS (3 g) (n = 8) | p-Value |
|---|---|---|---|---|
| Fetal weight (g) | 2.13 ± 0.04 | 2.13 ± 0.07 | 2.01 ± 0.05 | 0.28 |
| Fetal crown-rump length (cm) | 3.06 ± 0.04 | 3.03 ± 0.05 | 3.11 ± 0.03 | 0.51 |
| Viable litter size (n) | 10.71 ± 0.6 | 11.2 ± 0.5 | 11.3 ± 0.4 | 0.68 |
| Fetal resorptions (%) | 8.4 ± 1.9 | 5.9 ± 2.4 | 3.0 ± 1.5 | 0.24 |
| Placental weight (g) | 0.50 ± 0.01 | 0.51 ± 0.01 | 0.53 ± 0.01 | 0.14 |
| Fetal/placental weight ratio | 4.31 ± 0.08 a | 4.27 ± 0.16 | 3.83 ± 0.07 a | <0.01 |
| Parameter | CON (n = 13) | 2 g STS (n = 9) | 3 g STS (n = 8) | p-Value |
|---|---|---|---|---|
| Relative total kidney weight (%) | 0.72 ± 0.02 a | 0.75 ± 0.03 | 0.86 ± 0.04 a | <0.01 |
| Relative heart weight (%) | 0.34 ± 0.004 | 0.35 ± 0.01 | 0.33 ± 0.01 | 0.28 |
| Relative splenic weight (%) | 0.28 ± 0.01 | 0.30 ± 0.01 | 0.31 ± 0.01 | 0.07 |
| Hematocrit (%) | 36.5 ± 1.7 | 38.4 ± 0.9 | 33.4 ± 2.1 | 0.18 |
| Sample (n = 30) | r | p-Value |
|---|---|---|
| Plasma thiosulfate (uM) | −0.62 | <0.001 |
| Plasma sulfate concentration (uM) | −0.26 | 0.17 |
| Urinary thiosulfate excretion (umol/24 h) | −0.43 | 0.02 |
| Urinary sulfate excretion (umol/24 h) | −0.45 | 0.01 |
| Kidney Injury Scoring Parameter | CON (n = 13) | STS 2 g (n = 9) | STS 3 g (n = 7) | p-value |
|---|---|---|---|---|
| Glomerulosclerosis damage score (%) | 15.2 ± 2.5 | 10.2 ± 3.3 | 14.0 ± 5.8 | 0.58 |
| Glomerular area (µm2) | 10,906 ± 421 | 11,443 ± 516 | 10,279 ± 410 | 0.29 |
| Glomerular tuft (µm2) | 8944 ± 355 | 9390 ± 439 | 8502 ± 364 | 0.37 |
| Urinary space (µm2) | 1962 ± 84 | 2053 ± 129 | 1777 ± 155 | 0.31 |
| Placental Histology Scoring Parameter | CON (n = 12) | STS 2 g (n = 8) | STS 3 g (n = 8) | p-Value |
|---|---|---|---|---|
| Labyrinth zone (mm2) | 21.89 ± 0.74 | 20.60 ± 0.95 | 21.64 ± 1.45 | 0.65 |
| Basal zone (mm2) | 7.61 ± 0.44 | 6.9 ± 0.85 | 8.67 ± 0.57 | 0.17 |
| Proportion labyrinth zone of whole fetal placental area (%) | 74 ± 1 | 76 ± 2 | 71 ± 1 | 0.22 |
| Proportion basal zone of whole fetal placental area (%) | 26 ± 1 | 25 ± 2 | 29 ± 1 | 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terstappen, F.; Clarke, S.M.; Joles, J.A.; Ross, C.A.; Garrett, M.R.; Minnion, M.; Feelisch, M.; van Goor, H.; Sasser, J.M.; Lely, A.T. Sodium Thiosulfate in the Pregnant Dahl Salt-Sensitive Rat, a Model of Preeclampsia. Biomolecules 2020, 10, 302. https://doi.org/10.3390/biom10020302
Terstappen F, Clarke SM, Joles JA, Ross CA, Garrett MR, Minnion M, Feelisch M, van Goor H, Sasser JM, Lely AT. Sodium Thiosulfate in the Pregnant Dahl Salt-Sensitive Rat, a Model of Preeclampsia. Biomolecules. 2020; 10(2):302. https://doi.org/10.3390/biom10020302
Chicago/Turabian StyleTerstappen, Fieke, Sinéad M. Clarke, Jaap A. Joles, Courtney A Ross, Michael R. Garrett, Magdalena Minnion, Martin Feelisch, Harry van Goor, Jennifer M. Sasser, and A. Titia Lely. 2020. "Sodium Thiosulfate in the Pregnant Dahl Salt-Sensitive Rat, a Model of Preeclampsia" Biomolecules 10, no. 2: 302. https://doi.org/10.3390/biom10020302