Increased VA-ECMO Pump Speed Reduces Left Atrial Pressure: Insights from a Novel Biventricular Heart Model
Abstract
1. Background and Aims
2. Methods
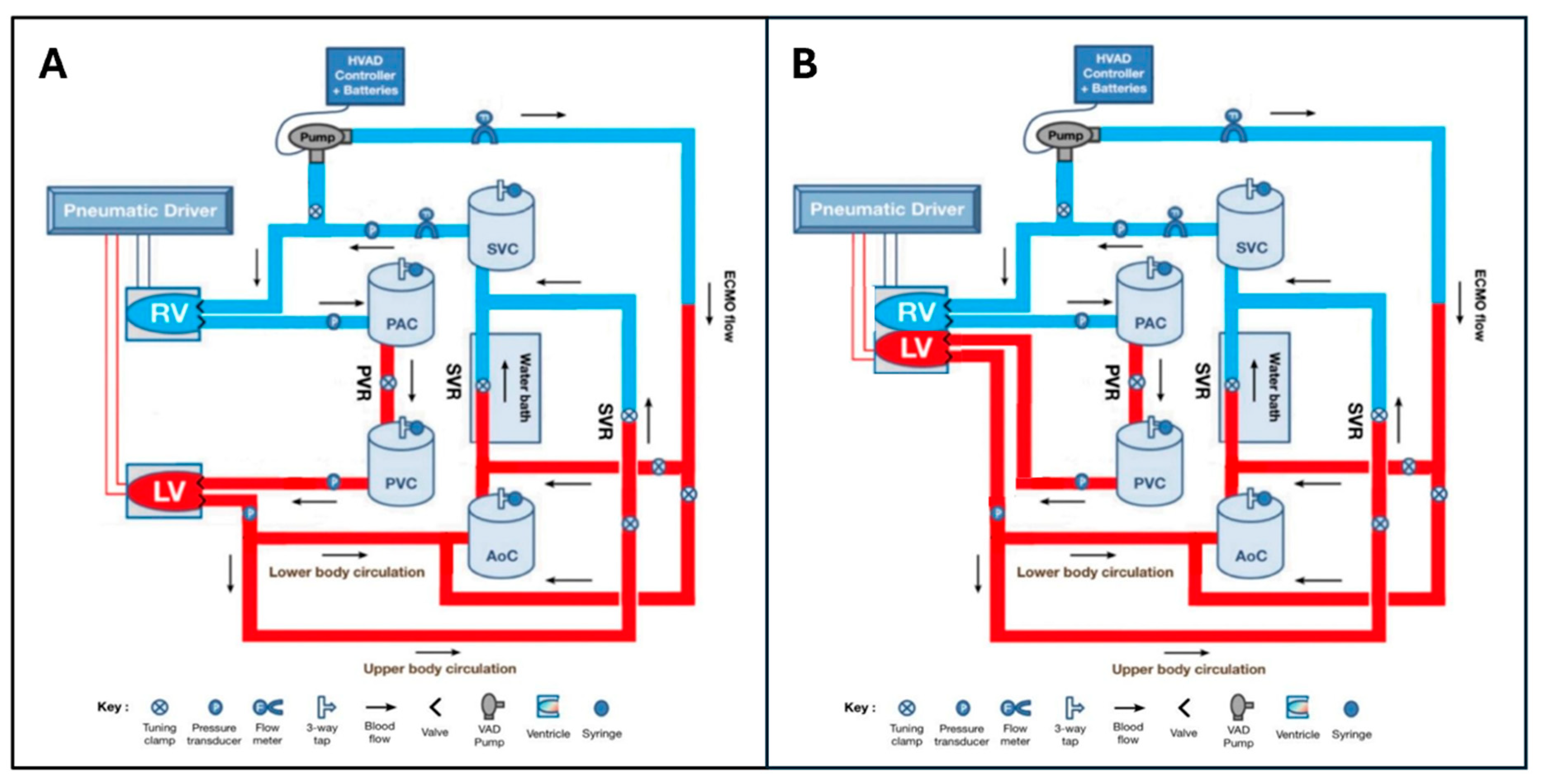
2.1. Mock Circulatory Loop Design
2.1.1. Separate Ventricle Model
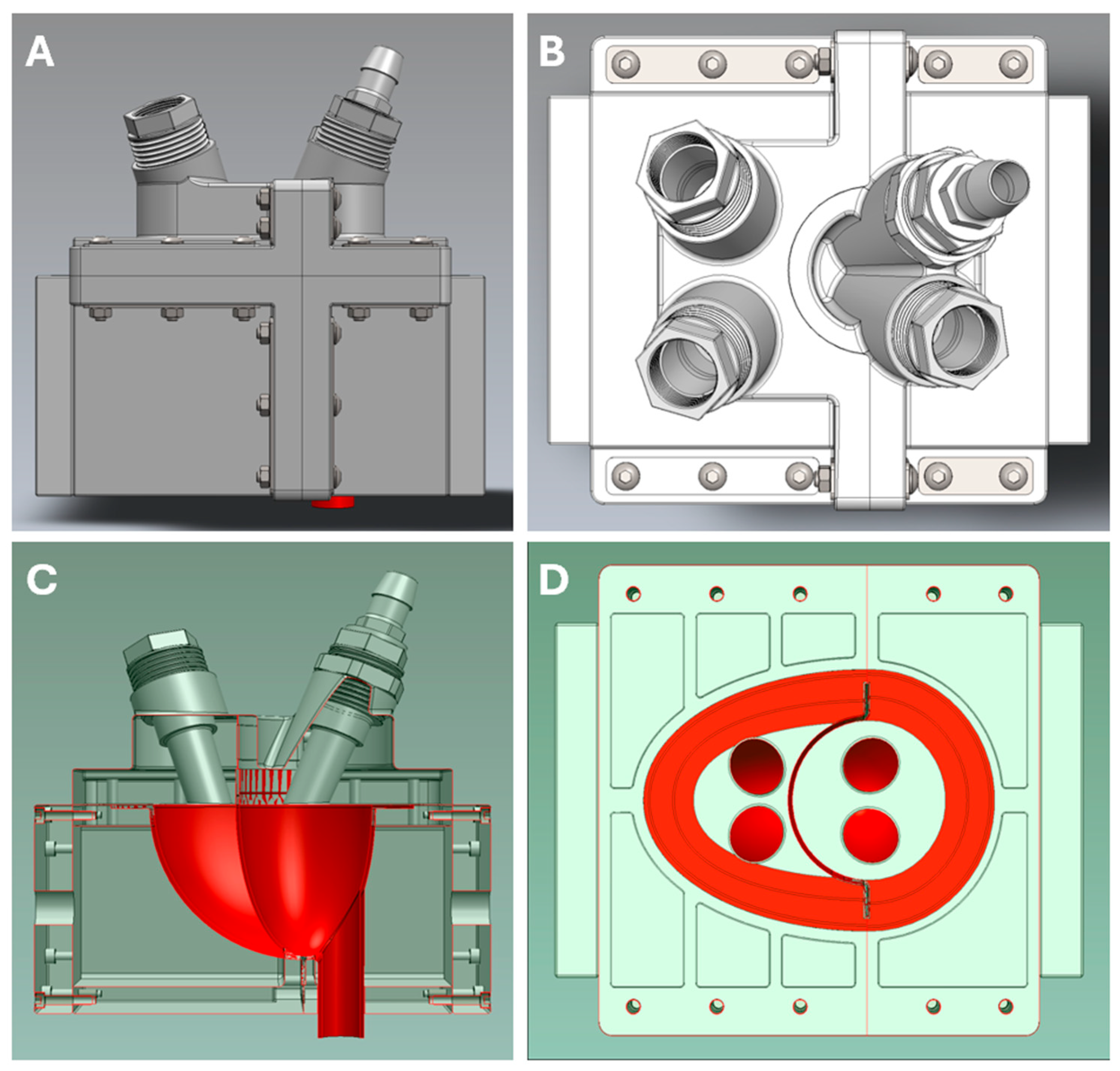
2.1.2. Biventricular Model
2.1.3. Frank–Starling Mechanism
2.1.4. Circulatory/Vascular Components
2.1.5. ECMO Components
2.2. Experimental Protocol
2.2.1. Validation of Biventricular Heart Model
- Ventricular interactions
- Frank–Starling mechanism
- Normal and pathological cardiac states
2.2.2. Effect of VA-ECMO on Left Atrial Pressure in Biventricular MCL
2.3. Data Acquisition and Analysis
3. Results
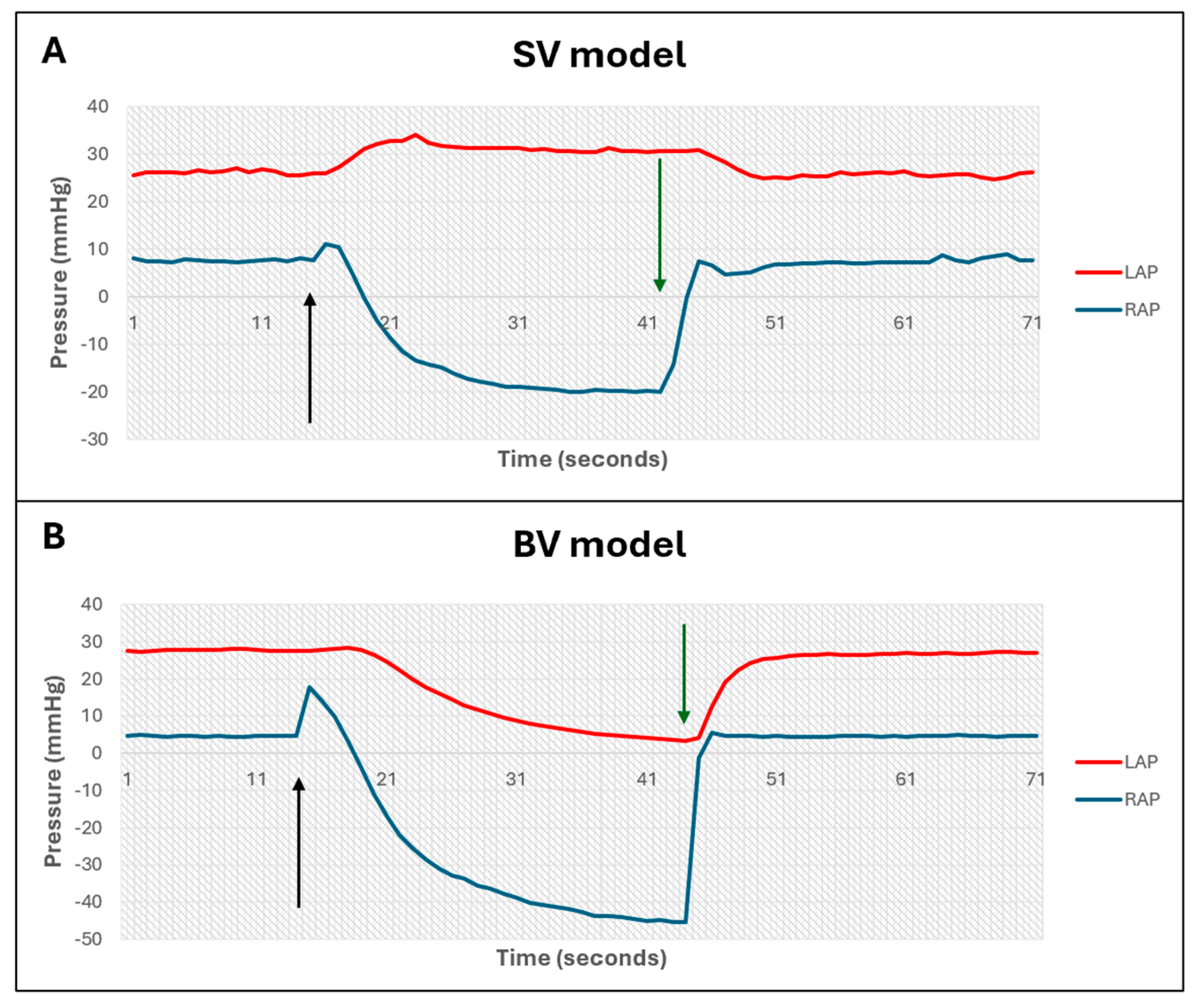
3.1. Validation of Biventricular MCL
- Ventricular interdependence
- Frank–Starling mechanism
- Reproduction of normal and diseased cardiac states
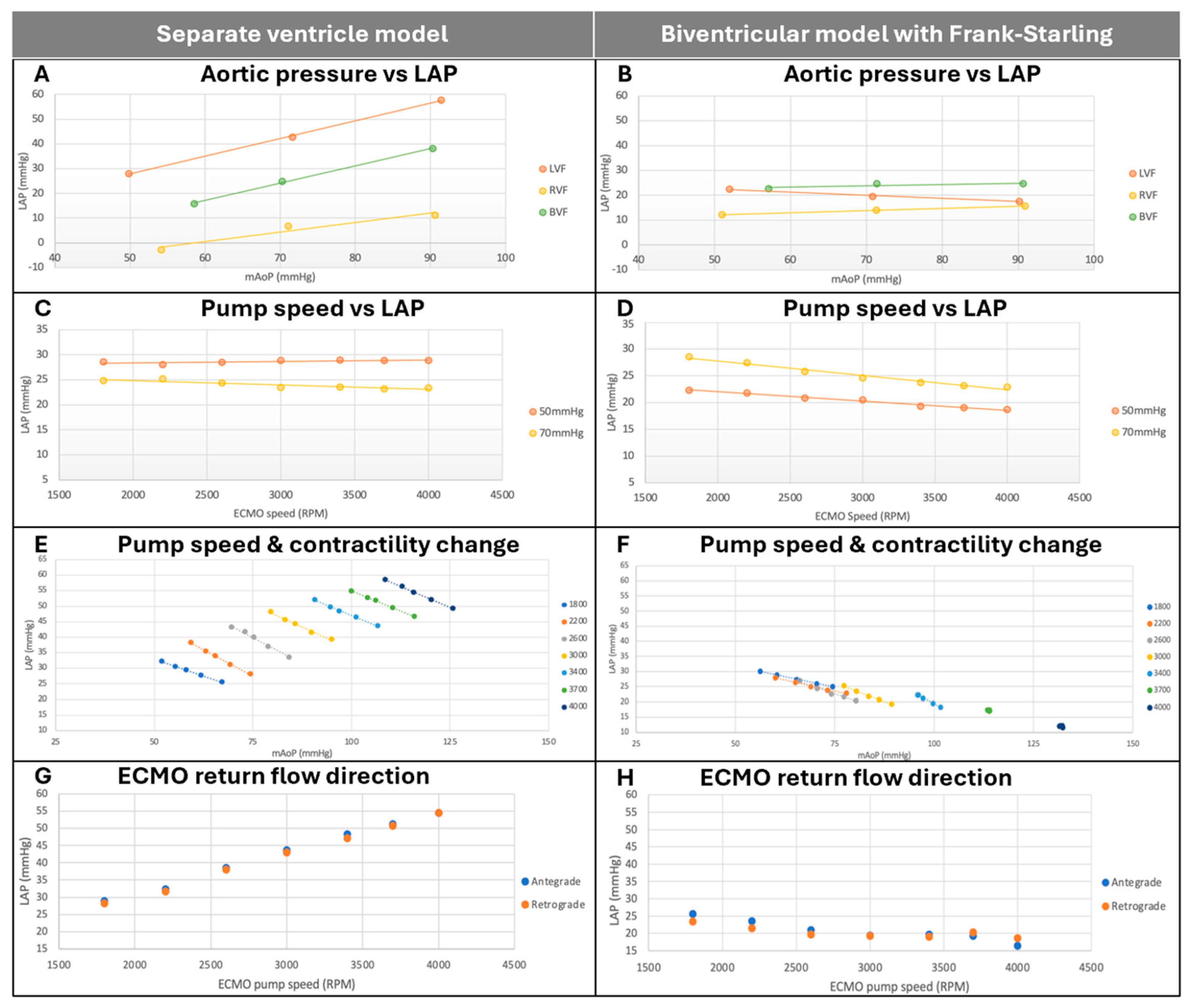
3.2. Determinants of Left Atrial Pressure During V-A ECMO
3.2.1. Effect of Aortic Pressure on Left Atrial Pressure
3.2.2. Effect of ECMO Pump Speed on Left Atrial Pressure
3.2.3. Effect of Changes in LV Contractility and Pump Speed on Left Atrial Pressure
3.2.4. Effect of ECMO Direction on Left Atrial Pressure
4. Discussion
4.1. Biventricular Interactions Mitigate the Effects of Arterial Pressure on LAP
4.2. Increased ECMO Speed Is Associated with Reduced LAP in the Presence of a Frank–Starling Mechanism
4.3. LAP and mAoP Are Stabilized at High ECMO Speed in a Biventricular Model with Frank–Starling Mechanism, Regardless of LV Contractility
4.4. ECMO Direction Does Not Affect LAP
4.5. Implications and Future Directions
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, H.R.; Hochman, J.S. Cardiogenic Shock Current Concepts and Improving Outcomes. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Puymirat, E.; Fagon, J.Y.; Aegerter, P.; Diehl, J.L.; Monnier, A.; Hauw-Berlemont, C.; Boissier, F.; Chatellier, G.; Guidet, B.; Danchin, N.; et al. Cardiogenic shock in intensive care units: Evolution of prevalence, patient profile, management and outcomes, 1997–2012. Eur. J. Heart Fail. 2017, 19, 192–200. [Google Scholar] [CrossRef]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Klocke, A.; Autschbach, R. Past, present, and future of long-term mechanical cardiac support in adults. J. Card. Surg. 2008, 23, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Farag, J.; Stephens, A.F.; Juene Chong, W.; Gregory, S.D.; Marasco, S.F. Intra-aortic Balloon Pump Use With Extra Corporeal Membrane Oxygenation-A Mock Circulation Loop Study. Am. Soc. Artif. Intern. Organs J. 2022, 68, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.; Narsule, C.K.; Frakes, M.A.; Ender, V.; Cohen, J.E.; Wilcox, S.R. ECMO cannulation across New England. Heart Lung 2025, 71, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef]
- Lafçı, G.; Budak, A.B.; Yener, A.Ü.; Cicek, O.F. Use of Extracorporeal Membrane Oxygenation in Adults. Heart Lung Circ. 2014, 23, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Pavlushkov, E.; Berman, M.; Valchanov, K. Cannulation techniques for extracorporeal life support. Ann. Transl. Med. 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jain, P.; Adji, A.; Stevens, M.; Vazquez, G.M.; Barua, S.; Jeyakumar, S.; Hayward, C. Afterload pressure and left ventricular contractility synergistically affect left atrial pressure during veno-arterial ECMO. JHLT Open 2024, 3, 100044. [Google Scholar] [CrossRef]
- Mascia, G.; Barca, L.; Sartori, P.; Bianco, D.; Della Bona, R.; Di Donna, P.; Porto, I. Provisional Circulatory Support with Extracorporeal Membrane Oxygenation during Ventricular Tachycardia Ablation in Intermediate Risk Patients: A Case Series. J. Clin. Med. 2024, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Noaman, S.; Andrianopoulos, N.; Brennan, A.L.; Dinh, D.; Reid, C.; Stub, D.; Biswas, S.; Clark, D.; Shaw, J.; Ajani, A.; et al. Outcomes of cardiogenic shock complicating acute coronary syndromes. Catheter. Cardiovasc. Interv. 2020, 96, E257–E267. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, M.L. The Starling Relationship and Veno-Arterial ECMO: Ventricular Distension Explained. Am. Soc. Artif. Intern. Organs J. 2018, 64, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Davila, C.D.; Chweich, H. Protecting the Vulnerable Left Ventricle: The Art of Unloading With VA-ECMO. Circ. Heart Fail. 2019, 12, e006581. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Meani, P.; Raffa, G.M.; Kowalewski, M. Extracorporeal membrane oxygenation and left ventricular unloading: What is the evidence? J. Thorac. Cardiovasc. Surg. Tech. 2022, 13, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Shimada, S.; Mullin, M.; Kreitler, K.; Cavarocchi, N.; Hirose, H. What Is the Optimal Blood Pressure on Veno-Arterial Extracorporeal Membrane Oxygenation? Impact of Mean Arterial Pressure on Survival. Am. Soc. Artif. Intern. Organs J. 2019, 65, 336–341. [Google Scholar] [CrossRef]
- Kalra, R.; Alexy, T.; Bartos, J.A.; Prisco, A.R.; Kosmopoulos, M.; Maharaj, V.R.; Bernal, A.G.; Elliott, A.M.; Garcia, S.; Raveendran, G.; et al. Left ventricular hemodynamics with veno-arterial extracorporeal membrane oxygenation. Catheter. Cardiovasc. Interv. 2024, 103, 472–481. [Google Scholar] [CrossRef] [PubMed]

| State | mAoP (mmHg) | LAP (mmHg) | RAP (mmHg) | Flow (L/min) |
|---|---|---|---|---|
| Normal | >60 | <15 | <10 | >4 |
| LVF | >60 | >25 | <10 | <3 |
| RVF | >60 | <15 | >25 | <3 |
| BVF | >60 | >25 | >25 | <3 |
| State | mAoP (mmHg) | LAP (mmHg) | RAP (mmHg) | Flow (L/min) |
|---|---|---|---|---|
| Normal | 63.8 | 12.0 | 6.4 | 4.10 |
| LVF | 61.7 | 40.8 | 3.5 | 2.44 |
| RVF | 62.7 | 13.2 | 32.2 | 2.32 |
| BVF | 64.5 | 32.9 | 34.0 | 1.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasavaraj, A.; Said, C.; Boss, L.A.; Matus Vazquez, G.; Stevens, M.; Jiang, J.; Adji, A.; Hayward, C.; Jain, P. Increased VA-ECMO Pump Speed Reduces Left Atrial Pressure: Insights from a Novel Biventricular Heart Model. Bioengineering 2025, 12, 237. https://doi.org/10.3390/bioengineering12030237
Kasavaraj A, Said C, Boss LA, Matus Vazquez G, Stevens M, Jiang J, Adji A, Hayward C, Jain P. Increased VA-ECMO Pump Speed Reduces Left Atrial Pressure: Insights from a Novel Biventricular Heart Model. Bioengineering. 2025; 12(3):237. https://doi.org/10.3390/bioengineering12030237
Chicago/Turabian StyleKasavaraj, Anirudhan, Christian Said, Laurence Antony Boss, Gabriel Matus Vazquez, Michael Stevens, Jacky Jiang, Audrey Adji, Christopher Hayward, and Pankaj Jain. 2025. "Increased VA-ECMO Pump Speed Reduces Left Atrial Pressure: Insights from a Novel Biventricular Heart Model" Bioengineering 12, no. 3: 237. https://doi.org/10.3390/bioengineering12030237
APA StyleKasavaraj, A., Said, C., Boss, L. A., Matus Vazquez, G., Stevens, M., Jiang, J., Adji, A., Hayward, C., & Jain, P. (2025). Increased VA-ECMO Pump Speed Reduces Left Atrial Pressure: Insights from a Novel Biventricular Heart Model. Bioengineering, 12(3), 237. https://doi.org/10.3390/bioengineering12030237






