Surgical Robots Improve Tunnel Angle and Graft Bending Angle in Anatomic ACL Reconstruction: A Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approvals
2.2. Study Population
2.3. Group Allocation
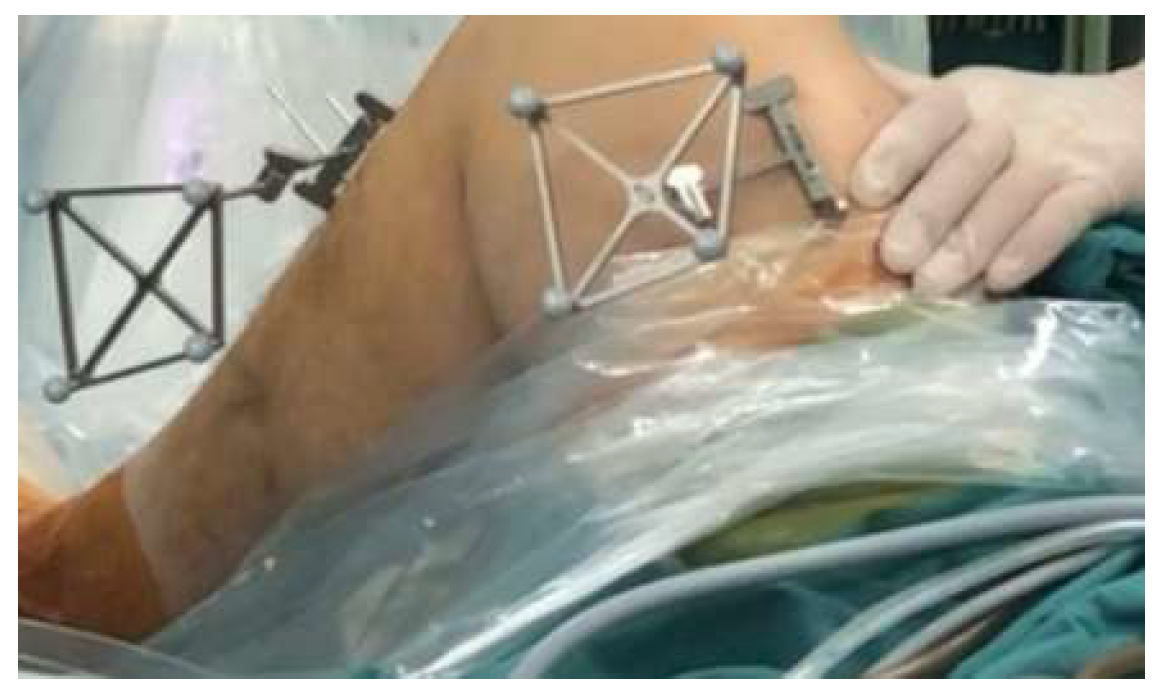
2.4. Surgical Robot Procedures
2.5. Traditional Arthroscopic Procedures
2.6. Postoperative Measurement
2.7. Statistical Analysis and Sample Size Calculation
3. Results
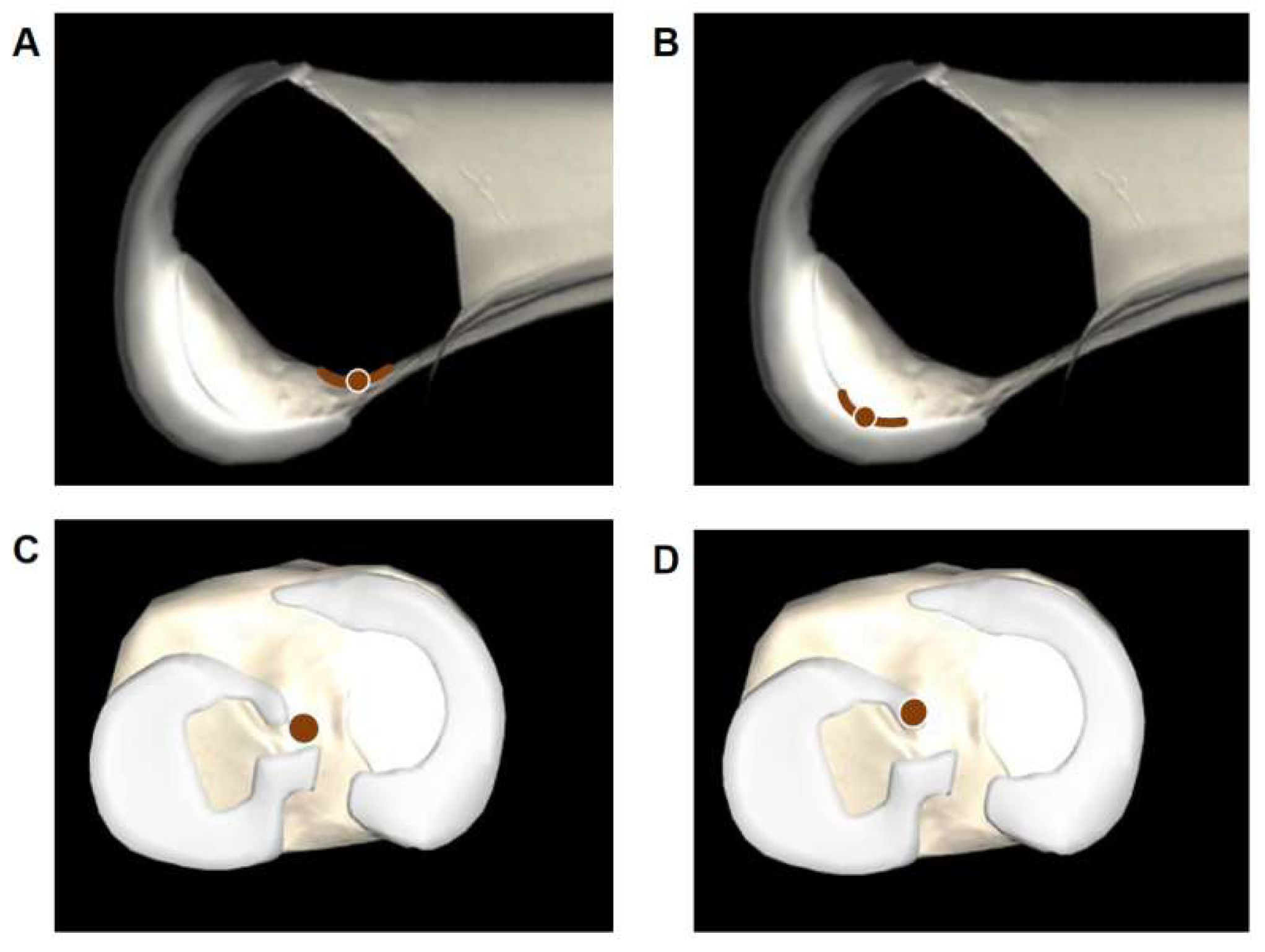
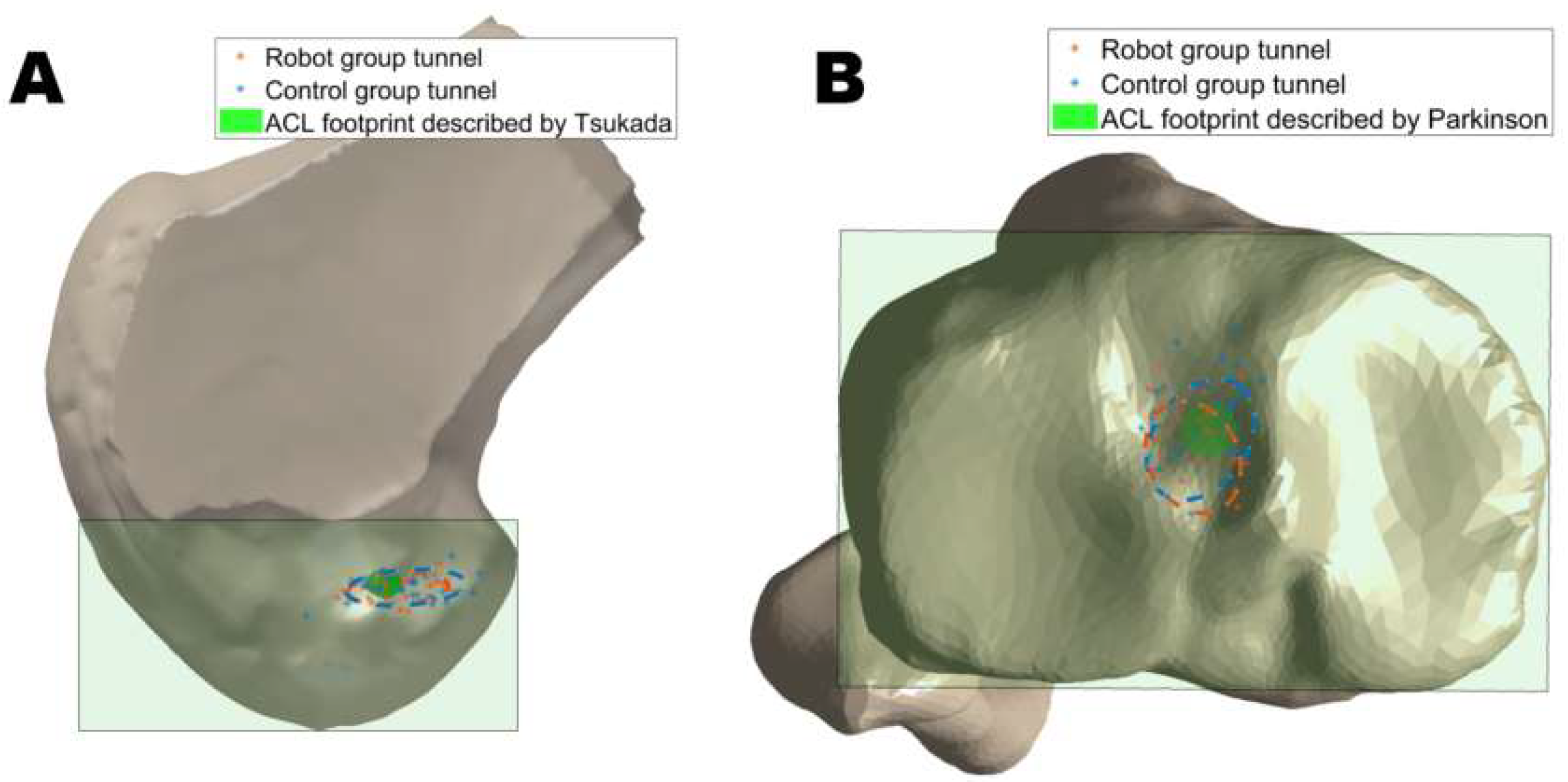
3.1. Tunnel Position
3.2. Tunnel Length
3.3. Tunnel and Graft Obliquity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Ambrosi, R.; Meena, A.; Arora, E.S.; Attri, M.; Schäfer, L.; Migliorini, F. Reconstruction of the anterior cruciate ligament: A historical view. Ann. Transl. Med. 2023, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Engler, I.D.; Zsidai, B.T.; Hughes, J.D.; Musahl, V. Anatomic anterior cruciate ligament reconstruction: Freddie Fu’s paradigm. J. ISAKOS 2023, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, S.; Balke, M.; Hagn, U.; Grote, S.; Bouillon, B.; Banerjee, M. Variability of Landmark Acquisition Affects Tunnel Calculation in Image-Free ACL Navigation. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1917–1924. [Google Scholar] [CrossRef]
- Wilson, W.T.; Hopper, G.P.; O’Boyle, M.; Henderson, L.; Blyth, M.J.G. Quantifying graft impingement in anterior cruciate ligament reconstruction. Knee 2022, 34, 270–278. [Google Scholar] [CrossRef]
- Kemler, B.; Coladonato, C.; Sonnier, J.H.; Campbell, M.; Darius, D.; Erickson, B.J.; Tjoumakaris, F.; Freedman, K.B. Evaluation of failed ACL reconstruction: An updated review. Open Access J. Sports Med. 2024, 15, 29–39. [Google Scholar] [CrossRef]
- Wolf, B.R.; Ramme, A.J.; Wright, R.W.; Brophy, R.H.; McCarty, E.C.; Vidal, A.R.; Parker, R.D.; Andrish, J.T.; Amendola, A.; MOON Knee Group; et al. Variability in ACL tunnel placement: Observational clinical study of surgeon ACL tunnel variability. Am. J. Sports Med. 2013, 41, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- McConkey, M.O.; Amendola, A.; Ramme, A.J.; Dunn, W.R.; Flanigan, D.C.; Britton, C.L.; MOON Knee Group; Wolf, B.R.; Spindler, K.P.; Carey, J.L.; et al. Arthroscopic agreement among surgeons on anterior cruciate ligament tunnel placement. Am. J. Sports Med. 2012, 40, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.; Chhabra, A.; Hassebrock, J.D.; Ford, E.; Amin, N.H. Risks and complications associated with intra-articular arthroscopy of the knee and shoulder in an office setting. Orthop. J. Sports Med. 2019, 7, 232596711986984. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, J.; Zhao, X.; Liu, M.; Hou, Y.; Zhang, Y.; Zhao, J.; Wang, S. Robotic-assisted anatomic anterior cruciate ligament reconstruction: A comparative analysis of modified transtibial and anteromedial portal techniques in cadaveric knees. Front. Bioeng. Biotechnol. 2024, 12, 1360560. [Google Scholar] [CrossRef]
- Yang, G.; Liu, D.; Zhou, G.; Wang, Q.; Zhang, X. Robot-assisted anterior cruciate ligament reconstruction based on three-dimensional images. J. Orthop. Surg. Res. 2024, 19, 246. [Google Scholar] [CrossRef]
- Ding, G.; Yang, G.; Zhang, J.; Huang, H.; Du, J.; Ren, S.; Wang, Q.; Zhou, Z.; Zhang, X.; Ao, Y. Feasibility and accuracy of orthopaedic surgical robot system for intraoperative navigation to locate bone tunnel in anterior cruciate ligament reconstruction. Robot. Comput. Surg. 2022, 18, e2354. [Google Scholar] [CrossRef] [PubMed]
- Inderhaug, E.; Larsen, A.; Waaler, P.A.; Strand, T.; Harlem, T.; Solheim, E. The effect of intraoperative fluoroscopy on the accuracy of femoral tunnel placement in single-bundle anatomic ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1211–1218. [Google Scholar] [CrossRef]
- Raposo, C.; Barreto, J.P.; Sousa, C.; Ribeiro, L.; Melo, R.; Oliveira, J.P.; Marques, P.; Fonseca, F.; Barrett, D. Video-based computer navigation in knee arthroscopy for patient-specific ACL Reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1529–1539. [Google Scholar] [CrossRef]
- Figueroa, F.; Figueroa, D.; Guiloff, R.; Putnis, S.; Fritsch, B.; Itriago, M. Navigation in anterior cruciate ligament reconstruction: State of the art. J. ISAKOS 2023, 8, 47–53. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Klos, T.V.; Bignozzi, S. Computer-assisted anterior cruciate ligament reconstruction: An evidence-based approach of the first 15 years. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kum, D.H.; Rhyu, I.J.; Kim, Y.; Cho, H.; Wang, J.H. Clinical advantages of image-free navigation system using surface-based registration in anatomical anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, G.; Eckerle, P.; Farrow, L.; Cutuk, A.; Bledsoe, G.; Kaar, S. Anterior cruciate ligament graft isometry is affected by the orientation of the femoral tunnel. J. Knee Surg. 2015, 29, 260–266. [Google Scholar] [CrossRef]
- Gelber, P.E.; Reina, F.; Torres, R.; Monllau, J.C. Effect of femoral tunnel length on the safety of anterior cruciate ligament graft fixation using cross-pin technique: A cadaveric study. Am. J. Sports Med. 2010, 38, 1877–1884. [Google Scholar] [CrossRef]
- Zhao, J. Anatomic double-bundle transtibial anterior cruciate ligament reconstruction. Arthrosc. Tech. 2021, 10, e683–e690. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Dean, C.S.; Chahla, J.; Menge, T.J.; Cram, T.R.; LaPrade, R.F. Posterior wall blowout in anterior cruciate ligament reconstruction: A review of anatomic and surgical considerations. Orthop. J. Sports Med. 2016, 4, 232596711665212. [Google Scholar] [CrossRef]
- Pastrone, A.; Ferro, A.; Bruzzone, M.; Bonasia, D.E.; Pellegrino, P.; D’Elicio, D.; Cottino, U.; Rossi, R. Anterior cruciate ligament reconstruction creating the femoral tunnel through the anteromedial portal. Surgical technique. Curr. Rev. Musculoskelet. Med. 2011, 4, 52–56. [Google Scholar] [CrossRef]
- Zhao, J. Anatomical single-bundle transtibial anterior cruciate ligament reconstruction. Arthrosc. Tech. 2020, 9, e1275–e1282. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Hertel, P.; Hornung, H.; Cierpinski, T. Femoral insertion of the ACL. Radiographic quadrant method. Am. J. Knee Surg. 1997, 10, 14–21. [Google Scholar] [PubMed]
- Robinson, J.; Inderhaug, E.; Harlem, T.; Spalding, T.; Brown, C.H. Anterior cruciate ligament femoral tunnel placement: An analysis of the intended versus achieved position for 221 international high-volume ACL surgeons. Am. J. Sports Med. 2020, 48, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Ishibashi, Y.; Tsuda, E.; Fukuda, A.; Toh, S. Anatomical analysis of the anterior cruciate ligament femoral and tibial footprints. J. Orthop. Sci. 2008, 13, 122–129. [Google Scholar] [CrossRef]
- Parkinson, B.; Gogna, R.; Robb, C.; Thompson, P.; Spalding, T. Anatomic ACL reconstruction: The normal central tibial footprint position and a standardised technique for measuring tibial tunnel location on 3D CT. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1568–1575. [Google Scholar] [CrossRef]
- Laux, C.J.; Ulbrich, E.J.; Andreisek, G.; Marcon, M.; Fischer, M.A.; Mehra, T.; Ciritsis, B.D. Impact of graft and tunnel orientation on patient-reported outcome in anterior cruciate ligament reconstruction using bone-patellar tendon-bone autografts. J. Orthop Surg. Res. 2018, 13, 245. [Google Scholar] [CrossRef]
- Haroun, H.K.; Abouelsoud, M.M.; Allam, M.R.; Abdelwahab, M.M. Transtibial versus independent femoral tunnel drilling techniques for anterior cruciate ligament reconstruction: Evaluation of femoral aperture positioning. J. Orthop. Surg. Res. 2022, 17, 166. [Google Scholar] [CrossRef]
- Weekes, D.; Campbell, R.E.; Shi, W.J.; Ciccotti, M.; Salvo, J.; Cohen, S.; Tucker, B.; Pepe, M.; Freedman, K.; Tjoumakaris, F. Are patient and surgeon expectations after ACL reconstruction realistic? Clin. Orthop. Relat. Res. 2020, 478, 619–628. [Google Scholar] [CrossRef]
- Besmens, I.S.; Politikou, O.; Giovanoli, P.; Calcagni, M.; Lindenblatt, N. Robotic microsurgery in extremity reconstruction—experience with a novel robotic system. Surg. Innov. 2024, 31, 42–47. [Google Scholar] [CrossRef]
- Allena, R.; Rémond, Y. Theramechanics: How acting on mechanics will help conceive new medical treatments. Math. Mech. Compl. Sys. 2023, 11, 541–566. [Google Scholar] [CrossRef]
- Giorgio, I.; dell’Isola, F.; Andreaus, U.; Misra, A. An orthotropic continuum model with substructure evolution for describing bone remodeling: An interpretation of the primary mechanism behind Wolff’s law. Biomech. Model Mechanobiol. 2023, 22, 2135–2152. [Google Scholar] [CrossRef]
- Geng, Y.; Gai, P. Comparison of 2 femoral tunnel drilling techniques in anterior cruciate ligament reconstruction. A prospective randomized comparative study. BMC Musculoskelet. Disord. 2018, 19, 454. [Google Scholar] [CrossRef]
- Piasecki, D.P.; Bach, B.R.; Espinoza Orias, A.A.; Verma, N.N. Anterior cruciate ligament reconstruction: Can anatomic femoral placement be achieved with a transtibial technique? Am. J. Sports Med. 2011, 39, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Trofa, D.P.; Saltzman, B.M.; Corpus, K.T.; Connor, P.M.; Fleischli, J.E.; Piasecki, D.P. A hybrid transtibial technique combines the advantages of anteromedial portal and transtibial approaches: A prospective randomized controlled trial. Am. J. Sports Med. 2020, 48, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Song, E.-K.; Kim, S.-K.; Lim, H.-A.; Seon, J.-K. Comparisons of tunnel-graft angle and tunnel length and position between transtibial and transportal techniques in anterior cruciate ligament reconstruction. Int. Orthop. 2014, 38, 2357–2362. [Google Scholar] [CrossRef]
- Simmons, R.; Howell, S.M.; Hull, M.L. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: An in vitro study. J. Bone Jt. Surg. 2003, 85, 1018–1029. [Google Scholar] [CrossRef]
- Tachibana, Y.; Mae, T.; Shino, K.; Ohori, T.; Amano, H.; Yoshikawa, H.; Nakata, K. Femoral tunnel enlargement after anatomic anterior cruciate ligament reconstruction: Bone-patellar tendon-bone/single rectangular tunnel versus hamstring tendon/double tunnels. J. Orthop. Sci. 2018, 23, 1011–1018. [Google Scholar] [CrossRef]
- Guo, N.; Yang, B.; Ji, X.; Wang, Y.; Hu, L.; Wang, T. Intensity-based 2D-3D registration for an ACL reconstruction navigation system. Robot. Comput. Surg. 2019, 15, e2008. [Google Scholar] [CrossRef]
- Li, T.; Badre, A.; Alambeigi, F.; Tavakoli, M. Robotic systems and navigation techniques in orthopedics: A historical review. Appl. Sci. 2023, 13, 9768. [Google Scholar] [CrossRef]
- Santhamoorthy, T.; Xavier, A.A.; Krun, K.; Dubey, D.K. The influence of tunnel parameters and graft inclination angle on clinical and radiological outcome at long-term follow-up after arthroscopic anterior cruciate ligament reconstruction. Rev. Bras. Ortop. 2024, 59, e189–e198. [Google Scholar] [CrossRef]
- Reddy, K.; Gharde, P.; Tayade, H.; Patil, M.; Reddy, L.S.; Surya, D. Advancements in robotic surgery: A comprehensive overview of current utilizations and upcoming Frontiers. Cureus 2023, 15, e50415. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Saithna, A.; Cavalier, M.; Kajetanek, C.; Temponi, E.F.; Daggett, M.; Helito, C.P.; Thaunat, M. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: A prospective comparative study of 502 patients from the SANTI study group. Am. J. Sports Med. 2017, 45, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marshall, B.; Tang, X.; Linde, M.A.; Fu, F.H.; Smolinski, P. ACL graft with extra-cortical fixation rotates around the femoral tunnel aperture during knee flexion. Knee. Surg. Sports Traumatol. Arthrosc. 2022, 30, 116–123. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, W.; Zhao, Q. Computational modelling of the graft-tunnel interaction in single-bundle ACL reconstructed knee. Biomed. Tech. 2023, 68, 573–582. [Google Scholar] [CrossRef]



| Robot Group (n = 35) | Control Group (n = 35) | p-Value | |
|---|---|---|---|
| Age, year | 36.1 ± 10.2 | 33.4 ± 13.0 | 0.317 |
| BMI, kg/m2 | 24.7 ± 4.1 | 25.6 ± 4.2 | 0.572 |
| Sex, male/female | 23/12 | 24/11 | 0.391 |
| Time from injury to surgery, week | 6.8 ± 4.3 | 7.4 ± 6.3 | 0.322 |
| Meniscal tears, medial/lateral/both | 8/2/9 | 4/4/9 | 0.793 |
| Robot Group (n = 35) | Control Group (n = 35) | p-Value | |
|---|---|---|---|
| Femoral aperture | |||
| Depth, % | 27.9 ± 5.2 | 25.5 ± 9.1 | 0.315 |
| Height, % | 32.5 ± 4.7 | 30.9 ± 6.7 | 0.700 |
| Tibial aperture | |||
| Anteroposterior, % | 43.0 ± 7.8 | 39.3 ± 9.1 | 0.110 |
| Mediolateral, % | 49.4 ± 2.9 | 48.4 ± 5.0 | 0.247 |
| Femoral tunnel length, mm | 35.6 ± 5.4 | 34.4 ± 5.4 | 0.386 |
| Tibial tunnel length, mm | 34.1 ± 7.5 | 31.5 ± 6.4 | 0.173 |
| Femoral coronal angle, ° | 51.7 ± 7.2 | 46.8 ± 8.5 | 0.012 * |
| Graft bending angle, ° | 121.1 ± 11.7 | 113.4 ± 13.1 | 0.008 * |
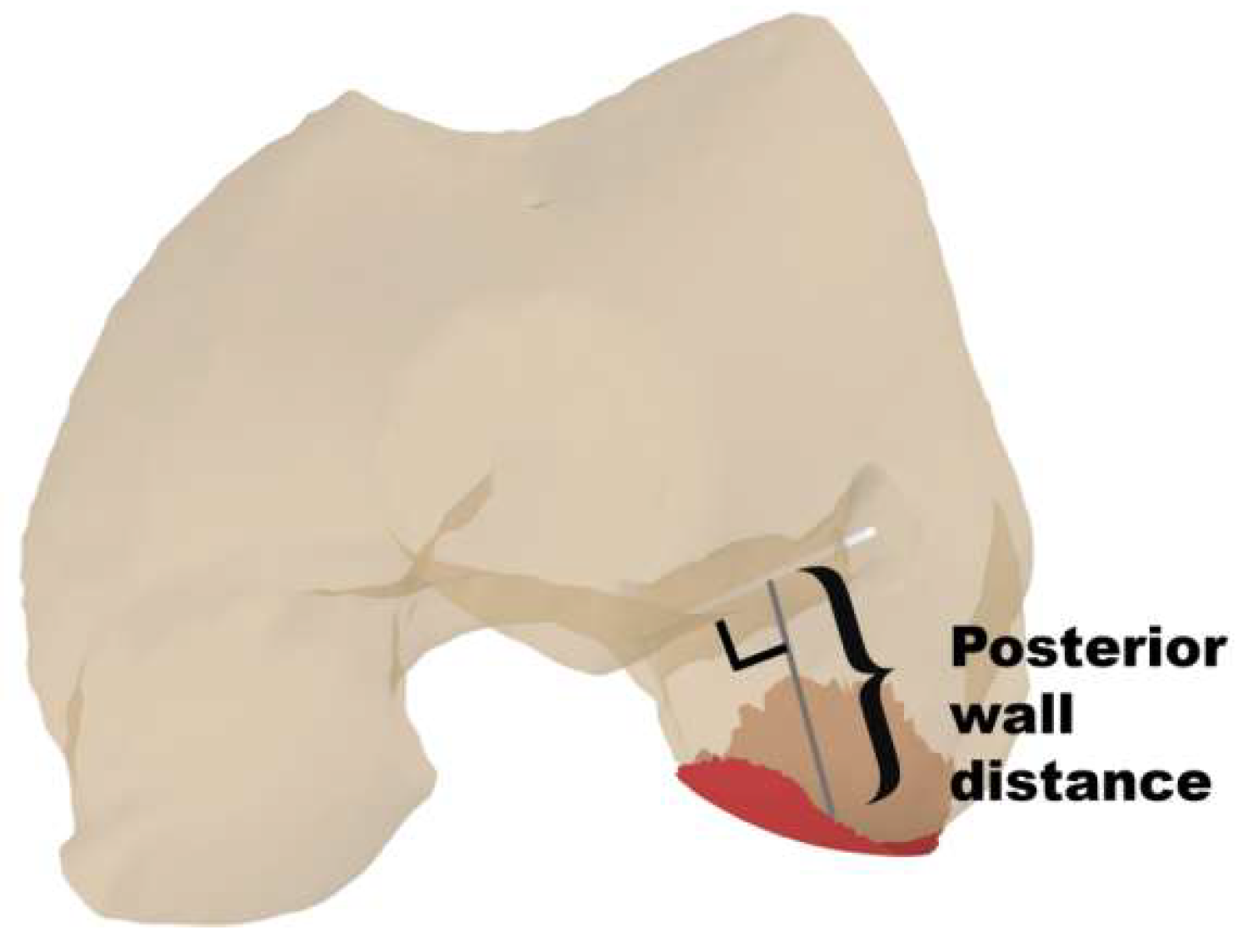
| Posterior wall distance, mm | 13.2 ± 2.9 | 10.3 ± 3.5 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Hu, H.; Huang, W.; Hu, M.; Li, Z.; Zhao, J.; Fei, W.; Wang, S. Surgical Robots Improve Tunnel Angle and Graft Bending Angle in Anatomic ACL Reconstruction: A Multicenter Study. Bioengineering 2025, 12, 338. https://doi.org/10.3390/bioengineering12040338
Zhang L, Hu H, Huang W, Hu M, Li Z, Zhao J, Fei W, Wang S. Surgical Robots Improve Tunnel Angle and Graft Bending Angle in Anatomic ACL Reconstruction: A Multicenter Study. Bioengineering. 2025; 12(4):338. https://doi.org/10.3390/bioengineering12040338
Chicago/Turabian StyleZhang, Ling, Hansheng Hu, Wennuo Huang, Mengling Hu, Zhuman Li, Jinzhong Zhao, Wenyong Fei, and Shaobai Wang. 2025. "Surgical Robots Improve Tunnel Angle and Graft Bending Angle in Anatomic ACL Reconstruction: A Multicenter Study" Bioengineering 12, no. 4: 338. https://doi.org/10.3390/bioengineering12040338
APA StyleZhang, L., Hu, H., Huang, W., Hu, M., Li, Z., Zhao, J., Fei, W., & Wang, S. (2025). Surgical Robots Improve Tunnel Angle and Graft Bending Angle in Anatomic ACL Reconstruction: A Multicenter Study. Bioengineering, 12(4), 338. https://doi.org/10.3390/bioengineering12040338







