The “Cocombola Study”: A Physical Phantom Model for tDCS-Induced Electric Field Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Watermelon Preparation
2.2. Recording Set Up
2.3. Graphical Representation
2.4. Validation
3. Results
3.1. CONDITION A (Monopolar Montage)
3.2. CONDITION B (Bipolar Antero–Posterior Montage)
3.3. CONDITION C (Bipolar Latero–Lateral Montage)
4. Discussion
4.1. Validation Experiments
4.2. General Considerations and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef]
- Maiorana, N.; Guidetti, M.; Dini, M.; Priori, A.; Ferrucci, R. Cerebellar tDCS as Therapy for Cerebellar Ataxias. Cerebellum 2022, 21, 755–761. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (tDCS); Elsevier: Amsterdam, The Netherlands, 2017; Volume 128, pp. 56–92. [Google Scholar]
- Matsumoto, H.; Ugawa, Y. Adverse Events of tDCS and tACS: A Review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef]
- Laakso, I.; Mikkonen, M.; Koyama, S.; Hirata, A.; Tanaka, S. Can Electric Fields Explain Inter-Individual Variability in Transcranial Direct Current Stimulation of the Motor Cortex? Sci. Rep. 2019, 9, 626. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, J.; Jeong, S.; Kim, S.; Jun, S.C.; Chung, E. Validation of Computational Studies for Electrical Brain Stimulation with Phantom Head Experiments. Brain Stimul. 2015, 8, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The Contribution of Interindividual Factors to Variability of Response in Transcranial Direct Current Stimulation Studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Bocci, T.; Caleo, M.; Tognazzi, S.; Francini, N.; Briscese, L.; Maffei, L.; Rossi, S.; Priori, A.; Sartucci, F. Evidence for Metaplasticity in the Human Visual Cortex. J. Neural Transm. 2014, 121, 221–231. [Google Scholar] [CrossRef]
- Siebner, H.R.; Lang, N.; Rizzo, V.; Nitsche, M.A.; Paulus, W.; Lemon, R.N.; Rothwell, J.C. Preconditioning of Low-Frequency Repetitive Transcranial Magnetic Stimulation with Transcranial Direct Current Stimulation: Evidence for Homeostatic Plasticity in the Human Motor Cortex. J. Neurosci. 2004, 24, 3379–3385. [Google Scholar] [CrossRef]
- Fridriksson, J.; Elm, J.; Stark, B.C.; Basilakos, A.; Rorden, C.; Sen, S.; George, M.S.; Gottfried, M.; Bonilha, L. BDNF Genotype and tDCS Interaction in Aphasia Treatment. Brain Stimul. 2018, 11, 1276–1281. [Google Scholar] [CrossRef]
- Plewnia, C.; Zwissler, B.; Längst, I.; Maurer, B.; Giel, K.; Krüger, R. Effects of Transcranial Direct Current Stimulation (tDCS) on Executive Functions: Influence of COMT Val/Met Polymorphism. Cortex 2013, 49, 1801–1807. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Wagner, T.A.; Miranda, P.C.; Nitsche, M.A.; Paulus, W.; Lisanby, S.H.; Pascual-Leone, A.; Bikson, M. Fundamentals of Transcranial Electric and Magnetic Stimulation Dose: Definition, Selection, and Reporting Practices. Brain Stimul. 2012, 5, 435–453. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-Precise Head Model of Transcranial Direct Current Stimulation: Improved Spatial Focality Using a Ring Electrode versus Conventional Rectangular Pad. Brain Stimul. 2009, 2, 201–207. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Guan, H.; Zhang, J.; Zhang, S. Validation of Numerical Simulation for Transcranial Direct Current Stimulation with Spherical Phantom. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar] [CrossRef]
- Bikson, M.; Rahman, A.; Datta, A. Computational Models of Transcranial Direct Current Stimulation. Clin. EEG Neurosci. 2012, 43, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family—Development of Surface-Based Anatomical Models of Two Adults and Two Children for Dosimetric Simulations. Phys. Med. Biol. 2010, 55, 23. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: II. Measurements in the Frequency Range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Gabriel, C.; Peyman, A.; Grant, E.H. Electrical Conductivity of Tissue at Frequencies below 1 MHz. Phys. Med. Biol. 2009, 54, 4863. [Google Scholar] [CrossRef]
- Datta, A.; Krause, M.R.; Pilly, P.K.; Choe, J.; Zanos, T.P.; Thomas, C.; Pack, C.C. On Comparing in Vivo Intracranial Recordings in Non-Human Primates to Predictions of Optimized Transcranial Electrical Stimulation. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 1774–1777. [Google Scholar] [CrossRef]
- Parazzini, M.; Fiocchi, S.; Liorni, I.; Rossi, E.; Cogiamanian, F.; Vergari, M.; Priori, A.; Ravazzani, P. Modeling the Current Density Generated by Transcutaneous Spinal Direct Current Stimulation (tsDCS). Clin. Neurophysiol. 2014, 125, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Morales-Quezada, L.; El-Hagrassy, M.M.; Costa, B.; McKinley, R.A.; Lv, P.; Fregni, F. Transcranial Direct Current Stimulation Optimization—From Physics-Based Computer Simulations to High-Fidelity Head Phantom Fabrication and Measurements. Front. Hum. Neurosci. 2019, 13, 388. [Google Scholar] [CrossRef]
- Puonti, O.; Saturnino, G.B.; Madsen, K.H.; Thielscher, A. Value and Limitations of Intracranial Recordings for Validating Electric Field Modeling for Transcranial Brain Stimulation. NeuroImage 2020, 208, 116431. [Google Scholar] [CrossRef]
- Saturnino, G.B.; Antunes, A.; Thielscher, A. On the Importance of Electrode Parameters for Shaping Electric Field Patterns Generated by tDCS. NeuroImage 2015, 120, 25–35. [Google Scholar] [CrossRef]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-Subject Variability in Electric Fields of Motor Cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef]
- Chen, X.-L.; Benkler, S.; Chavannes, N.; De Santis, V.; Bakker, J.; van Rhoon, G.; Mosig, J.; Kuster, N. Analysis of Human Brain Exposure to Low-Frequency Magnetic Fields: A Numerical Assessment of Spatially Averaged Electric Fields and Exposure Limits. Bioelectromagnetics 2013, 34, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Arlotti, M.; Bocci, T.; Bianchi, A.M.; Parazzini, M.; Ferrucci, R.; Priori, A. Electric Fields Induced in the Brain by Transcranial Electric Stimulation: A Review of In Vivo Recordings. Biomedicines 2022, 10, 2333. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, J.H.; Kim, D.; Im, C.H. An Image-Guided Transcranial Direct Current Stimulation System: A Pilot Phantom Study. Physiol. Meas. 2013, 34, 937. [Google Scholar] [CrossRef] [PubMed]
- Hunold, A.; Strohmeier, D.; Fiedler, P.; Haueisen, J. Head Phantoms for Electroencephalography and Transcranial Electric Stimulation: A Skull Material Study. Biomed. Tech. 2018, 63, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Veniero, D.; Bortoletto, M.; Miniussi, C. TMS-EEG Co-Registration: On TMS-Induced Artifact. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2009, 120, 1392–1399. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Thomson, R.H.; Daskalakis, Z.J.; Fitzgerald, P.B. Short-Latency Artifacts Associated with Concurrent TMS-EEG. Brain Stimul. 2013, 6, 868–876. [Google Scholar] [CrossRef]
- Politte, D.; Prior, F.; Ponton, C.; Nolan, T.; Larson-Prior, L. Sources of Non-Physiologic Noise in Simultaneous EEG-fMRI Data: A Phantom Study. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August—4 September 2010; pp. 5129–5132. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A Technical Guide to tDCS, and Related Non-Invasive Brain Stimulation Tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Nardo, D.; Creasey, M.; Negus, C.; Pappa, K.; Reid, A.; Josephs, O.; Callaghan, M.F.; Crinion, J.T.; Violante, I.; Moisa, M. Transcranial Direct Current Stimulation with Functional Magnetic Resonance Imaging: A Detailed Validation and Operational Guide. Wellcome Open Res. 2021, 6, 143. [Google Scholar] [CrossRef]
- Roy, A.; Baxter, B.; He, B. High Definition Transcranial Direct Current Stimulation Induces Both Acute and Persistent Changes in Broadband Cortical Synchronization: A Simultaneous tDCS-EEG Study. IEEE Trans. Biomed. Eng. 2014, 61, 1967. [Google Scholar] [CrossRef]
- Nelson, S.O. Dielectric Properties of Agricultural Products: Measurements and Applications. IEEE Trans. Electr. Insul. 1991, 26, 845–869. [Google Scholar] [CrossRef]
- Nelson, S.O.; Trabelsi, S.; Kays, S.J. Dielectric Spectroscopy of Melons for Potential Quality Sensing. Trans. ASABE 2008, 51, 2209–2214. [Google Scholar] [CrossRef]
- Sunaga, T.; Ikehira, H.; Furukawa, S.; Shinkai, H.; Kobayashi, H.; Matsumoto, Y.; Yoshitome, E.; Obata, T.; Tanada, S.; Murata, H.; et al. Measurement of the Electrical Properties of Human Skin and the Variation among Subjects with Certain Skin Conditions. Phys. Med. Biol. 2002, 47, N11. [Google Scholar] [CrossRef]
- Youngs, I.J.; Treen, A.S.; Fixter, G.; Holden, S. Design of Solid Broadband Human Tissue Simulant Materials. IEE Proc. Sci. Meas. Technol. 2002, 149, 323–328. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, S.; Istook, C. Analysis of Human Head Shapes in the United States. Int. J. Hum. Ecol. 2006, 7, 77–83. [Google Scholar]
- Yoganandan, N.; Pintar, F.A.; Zhang, J.; Baisden, J.L. Physical Properties of the Human Head: Mass, Center of Gravity and Moment of Inertia. J. Biomech. 2009, 42, 1177–1192. [Google Scholar] [CrossRef]
- Report of the Committee on Methods of Clinical Examination in Electroencephalography: 1957. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [CrossRef]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the Electric Field during Transcranial Direct Current Stimulation. NeuroImage 2015, 109, 140–150. [Google Scholar] [CrossRef]
- Im, C.-H.; Jung, H.-H.; Choi, J.-D.; Lee, S.Y.; Jung, K.-Y. Determination of Optimal Electrode Positions for Transcranial Direct Current Stimulation (tDCS). Phys. Med. Biol. 2008, 53, N219. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, X.; Nelson, S.O. Permittivities of Watermelon Pulp and Juice and Correlation with Quality Indicators. Int. J. Food Prop. 2013, 16, 475–484. [Google Scholar] [CrossRef]
- Noetscher, G.M.; Yanamadala, J.; Makarov, S.N.; Pascual-Leone, A. Comparison of Cephalic and Extracephalic Montages for Transcranial Direct Current Stimulation-a Numerical Study. IEEE Trans. Biomed. Eng. 2014, 61, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.C.; Lomarev, M.; Hallett, M. Modeling the Current Distribution during Transcranial Direct Current Stimulation. Clin. Neurophysiol. 2006, 117, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Dokos, S.; Ho, K.A.; Loo, C. A Computational Modelling Study of Transcranial Direct Current Stimulation Montages Used in Depression. NeuroImage 2014, 87, 332–344. [Google Scholar] [CrossRef]
- Parazzini, M.; Rossi, E.; Rossi, L.; Priori, A.; Ravazzani, P.; Cogiamanian, F.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Evaluation of the Current Density in the Brainstem during Transcranial Direct Current Stimulation with Extra-Cephalic Reference Electrode. Clin. Neurophysiol. 2013, 124, 1039–1040. [Google Scholar] [CrossRef]
- Mendonca, M.E.; Santana, M.B.; Baptista, A.F.; Datta, A.; Bikson, M.; Fregni, F.; Araujo, C.P. Transcranial DC Stimulation in Fibromyalgia: Optimized Cortical Target Supported by High-Resolution Computational Models. J. Pain 2011, 12, 610–617. [Google Scholar] [CrossRef]
- Im, C.-H.; Park, J.-H.; Shim, M.; Chang, W.H.; Kim, Y.-H. Evaluation of Local Electric Fields Generated by Transcranial Direct Current Stimulation with an Extracephalic Reference Electrode Based on Realistic 3D Body Modeling. Phys. Med. Biol. 2012, 57, 2137. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, M.; Fiocchi, S.; Liorni, I.; Ravazzani, P. Effect of the Interindividual Variability on Computational Modeling of Transcranial Direct Current Stimulation. Comput. Intell. Neurosci. 2015, 2015, 963293. [Google Scholar] [CrossRef]
- Fiocchi, S.; Ravazzani, P.; Priori, A.; Parazzini, M. Cerebellar and Spinal Direct Current Stimulation in Children: Computational Modeling of the Induced Electric Field. Front. Hum. Neurosci. 2016, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Truong, D.; Minhas, P.; Parra, L.C.; Bikson, M. Inter-Individual Variation During Transcranial Direct Current Stimulation and Normalization of Dose Using MRI-Derived Computational Models. Front. Psychiatry 2012, 3, 91. [Google Scholar] [CrossRef]
- Truong, D.Q.; Magerowski, G.; Blackburn, G.L.; Bikson, M.; Alonso-Alonso, M. Computational Modeling of Transcranial Direct Current Stimulation (tDCS) in Obesity: Impact of Head Fat and Dose Guidelines. NeuroImage Clin. 2013, 2, 759–766. [Google Scholar] [CrossRef]
- Parazzini, M.; Rossi, E.; Ferrucci, R.; Liorni, I.; Priori, A.; Ravazzani, P. Modelling the Electric Field and the Current Density Generated by Cerebellar Transcranial DC Stimulation in Humans. Clin. Neurophysiol. 2014, 125, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ciechanski, P.; Carlson, H.L.; Yu, S.S.; Kirton, A. Modeling Transcranial Direct-Current Stimulation-Induced Electric Fields in Children and Adults. Front Hum Neurosci 2018, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and Models of Electric Fields in the in Vivo Human Brain during Transcranial Electric Stimulation. eLife 2017, 6, e18834. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Manshaii, F.; Xiao, X.; Yin, J.; Chen, J. Triboelectric Nanogenerators for Self-Powered Neurostimulation. Nano Res. 2024, 17, 8926–8941. [Google Scholar] [CrossRef]



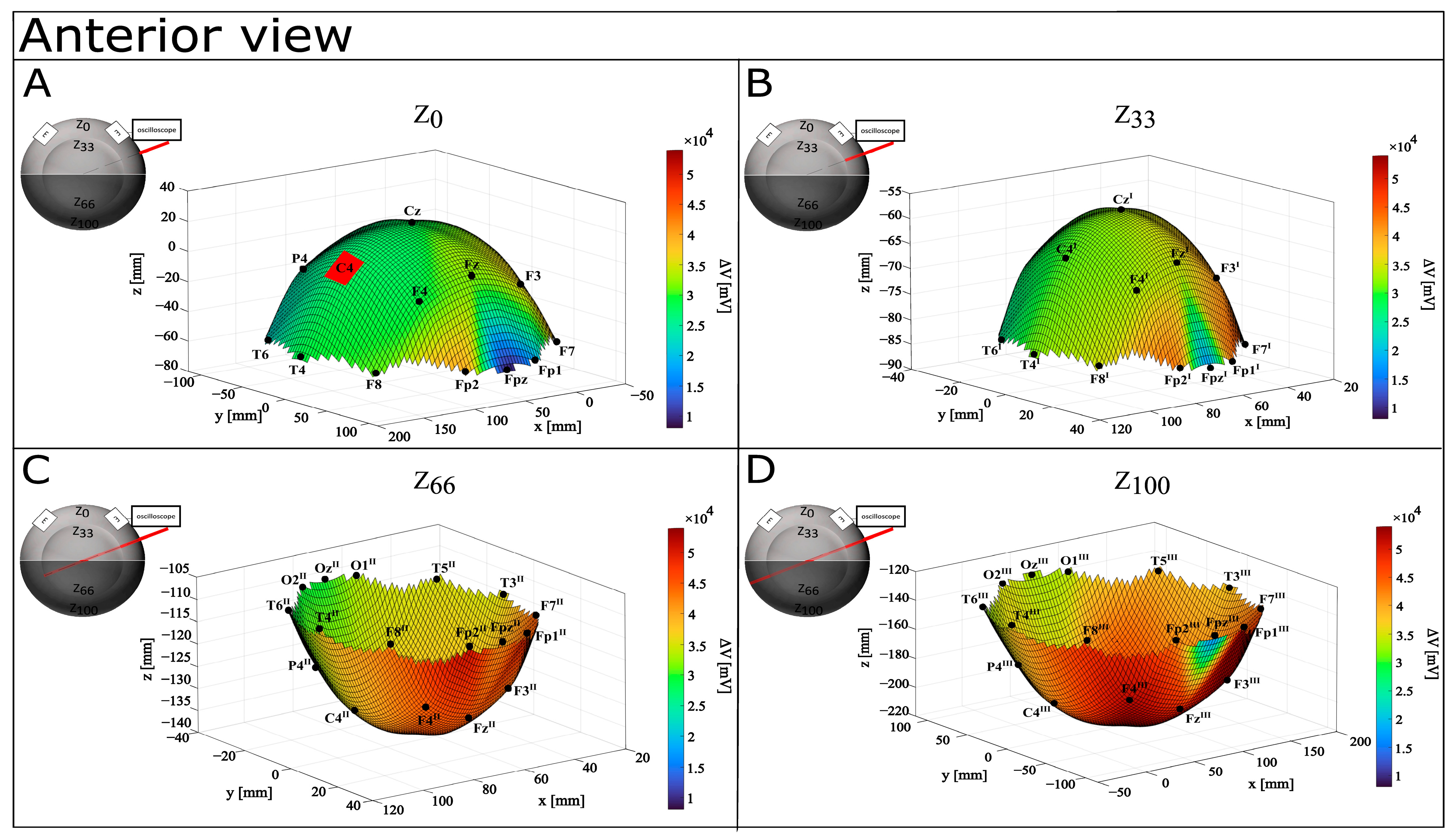


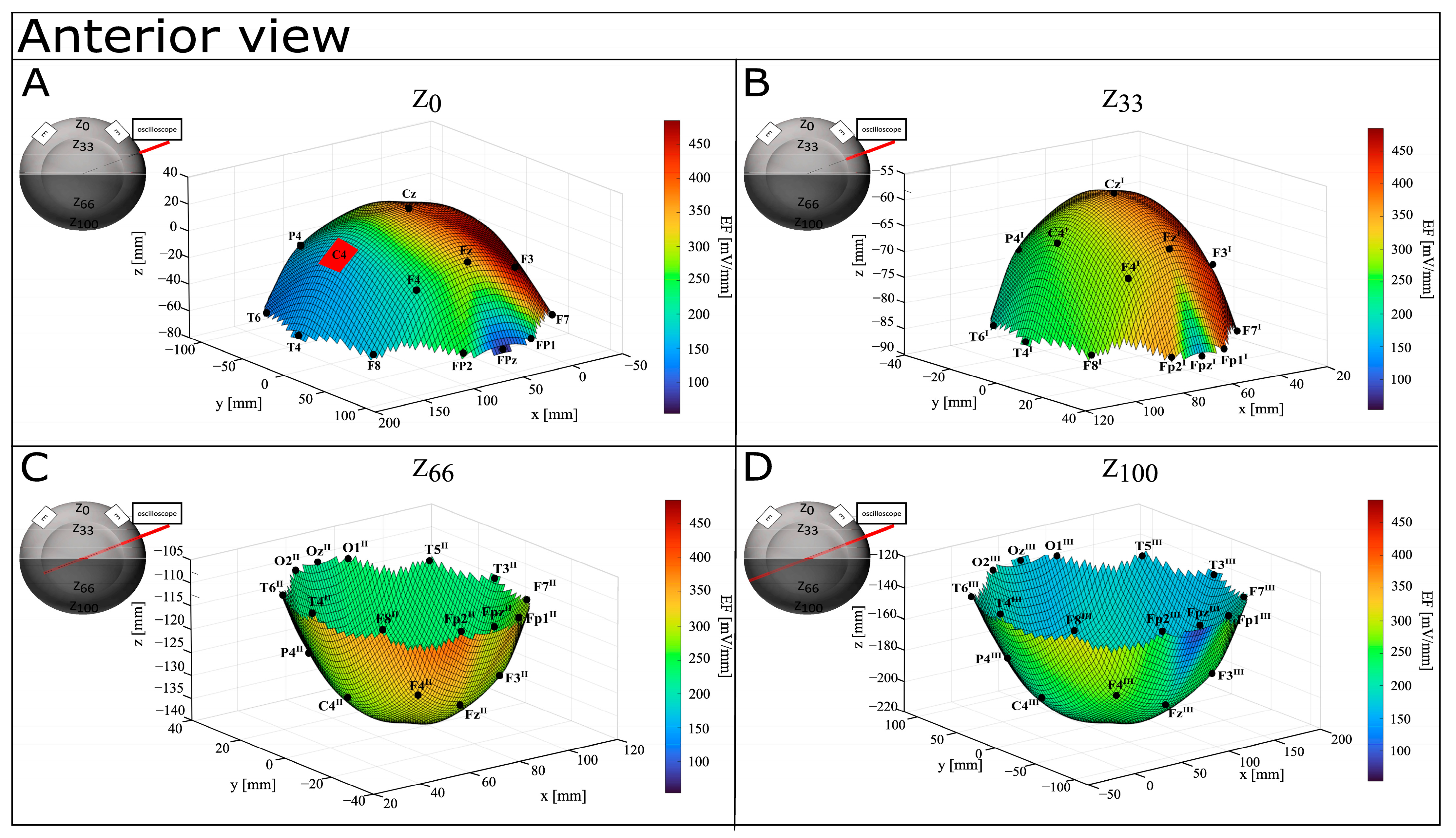


| Electrode Placement on Surface | Depth Z | X (mm) | Y (mm) | Z (mm) | C3E | ΔV (mV) | EF (mV/mm) |
|---|---|---|---|---|---|---|---|
| Fp1 | Z0 | 35.38 | 108.90 | −60.20 | 129.37 | 15,800.00 | 122.13 |
| Z33 | 58.97 | 36.30 | −85.00 | 109.64 | 41,200.00 | 375.77 | |
| Z66 | 58.97 | −36.30 | −109.81 | 129.82 | 48,400.00 | 372.82 | |
| Z100 | 35.38 | −108.90 | −134.61 | 176.73 | 53,200.00 | 301.03 | |
| Fpz | Z0 | 70.77 | 114.51 | −60.20 | 147.46 | 8080.00 | 54.79 |
| Z33 | 70.77 | 38.17 | −85.00 | 117.01 | 15,600.00 | 133.32 | |
| Z66 | 70.77 | −38.17 | −109.81 | 136.10 | 41,600.00 | 305.66 | |
| Z100 | 70.77 | −114.51 | −134.61 | 190.37 | 16,600.00 | 87.20 | |
| Fp2 | Z0 | 106.15 | 108.90 | −60.20 | 163.56 | 39,600.00 | 242.11 |
| Z33 | 82.56 | 36.30 | −85.00 | 123.94 | 42,800.00 | 345.34 | |
| Z66 | 82.56 | −36.30 | −109.81 | 142.10 | 50,400.00 | 354.68 | |
| Z100 | 106.15 | −108.90 | −134.61 | 203.10 | 42,400.00 | 208.77 | |
| F7 | Z0 | −21.87 | 67.31 | −60.20 | 92.91 | 39,200.00 | 421.91 |
| Z33 | 39.89 | 22.44 | −85.00 | 96.54 | 40,800.00 | 422.62 | |
| Z66 | 39.89 | −22.44 | −109.81 | 118.96 | 44,000.00 | 369.86 | |
| Z100 | −21.87 | −67.31 | −134.61 | 152.08 | 42,400.00 | 278.80 | |
| F3 | Z0 | 13.52 | 78.80 | −26.64 | 84.27 | 35,600.00 | 484.73 |
| Z33 | 51.68 | 26.27 | −73.82 | 93.86 | 39,600.00 | 436.23 | |
| Z66 | 51.68 | −26.27 | −121.00 | 134.17 | 40,400.00 | 291.43 | |
| Z100 | 13.52 | −78.80 | −168.17 | 186.21 | 54,000.00 | 275.85 | |
| F7 | Z0 | 70.77 | 70.77 | 0.00 | 100.08 | 32,000.00 | 319.73 |
| Z33 | 70.77 | 23.59 | −64.94 | 98.90 | 34,400.00 | 347.82 | |
| Z66 | 70.77 | −23.59 | −129.87 | 149.77 | 44,000.00 | 293.78 | |
| Z100 | 70.77 | −70.77 | −194.81 | 219.02 | 49,600.00 | 226.47 | |
| F4 | Z0 | 128.02 | 78.80 | −26.64 | 152.67 | 29,200.00 | 208.24 |
| Z33 | 89.85 | 26.27 | −73.82 | 119.22 | 33,600.00 | 294.73 | |
| Z66 | 89.85 | −26.27 | −121.00 | 152.98 | 44,400.00 | 286.76 | |
| Z100 | 128.02 | −78.80 | −168.17 | 225.57 | 51,600.00 | 225.01 | |
| F8 | Z0 | 163.41 | 67.31 | −60.20 | 186.70 | 31,600.00 | 169.26 |
| Z33 | 101.65 | 22.44 | −85.00 | 134.39 | 35,600.00 | 264.90 | |
| Z66 | 101.65 | −22.44 | −109.81 | 151.31 | 42,800.00 | 282.87 | |
| Z100 | 163.41 | −67.31 | −134.61 | 222.15 | 47,600.00 | 214.27 | |
| T3 | Z0 | −43.74 | 0.00 | −60.20 | 74.41 | 30,800.00 | 413.92 |
| Z33 | 32.60 | 0.00 | −85.00 | 91.04 | 32,000.00 | 351.49 | |
| Z66 | 32.60 | −0.00 | −109.81 | 114.54 | 35,200.00 | 307.30 | |
| Z100 | −43.74 | −0.00 | −134.61 | 141.54 | 36,800.00 | 260.00 | |
| C3 | Z0 | −0.00 | 0.00 | 0.00 | 0.00 | N.R. * | 404.08 |
| Z33 | 47.18 | 0.00 | −64.94 | 80.27 | N.R. * | 436.54 | |
| Z66 | 47.18 | −0.00 | −129.87 | 138.18 | N.R. * | 275.59 | |
| Z100 | −0.00 | −0.00 | −194.81 | 194.81 | N.R. * | 219.70 | |
| Cz | Z0 | 70.77 | 0.00 | 22.99 | 74.41 | 28,000.00 | 376.29 |
| Z33 | 70.77 | 0.00 | −57.27 | 91.04 | 33,200.00 | 364.67 | |
| Z66 | 70.77 | 0.00 | −137.54 | 154.68 | 43,600.00 | 281.88 | |
| Z100 | 70.77 | 0.00 | −217.81 | 229.01 | 52,000.00 | 227.06 | |
| C4 | Z0 | 141.54 | 0.00 | 0.00 | 141.54 | N.R. * | 0.00 |
| Z33 | 94.36 | 0.00 | −64.94 | 114.54 | N.R. * | 267.49 | |
| Z66 | 94.36 | 0.00 | −129.87 | 160.53 | N.R. * | 229.73 | |
| Z100 | 141.54 | 0.00 | −194.81 | 240.80 | N.R. * | 170.76 | |
| T4 | Z0 | 185.28 | 0.00 | −60.20 | 194.81 | 29,200.00 | 149.89 |
| Z33 | 108.94 | 0.00 | −85.00 | 138.18 | 32,000.00 | 231.59 | |
| Z66 | 108.94 | 0.00 | −109.81 | 154.68 | 36,800.00 | 237.91 | |
| Z100 | 185.28 | 0.00 | −134.61 | 229.01 | 38,000.00 | 165.93 | |
| T5 | Z0 | −21.87 | −67.31 | −60.20 | 92.91 | 29,600.00 | 318.59 |
| Z33 | 39.89 | −22.44 | −85.00 | 96.54 | 32,800.00 | 339.75 | |
| Z66 | 39.89 | 22.44 | −109.81 | 118.96 | 34,800.00 | 292.53 | |
| Z100 | −21.87 | 67.31 | −134.61 | 152.08 | 37,600.00 | 247.24 | |
| P3 | Z0 | 13.52 | −78.80 | −26.64 | 84.27 | 28,000.00 | 381.25 |
| Z33 | 51.68 | −26.27 | −73.82 | 93.86 | 30,000.00 | 330.48 | |
| Z66 | 51.68 | 26.27 | −121.00 | 134.17 | 36,000.00 | 259.69 | |
| Z100 | 13.52 | 78.80 | −168.17 | 186.21 | 43,200.00 | 220.68 | |
| Pz | Z0 | 70.77 | −70.77 | 0.00 | 100.08 | 24,400.00 | 243.80 |
| Z33 | 70.77 | −23.59 | −64.94 | 98.90 | 27,600.00 | 279.06 | |
| Z66 | 70.77 | 23.59 | −129.87 | 149.77 | 34,400.00 | 229.68 | |
| Z100 | 70.77 | 70.77 | −194.81 | 219.02 | 36,700.00 | 167.57 | |
| P4 | Z0 | 128.02 | −78.80 | −26.64 | 152.67 | 24,000.00 | 171.16 |
| Z33 | 89.85 | −26.27 | −73.82 | 119.22 | 27,200.00 | 238.59 | |
| Z66 | 89.85 | 26.27 | −121.00 | 152.98 | 32,400.00 | 209.26 | |
| Z100 | 128.02 | 78.80 | −168.17 | 225.57 | 36,800.00 | 160.47 | |
| T6 | Z0 | 163.41 | −67.31 | −60.20 | 186.70 | 21,600.00 | 115.69 |
| Z33 | 101.65 | −22.44 | −85.00 | 134.39 | 24,800.00 | 184.53 | |
| Z66 | 101.65 | 22.44 | −109.81 | 151.31 | 28,000.00 | 185.06 | |
| Z100 | 163.41 | 67.31 | −134.61 | 222.15 | 31,600.00 | 142.24 | |
| O1 | Z0 | 35.38 | −108.90 | −60.20 | 129.37 | 26,400.00 | 204.07 |
| Z33 | 58.97 | −36.30 | −85.00 | 109.64 | 28,800.00 | 262.67 | |
| Z66 | 58.97 | 36.30 | −109.81 | 129.82 | 33,200.00 | 255.74 | |
| Z100 | 35.38 | 108.90 | −134.61 | 176.73 | 33,200.00 | 187.86 | |
| Oz | Z0 | 70.77 | −114.51 | −60.20 | 147.46 | 22,800.00 | 154.62 |
| Z33 | 70.77 | −38.17 | −85.00 | 117.01 | 25,200.00 | 215.37 | |
| Z66 | 70.77 | 38.17 | −109.81 | 136.10 | 29,200.00 | 214.55 | |
| Z100 | 70.77 | 114.51 | −134.61 | 190.37 | 33,200.00 | 174.40 | |
| O2 | Z0 | 106.15 | −108.90 | −60.20 | 163.56 | 22,000.00 | 134.51 |
| Z33 | 82.56 | −36.30 | −85.00 | 123.94 | 24,400.00 | 196.88 | |
| Z66 | 82.56 | 36.30 | −109.81 | 142.10 | 28,800.00 | 202.68 | |
| Z100 | 106.15 | 108.90 | −134.61 | 203.10 | 34,800.00 | 171.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidetti, M.; Ferrara, R.; Montemagno, K.; Maiorana, N.V.; Bocci, T.; Marceglia, S.; Oliveri, S.; Bianchi, A.M.; Priori, A. The “Cocombola Study”: A Physical Phantom Model for tDCS-Induced Electric Field Distribution. Bioengineering 2025, 12, 346. https://doi.org/10.3390/bioengineering12040346
Guidetti M, Ferrara R, Montemagno K, Maiorana NV, Bocci T, Marceglia S, Oliveri S, Bianchi AM, Priori A. The “Cocombola Study”: A Physical Phantom Model for tDCS-Induced Electric Field Distribution. Bioengineering. 2025; 12(4):346. https://doi.org/10.3390/bioengineering12040346
Chicago/Turabian StyleGuidetti, Matteo, Rosanna Ferrara, Kora Montemagno, Natale Vincenzo Maiorana, Tommaso Bocci, Sara Marceglia, Serena Oliveri, Anna Maria Bianchi, and Alberto Priori. 2025. "The “Cocombola Study”: A Physical Phantom Model for tDCS-Induced Electric Field Distribution" Bioengineering 12, no. 4: 346. https://doi.org/10.3390/bioengineering12040346
APA StyleGuidetti, M., Ferrara, R., Montemagno, K., Maiorana, N. V., Bocci, T., Marceglia, S., Oliveri, S., Bianchi, A. M., & Priori, A. (2025). The “Cocombola Study”: A Physical Phantom Model for tDCS-Induced Electric Field Distribution. Bioengineering, 12(4), 346. https://doi.org/10.3390/bioengineering12040346








