Computational Fluid Dynamic Optimization of Micropatterned Surfaces: Towards Biofunctionalization of Artificial Organs
Abstract
1. Introduction
2. Materials and Methods
Flow Field Simulation and the Optimization of Microtrenches
3. Results
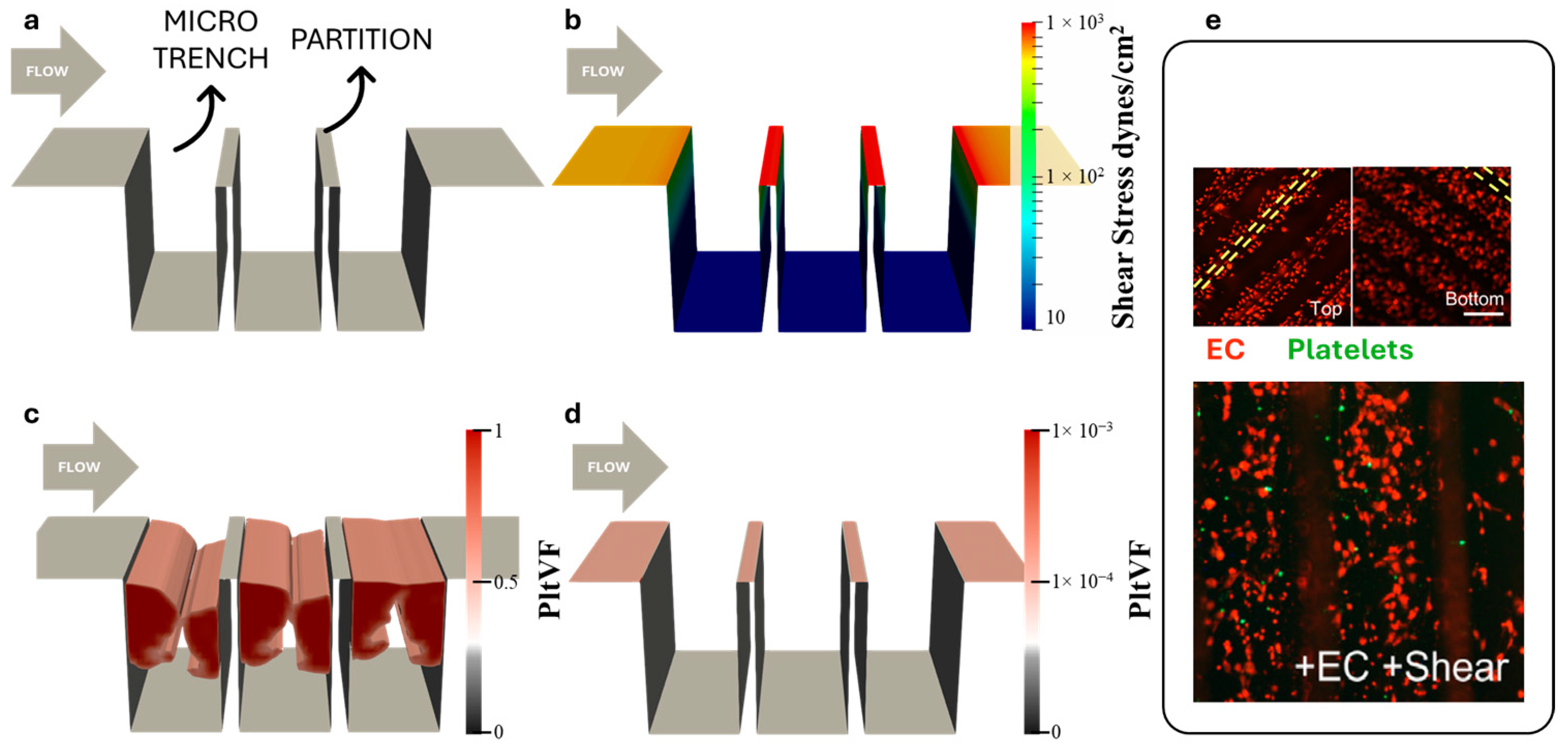
3.1. Simulation of Platelet Adhesion
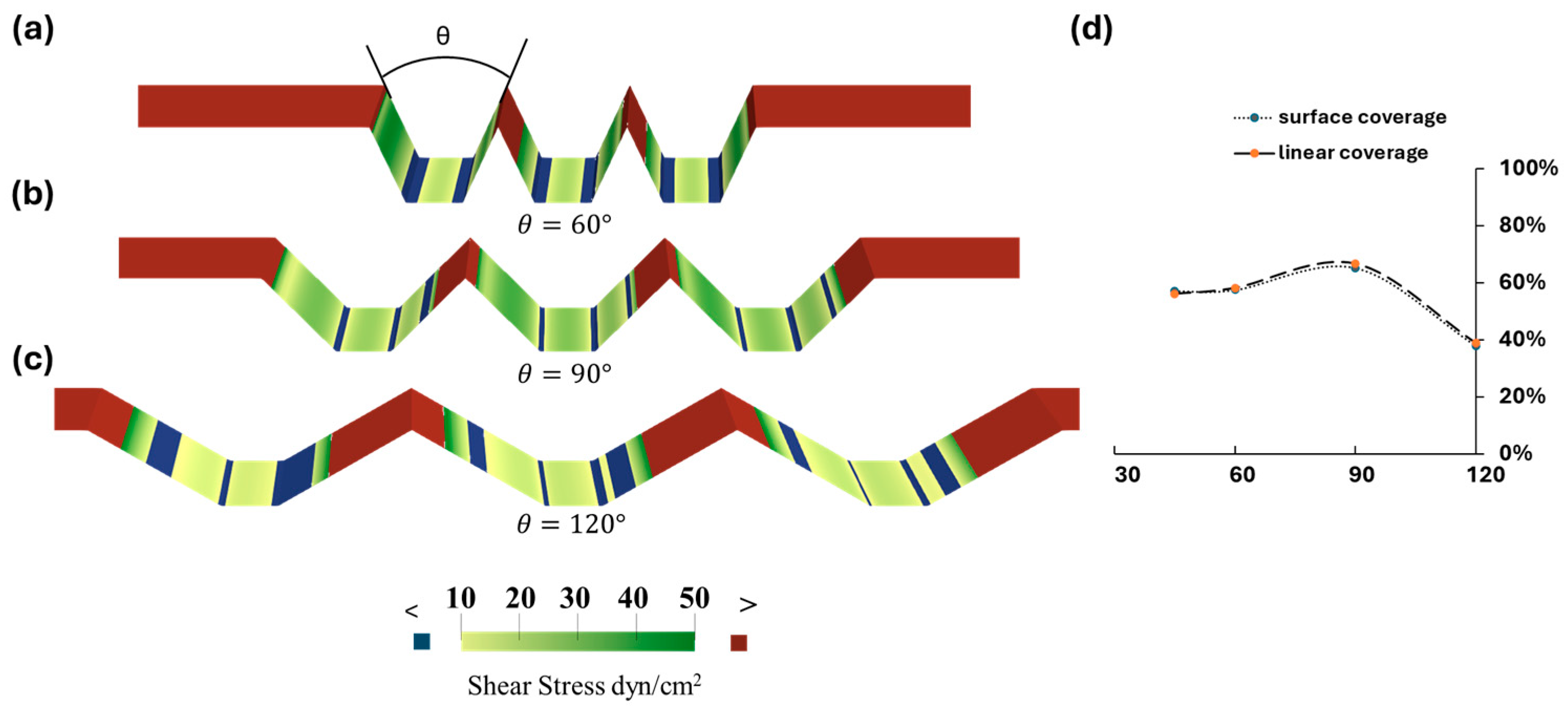
3.2. Optimization of Microtrenches
3.3. Optimization of Trapezoidal Trench Geometry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. In The Biomaterials: Silver Jubilee Compendium; 2004; pp. 219–241. ISBN 9780080451541. [Google Scholar]
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, H.J.; Benjamin, E.J.; Callaway, C.W.; Carson, A.P.; Cheng, S.; Elkind, M.S.V.; Evenson, K.R.; Ferguson, J.F.; Knutson, K.L.; Lee, C.D.; et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef]
- Cannegieter, S.C.; Rosendaal, F.R.; Briët, E. Thromboembolic and Bleeding Complications in Patients with Mechanical Heart Valve Prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact Activation of Blood-Plasma Coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef]
- Furukawa, K.S.; Ushida, T.; Sugano, H.; Tamaki, T.; Ohshima, N.; Tateishi, T. Effect of Shear Stress on Platelet Adhesion to Expanded Polytetrafluoroethylene, a Silicone Sheet, and an Endothelial Cell Monolayer. ASAIO J. 2000, 46, 696–701. [Google Scholar] [CrossRef]
- Ahmann, K.A.; Johnson, S.L.; Hebbel, R.P.; Tranquillo, R.T. Shear Stress Responses of Adult Blood Outgrowth Endothelial Cells Seeded on Bioartificial Tissue. Tissue Eng.-Part A 2011, 17, 2511–2521. [Google Scholar] [CrossRef]
- Jin, R.C.; Voetsch, B.; Loscalzo, J. Endogenous Mechanisms of Inhibition of Platelet Function. Microcirculation 2005, 12, 247–258. [Google Scholar] [CrossRef]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prostheticurrentc Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Wolfe, J.T.; Shradhanjali, A.; Tefft, B.J. Strategies for Improving Endothelial Cell Adhesion to Blood-Contacting Medical Devices. Tissue Eng. Part B Rev. 2022, 28, 1067–1092. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Butcher, J.T.; Rowlands, G.W.; Antaki, J.F. Biological Response to Sintered Titanium in Left Ventricular Assist Devices: Pseudoneointima, Neointima, and Pannus. ASAIO J. 2023, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zapanta, C.M.; Griffith, J.W.; Hess, G.D.; Doxtater, B.J.; Khalapyan, T.; Pae, W.E.; Rosenberg, G. Microtextured Materials for Circulatory Support Devices: Preliminary Studies. ASAIO J. 2006, 52, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Nishinaka, T.; Mizuno, T.; Tatsumi, E.; Yamazaki, K. LVAD Inflow Cannula Covered with a Titanium Mesh Induces Neointimal Tissue with Neovessels. Int. J. Artif. Organs 2015, 38, 316–324. [Google Scholar] [CrossRef]
- Robotti, F.; Franco, D.; Bänninger, L.; Wyler, J.; Starck, C.T.; Falk, V.; Poulikakos, D.; Ferrari, A. The Influence of Surface Micro-Structure on Endothelialization under Supraphysiological Wall Shear Stress. Biomaterials 2014, 35, 8479–8486. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; He, H.; Zhao, X.; Liu, X.; Zhao, F.; Sun, Y.; Fan, Y. Combination of Flow and Micropattern Alignment Affecting Flow-Resistant Endothelial Cell Adhesion. J. Mech. Behav. Biomed. Mater. 2017, 74, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yoganathan, A.P.; Chaux, A.; Gray, R.J.; Woo, Y.R.; DeRobertis, M.; Williams, F.P.; Matloff, J.M. Bileaflet, Tilting Disc and Porcine Aortic Valve Substitutes: In Vitro Hydrodynamic Characteristics. J. Am. Coll. Cardiol. 1984, 3, 313–320. [Google Scholar] [CrossRef]
- Franco, D.; Milde, F.; Klingauf, M.; Orsenigo, F.; Dejana, E.; Poulikakos, D.; Cecchini, M.; Koumoutsakos, P.; Ferrari, A.; Kurtcuoglu, V. Biomaterials Accelerated Endothelial Wound Healing on Microstructured Substrates under Fl Ow. Biomaterials 2013, 34, 1488–1497. [Google Scholar] [CrossRef]
- Stefopoulos, G.; Robotti, F.; Falk, V.; Poulikakos, D.; Ferrari, A. Endothelialization of Rationally Microtextured Surfaces with Minimal Cell Seeding Under Flow. Small 2016, 12, 4113–4126. [Google Scholar] [CrossRef]
- Bachmann, B.J.; Giampietro, C.; Bayram, A.; Stefopoulos, G.; Michos, C.; Graeber, G.; Falk, M.V.; Poulikakos, D.; Ferrari, A. Honeycomb-Structured Metasurfaces for the Adaptive Nesting of Endothelial Cells under Hemodynamic Loads. Biomater. Sci. 2018, 6, 2726–2737. [Google Scholar] [CrossRef]
- Daxini, S.C.; Nichol, J.W.; Sieminski, A.L.; Smith, G.; Gooch, K.J.; Shastri, V.P. Micropatterned Polymer Surfaces Improve Retention of Endothelial Cells Exposed to Flow-Induced Shear Stress. Biorheology 2006, 43, 45–55. [Google Scholar] [PubMed]
- Frendl, C.M.; Tucker, S.M.; Khan, N.A.; Esch, M.B.; Kanduru, S.; Cao, T.M.; García, A.J.; King, M.R.; Butcher, J.T. Endothelial Retention and Phenotype on Carbonized Cardiovascular Implant Surfaces. Biomaterials 2014, 35, 7714–7723. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Webster, T.J. Increased Endothelial Cell Adhesion and Elongation on Micron-Patterned Nano-Rough Poly(Dimethylsiloxane) Films. Nanotechnology 2009, 20, 305102. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Jamiolkowski, M.A.; Wagner, W.R.; Aubry, N.; Massoudi, M.; Antaki, J.F. Multi-Constituent Simulation of Thrombus Deposition. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]



| Design Variables | Lower Bound | Upper Bound | Average Interval |
|---|---|---|---|
| Height (mm) | 0.01 | 3 | 0.1 |
| Width (mm) | 0.01 | 3 | 0.1 |
| Angle * (°) | 0 | 90 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Ibrahim, A.M.; Karmakar, A.; Tuli, S.; Butcher, J.T.; Antaki, J.F. Computational Fluid Dynamic Optimization of Micropatterned Surfaces: Towards Biofunctionalization of Artificial Organs. Bioengineering 2024, 11, 1092. https://doi.org/10.3390/bioengineering11111092
He W, Ibrahim AM, Karmakar A, Tuli S, Butcher JT, Antaki JF. Computational Fluid Dynamic Optimization of Micropatterned Surfaces: Towards Biofunctionalization of Artificial Organs. Bioengineering. 2024; 11(11):1092. https://doi.org/10.3390/bioengineering11111092
Chicago/Turabian StyleHe, Wenxuan, Aminat M. Ibrahim, Abhishek Karmakar, Shivani Tuli, Jonathan T. Butcher, and James F. Antaki. 2024. "Computational Fluid Dynamic Optimization of Micropatterned Surfaces: Towards Biofunctionalization of Artificial Organs" Bioengineering 11, no. 11: 1092. https://doi.org/10.3390/bioengineering11111092
APA StyleHe, W., Ibrahim, A. M., Karmakar, A., Tuli, S., Butcher, J. T., & Antaki, J. F. (2024). Computational Fluid Dynamic Optimization of Micropatterned Surfaces: Towards Biofunctionalization of Artificial Organs. Bioengineering, 11(11), 1092. https://doi.org/10.3390/bioengineering11111092






