Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration and Reporting Format
2.2. Focus Question
2.3. PICO Strategy
- The participants (P) included were women undergoing assisted reproduction with a history of embryo transfer failure;
- The intervention (I) was intrauterine PRP infusion before embryo implantation;
- The comparison (C) was to no intervention or placebo;
- The outcomes (O) were implantation rate, biochemical pregnancy rate, clinical pregnancy rate, live birth rate and miscarriage rate.
2.4. Eligibility Criteria
2.5. Data Sources and Search Strategy
2.6. Study Selection
2.7. Data Extraction
2.8. Risk of Bias Assessment
2.9. Outcomes
2.10. Quality of Evidence
2.11. Statistical Analysis
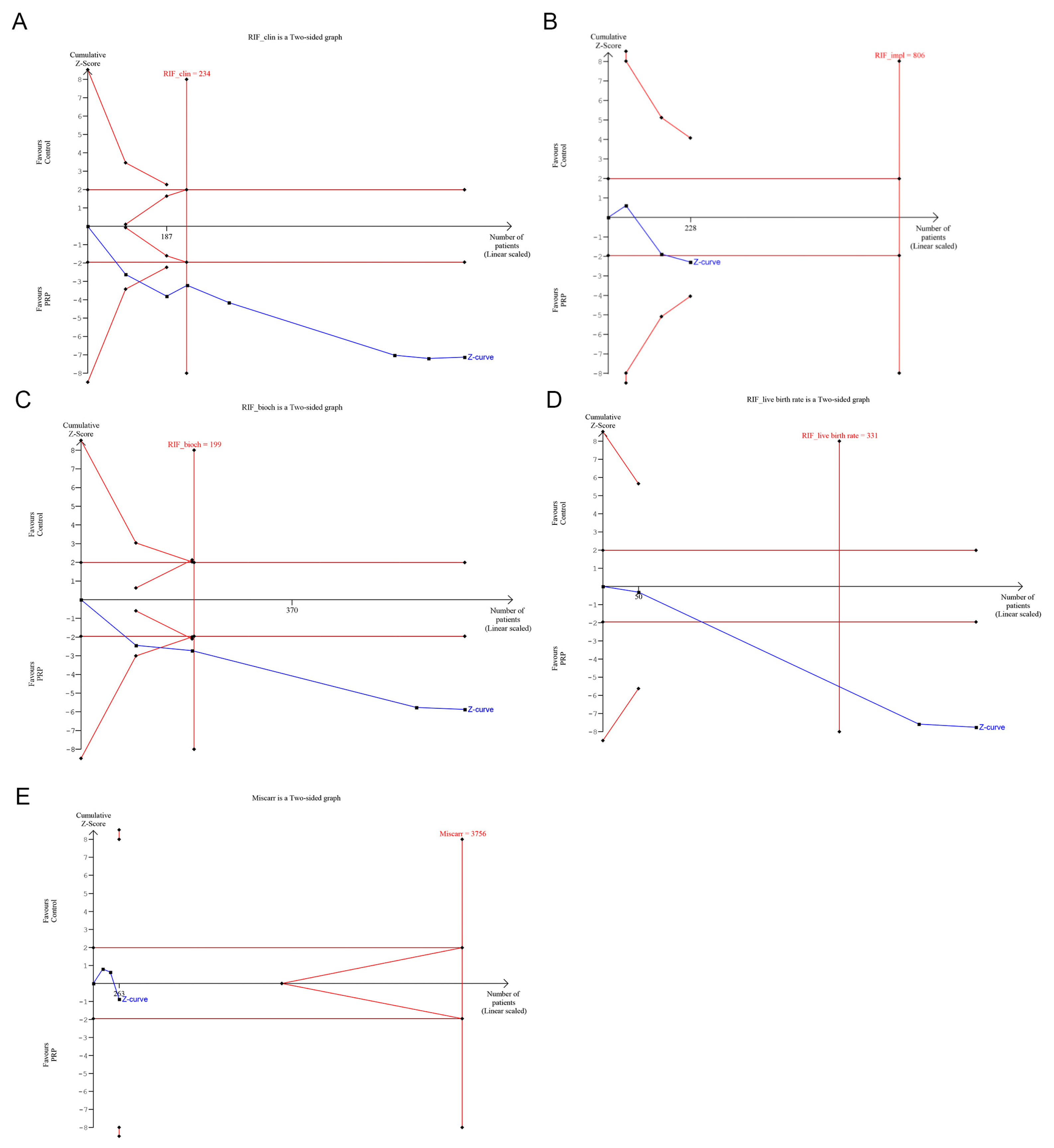
3. Trial Sequential Analysis
4. Results
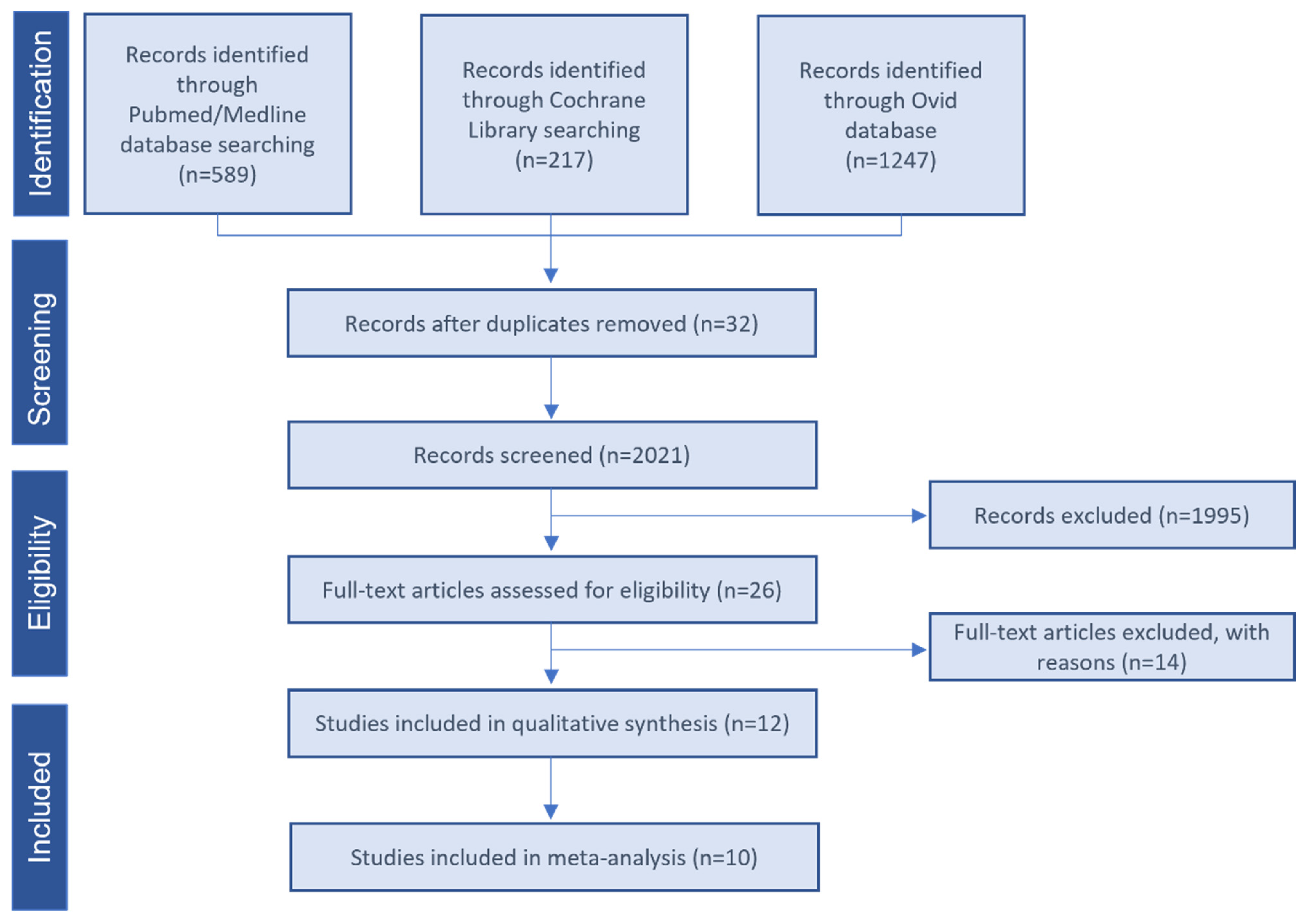
4.1. Summary of the Literature Search
4.2. Study Characteristics
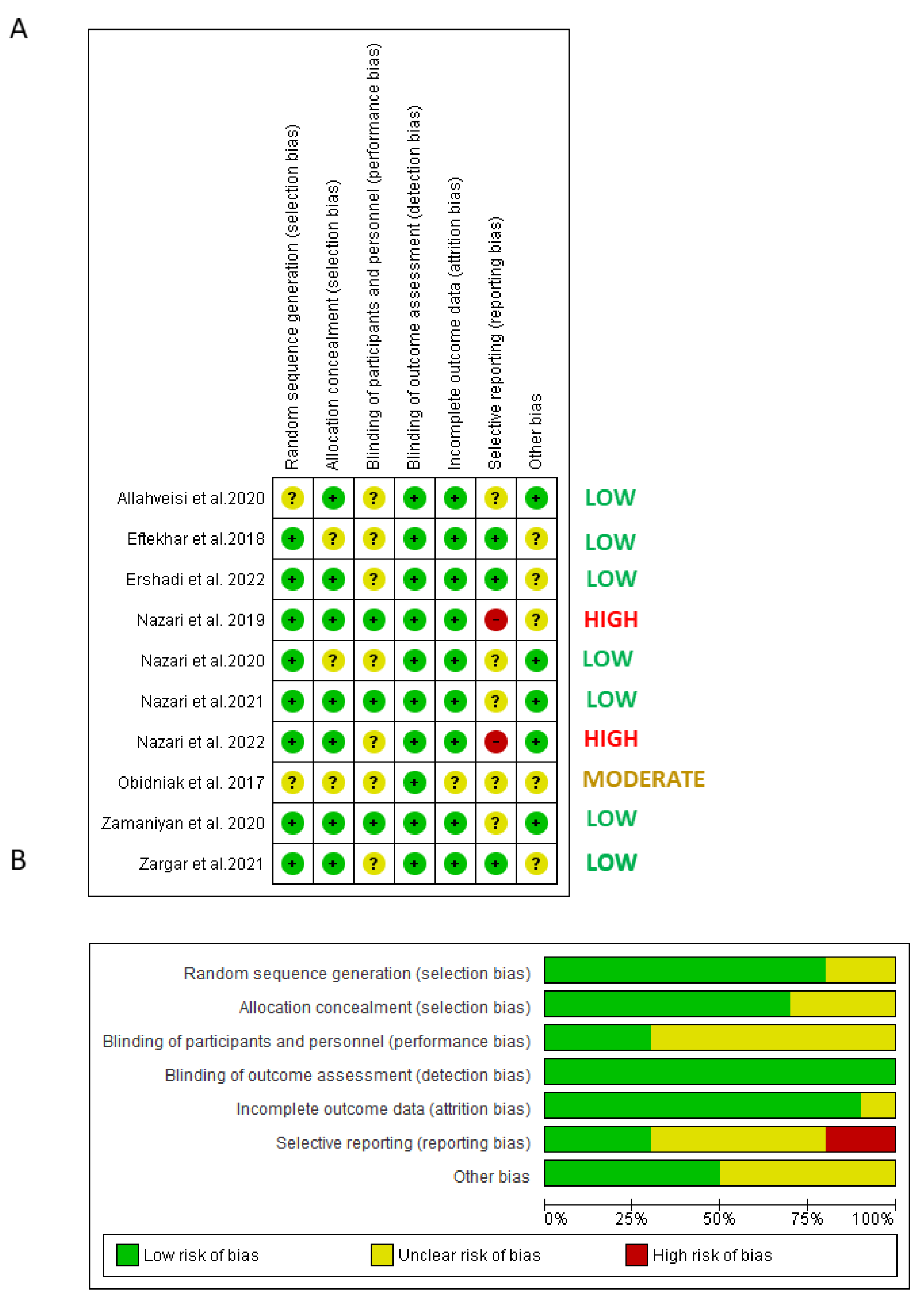
4.3. Risk of Bias of Included Trials
| Study | Sample Size | Implantation Rate | Biochemical Pregnancy Rate | Clinical Pregnancy Rate | Live Birth Rate | Miscarriage Rate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PRP | Control | PRP | Control | PRP | Control | PRP | Control | PRP | Control | PRP | |
| Allahveisi et al., 2020 | 25 | 25 | 36% (n = 9) | 28% (n = 7) | NR | NR | 36% (n = 9) | 28% (n = 7) | 28% (n = 6) | 24% (n = 7) | NR | NR |
| Eftekhar et al., 2018 | 43 | 40 | 9.37% * | 21% * | 24.2% (n = 8) | 42.4% (n = 14) | 18.2% (n = 6) | 39.4% (n = 13) | 18.2% (n = 6) | 33.3% (n = 11) | 6% (n = 2) | 9% (n = 3) |
| Ershadi et al., 2022 | 45 | 40 | 0.38 ± 0.16% | 0.36 ± 0.24% | 27% (n = 12) | 40% (n = 16) | 24% (n = 11) | 33% (n = 13) | NR | NR | 31.25% (n = 5) | 8.33% (n = 1) |
| Nazari et al., 2020 | 48 | 49 | NR | NR | 27.08% (n = 13) | 53.06% (n = 26) | 16.66% (n = 8) | 44.89% (n = 22) | NR | NR | NR | NR |
| Nazari et al., 2019 | 30 | 30 | NR | NR | 6.7% (n = 2) | 40% (n = 12) | 3.3% (n = 1) | 33.3% (n = 10) | NR | NR | NR | NR |
| Nazari et al., 2021 | 197 | 196 | NR | NR | 24.87% (n = 49) | 51.53% (n = 101) | 19.28% (n = 38) | 48.97% (n = 96) | 5.58% (n = 11) | 39.28% (n = 77) | NR | NR |
| Nazari et al., 2022 | 20 | 20 | NR | NR | NR | NR | 20% (n = 4) | 35% (n = 7) | 0% (n = 0) | 15% (n = 3) | 20% (n = 4) | 20% (n = 4) |
| Obidniak et al., 2017 | 45 | 45 | 20.9% * | 40.5% * | NR | NR | 24.4% (n = 11) | 53.3% (n = 24) | NR | NR | NR | NR |
| Zamaniyan et al., 2020 | 43 | 55 | 34.9% (n = 15) | 63.6% (n = 35) | 23.3% (n = 10) | 36.2% (n = 20) | 23.3% (n = 10) | 52.7% (n = 29) | NR | NR | 4.6% (n = 2) | 1.8% (n = 1) |
| Zargar et al., 2021 | 40 | 40 | 5% (n = 2) | 15% (n = 6) | NR | NR | 2.5% (n = 1) | 12.5% (n = 5) | 2.5% (n = 1) | 12.5% (n = 5) | 2.5% (n = 1) | 2.5% (n = 1) |
4.4. Quality of Evidence
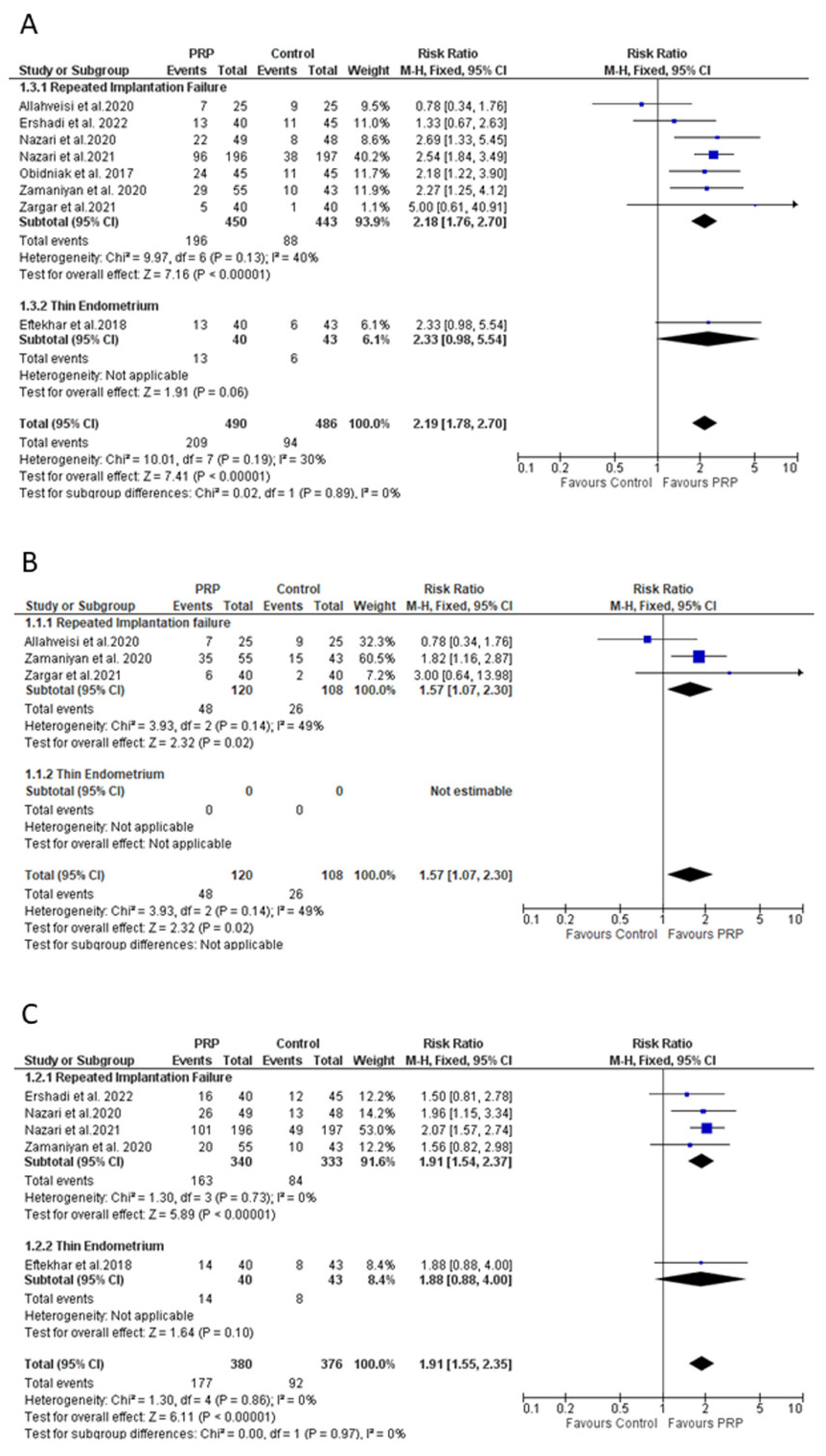
4.5. Effect of PRP on Clinical Pregnancy Rate
4.6. Effect of PRP on Implantation Rate
4.7. Effect of PRP on Biochemical Pregnancy Rate
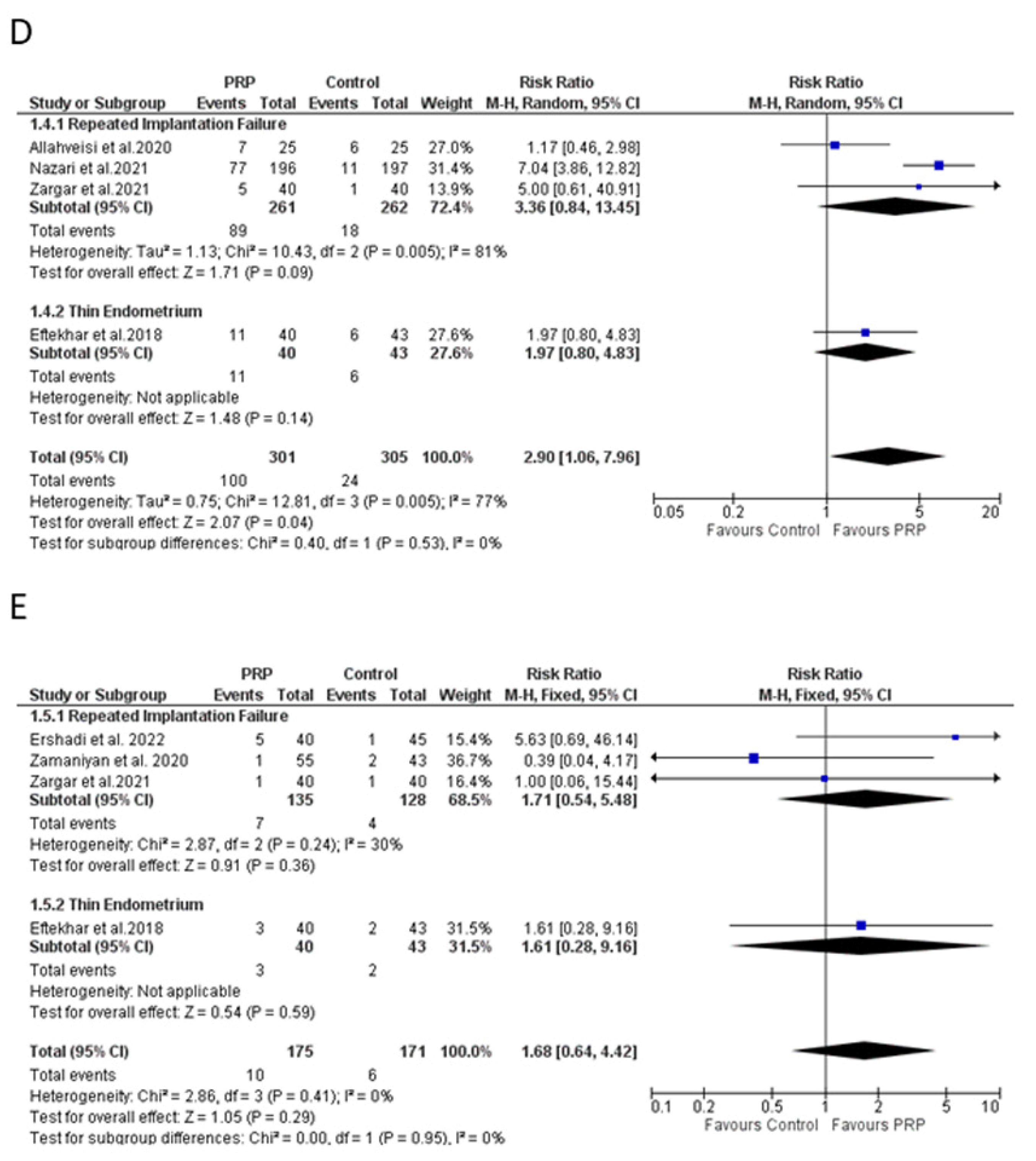
4.8. Effect of PRP on Live Birth Rate
4.9. Effect of PRP on Miscarriage Rate
4.10. Trial Sequential Analysis
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.K.; Vandekerckhove, P.; Kennedy, R.; Keay, S.D. Investigation and Current Management of Recurrent IVF Treatment Failure in the UK. BJOG 2005, 112, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Garneau, A.S.; Young, S.L. Defining Recurrent Implantation Failure: A Profusion of Confusion or Simply an Illusion? Fertil. Steril. 2021, 116, 1432–1435. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of Infertility and Recurrent Pregnancy Loss: A Committee Opinion. Fertil. Steril. 2013, 99, 63. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Alecsandru, D.; Forman, E.J.; Gemmell, L.C.; Goldberg, J.M.; Llarena, N.; Margolis, C.; Laven, J.; Schoenmakers, S.; Seli, E. A Review of the Pathophysiology of Recurrent Implantation Failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-Update Overview on Etiology, Diagnosis, Treatment and Future Directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [Green Version]
- Aghajanova, L.; Houshdaran, S.; Balayan, S.; Manvelyan, E.; Irwin, J.C.; Huddleston, H.G.; Giudice, L.C. In Vitro Evidence That Platelet-Rich Plasma Stimulates Cellular Processes Involved in Endometrial Regeneration. J. Assist. Reprod. Genet. 2018, 35, 757–770. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous Platelet-Rich Plasma Promotes Endometrial Growth and Improves Pregnancy Outcome during in Vitro Fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286–1290. [Google Scholar]
- Coksuer, H.; Akdemir, Y.; Ulas Barut, M. Improved in Vitro Fertilization Success and Pregnancy Outcome with Autologous Platelet-Rich Plasma Treatment in Unexplained Infertility Patients That Had Repeated Implantation Failure History. Gynecol. Endocrinol. 2019, 35, 815–818. [Google Scholar] [CrossRef]
- Farimani, M.; Poorolajal, J.; Rabiee, S.; Bahmanzadeh, M. Successful Pregnancy and Live Birth after Intrauterine Administration of Autologous Platelet-Rich Plasma in a Woman with Recurrent Implantation Failure: A Case Report. Int. J. Reprod. Biomed. 2017, 15, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Nazari, L.; Salehpour, S.; Hoseini, S.; Zadehmodarres, S.; Azargashb, E. Effects of Autologous Platelet-Rich Plasma on Endometrial Expansion in Patients Undergoing Frozen-Thawed Embryo Transfer: A Double-Blind RCT. Int. J. Reprod. Biomed. 2019, 17, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar, M.; Neghab, N.; Naghshineh, E.; Khani, P. Can Autologous Platelet Rich Plasma Expand Endometrial Thickness and Improve Pregnancy Rate during Frozen-Thawed Embryo Transfer Cycle? A Randomized Clinical Trial. Taiwan J. Obstet. Gynecol. 2018, 57, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.B.; Jeelall, Y.; Pennell, C.E.; Hart, R.; McLean-Tooke, A.; Lucas, M. The Role of Immunological Testing and Intervention in Reproductive Medicine: A Fertile Collaboration? Am. J. Reprod. Immunol. 2018, 79, e12784. [Google Scholar] [CrossRef] [PubMed]
- Ghaebi, M.; Abdolmohammadi-Vahid, S.; Ahmadi, M.; Eghbal-Fard, S.; Dolati, S.; Nouri, M.; Talebi, M.; Hamdi, K.; Marofi, F.; Aghebati-Maleki, L.; et al. T Cell Subsets in Peripheral Blood of Women with Recurrent Implantation Failure. J. Reprod. Immunol. 2019, 131, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Petitbarat, M.; Chevrier, L.; Vitoux, D.; Vezmar, K.; Rahmati, M.; Dubanchet, S.; Gahéry, H.; Bensussan, A.; Chaouat, G. The Uterine Immune Profile May Help Women with Repeated Unexplained Embryo Implantation Failure after In Vitro Fertilization. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davar, R.; Janati, S.; Mohseni, F.; Khabazkhoob, M.; Asgari, S. A Comparison of the Effects of Transdermal Estradiol and Estradiol Valerate on Endometrial Receptivity in Frozen-Thawed Embryo Transfer Cycles: A Randomized Clinical Trial. J. Reprod. Infertil. 2016, 17, 97–103. [Google Scholar]
- Weckstein, L.N.; Jacobson, A.; Galen, D.; Hampton, K.; Hammel, J. Low-Dose Aspirin for Oocyte Donation Recipients with a Thin Endometrium: Prospective, Randomized Study. Fertil. Steril. 1997, 68, 927–930. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Li, X.; Lv, F.; He, X.; Pan, Y.; Wang, L.; Zhang, X. Efficacy Evaluation of Low-Dose Aspirin in IVF/ICSI Patients Evidence from 13 RCTs: A Systematic Review and Meta-Analysis. Medicine 2017, 96, e7720. [Google Scholar] [CrossRef]
- Acharya, S.; Yasmin, E.; Balen, A.H. The Use of a Combination of Pentoxifylline and Tocopherol in Women with a Thin Endometrium Undergoing Assisted Conception Therapies—A Report of 20 Cases. Hum. Fertil. 2009, 12, 198–203. [Google Scholar] [CrossRef]
- Zinger, M.; Liu, J.H.; Thomas, M.A. Successful Use of Vaginal Sildenafil Citrate in Two Infertility Patients with Asherman’s Syndrome. J. Women’s Health 2006, 15, 442–444. [Google Scholar] [CrossRef]
- Sher, G.; Fisch, J.D. Effect of Vaginal Sildenafil on the Outcome of in Vitro Fertilization (IVF) after Multiple IVF Failures Attributed to Poor Endometrial Development. Fertil. Steril. 2002, 78, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Refai, H.; Hassan, D.; Abdelmonem, R. Development and Characterization of Polymer-Coated Liposomes for Vaginal Delivery of Sildenafil Citrate. Drug Deliv. 2017, 24, 278–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, H.; Jiang, J.; Xia, M.; Tang, R.; Qin, Y.; Chen, Z.-J. The Effect of Tamoxifen on Thin Endometrium in Patients Undergoing Frozen-Thawed Embryo Transfer. Reprod. Sci. 2018, 25, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Kunicki, M.; Łukaszuk, K.; Liss, J.; Skowrońska, P.; Szczyptańska, J. Granulocyte Colony Stimulating Factor Treatment of Resistant Thin Endometrium in Women with Frozen-Thawed Blastocyst Transfer. Syst. Biol. Reprod. Med. 2017, 63, 49–57. [Google Scholar] [CrossRef]
- Bos-Mikich, A.; De Oliveira, R.; Frantz, N. Platelet-Rich Plasma Therapy and Reproductive Medicine. J. Assist. Reprod. Genet. 2018, 35, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Gat, I.; Levron, J.; Yerushalmi, G.; Dor, J.; Brengauz, M.; Orvieto, R. Should Zygote Intrafallopian Transfer Be Offered to All Patients with Unexplained Repeated In-Vitro Fertilization Cycle Failures? J. Ovarian Res. 2014, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-Rich Plasma and Regenerative Dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Merchán, W.H.; Gómez, L.A.; Chasoy, M.E.; Alfonso-Rodríguez, C.A.; Muñoz, A.L. Platelet-Rich Plasma, a Powerful Tool in Dermatology. J. Tissue Eng. Regen. Med. 2019, 13, 892–901. [Google Scholar] [CrossRef]
- Urits, I.; Viswanath, O.; Galasso, A.C.; Sottosani, E.R.; Mahan, K.M.; Aiudi, C.M.; Kaye, A.D.; Orhurhu, V.J. Platelet-Rich Plasma for the Treatment of Low Back Pain: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 52. [Google Scholar] [CrossRef]
- Sanchez-Avila, R.M.; Merayo-Lloves, J.; Muruzabal, F.; Orive, G.; Anitua, E. Plasma Rich in Growth Factors for the Treatment of Dry Eye from Patients with Graft versus Host Diseases. Eur. J. Ophthalmol. 2020, 30, 94–103. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, M.S.; Hashemi Moghanjoughi, P. The Effects of Autologous Platelet-Rich Plasma in Repeated Implantation Failure: A Randomized Controlled Trial. Hum. Fertil. 2020, 23, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Sheibani, S.; Hosseinirad, H. The Effects of Autologous Platelet-Rich Plasma on Pregnancy Outcomes in Repeated Implantation Failure Patients Undergoing Frozen Embryo Transfer: A Randomized Controlled Trial. Reprod. Sci. 2022, 29, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; de la Fuente, M.; Ferrando, M.; Quintana, F.; Larreategui, Z.; Matorras, R.; Orive, G. Biological Effects of Plasma Rich in Growth Factors (PRGF) on Human Endometrial Fibroblasts. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, L.; Mou, S.; Zhao, H.; Fang, J.; Xiang, Y.; Zhao, T.; Sha, T.; Ding, J.; Hao, C. Investigation of Platelet-Rich Plasma in Increasing Proliferation and Migration of Endometrial Mesenchymal Stem Cells and Improving Pregnancy Outcome of Patients with Thin Endometrium. J. Cell. Biochem. 2018, 120, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.; Navarro, C.; Jones, C.; Coward, K.; Coleman, L. The Use of Autologous Platelet-Rich Plasma (PRP) versus No Intervention in Women with Low Ovarian Reserve Undergoing Fertility Treatment: A Non-Randomized Interventional Study. J. Assist. Reprod. Genet. 2020, 37, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Pantos, K.; Simopoulou, M.; Pantou, A.; Rapani, A.; Tsioulou, P.; Nitsos, N.; Syrkos, S.; Pappas, A.; Koutsilieris, M.; Sfakianoudis, K. A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transplant. 2019, 28, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Allahveisi, A.; Seyedoshohadaei, F.; Rezaei, M.; Bazrafshan, N.; Rahimi, K. The Effect of Platelet-Rich Plasma on the Achievement of Pregnancy during Frozen Embryo Transfer in Women with a History of Failed Implantation. Heliyon 2020, 6, e03577. [Google Scholar] [CrossRef]
- Zargar, M.; Pazhouhanfar, R.; Najafian, M.; Choghakabodi, P.M. Effects of Intrauterine Autologous Platelet-Rich Plasma Infusions on Outcomes in Women with Repetitive in Vitro Fertilization Failures: A Prospective Randomized Study. Clin. Exp. Obstet. Gynecol. 2021, 48, 180–185. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; The GRADE Working Group, McMaster University: Hamilton, ON, Canada, 2013; Available online: https://www.guidelinedevelopment.org/handbook (accessed on 8 February 2023).
- Bakhsh, A.S.; Maleki, N.; Sadeghi, M.R.; SadeghiTabar, A.; Tavakoli, M.; Zafardoust, S.; Karimi, A.; Askari, S.; Jouhari, S.; Mohammadzadeh, A. Effects of Autologous Platelet-Rich Plasma in Women with Repeated Implantation Failure Undergoing Assisted Reproduction. JBRA Assist. Reprod. 2021, 26, 84–87. [Google Scholar] [CrossRef]
- Ghasemi, M. Evaluation of the Effect of Intrauterine Injection of Platelet-Rich Plasma on the Pregnancy Rate of Patients with a History of Implantation Failure in the in Vitro Fertilization Cycle. Int. J. Gynecol. Cancer 2020, 30, A1–A142. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Hashemi, T.; Borumandnia, N.; Azizi, E. Effect of Autologous Platelet-Rich Plasma for Treatment of Recurrent Pregnancy Loss: A Randomized Controlled Trial. Obstet. Gynecol. Sci. 2022, 65, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ershadi, S.; Noori, N.; Dashipoor, A.; Ghasemi, M.; Shamsa, N. Evaluation of the Effect of Intrauterine Injection of Platelet-Rich Plasma on the Pregnancy Rate of Patients with a History of Implantation Failure in the in Vitro Fertilization Cycle. J. Fam. Med. Prim. Care 2022, 11, 2162–2166. [Google Scholar] [CrossRef]
- Zamaniyan, M.; Peyvandi, S.; Heidaryan Gorji, H.; Moradi, S.; Jamal, J.; Yahya Poor Aghmashhadi, F.; Hossein Mohammadi, M. Effect of Platelet-Rich Plasma on Pregnancy Outcomes in Infertile Women with Recurrent Implantation Failure: A Randomized Controlled Trial. Gynecol. Endocrinol. 2021, 37, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Obidniak, D.; Gzgzyan, A.; Feoktistov, A.; Niauri, D. Randomized Controlled Trial Evaluating Efficacy of Autologous Platelet-Rich Plasma Therapy for Patients with Recurrent Implantation Failure. Fertil. Steril. 2017, 108, e370. [Google Scholar] [CrossRef] [Green Version]
- Baum, M.; Yerushalmi, G.M.; Maman, E.; Kedem, A.; Machtinger, R.; Hourvitz, A.; Dor, J. Does Local Injury to the Endometrium before IVF Cycle Really Affect Treatment Outcome? Results of a Randomized Placebo Controlled Trial. Gynecol. Endocrinol. 2012, 28, 933–936. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of Chronic Endometritis in Repeated Unexplained Implantation Failure and the IVF Success Rate after Antibiotic Therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Sun, Y.; Xie, H.; Fang, S.; Zhao, X. Hysteroscopy Prior to Repeat Embryo Transfer May Improve Pregnancy Outcomes for Asymptomatic Women with Repeated Implantation Failure. J. Obstet. Gynaecol. Res. 2015, 41, 1569–1576. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, J.; Chen, Q.; Kuang, Y.; Zhang, S. Short-Term Copper Intrauterine Device Placement Improves the Implantation and Pregnancy Rates in Women with Repeated Implantation Failure. Fertil. Steril. 2017, 108, 55–61.e1. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wu, H.; He, X.; Xing, Q.; Zhou, P.; Cao, Y.; Wei, Z. Application of Atosiban in Frozen-Thawed Cycle Patients with Different Times of Embryo Transfers. Gynecol. Endocrinol. 2016, 32, 811–815. [Google Scholar] [CrossRef]
- Tehraninejad, E.S.; Raisi, E.; Ghaleh, F.B.; Rashidi, B.H.; Aziminekoo, E.; Kalantari, V.; Haghollahi, F.; Shariat, M. The Sequential Embryo Transfer Compared to Blastocyst Embryo Transfer in in Vitro Fertilization (IVF) Cycle in Patients with the Three Repeated Consecutive IVF. A Randomized Controlled Trial. Gynecol. Endocrinol. 2019, 35, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Yu, M.; Zhang, X.-J. Effect of Hyaluronic Acid-Enriched Transfer Medium on Frozen-Thawed Embryo Transfer Outcomes. J. Obstet. Gynaecol. Res. 2018, 44, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Benkhalifa, M.; Demirol, A.; Sari, T.; Balashova, E.; Tsouroupaki, M.; Giakoumakis, Y.; Gurgan, T. Autologous Embryo-Cumulus Cells Co-Culture and Blastocyst Transfer in Repeated Implantation Failures: A Collaborative Prospective Randomized Study. Zygote 2012, 20, 173–180. [Google Scholar] [CrossRef]
- Delaroche, L.; Yazbeck, C.; Gout, C.; Kahn, V.; Oger, P.; Rougier, N. Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) after Repeated IVF or ICSI Failures: A Prospective Comparative Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Liu, W.; Liu, J.; Wu, L.; Xiong, S.; Zhu, J.; Han, W.; Wang, J.; Hao, X.; et al. Frozen Blastocyst Embryo Transfer vs. Frozen Cleavage-Stage Embryo Transfer in Couples with Recurrent Implantation Failure: A Cohort Study. Hum. Fertil. 2021, 24, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Levran, D.; Mashiach, S.; Dor, J.; Levron, J.; Farhi, J. Zygote Intrafallopian Transfer May Improve Pregnancy Rate in Patients with Repeated Failure of Implantation. Fertil. Steril. 1998, 69, 26–30. [Google Scholar] [CrossRef]
- Edirisinghe, W.R.; Ahnonkitpanit, V.; Promviengchai, S.; Suwajanakorn, S.; Pruksananonda, K.; Chinpilas, V.; Virutamasen, P. A Study Failing to Determine Significant Benefits from Assisted Hatching: Patients Selected for Advanced Age, Zonal Thickness of Embryos, and Previous Failed Attempts. J. Assist. Reprod. Genet. 1999, 16, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Bellver, J.; Rodrigo, L.; Bosch, E.; Mercader, A.; Vidal, C.; De los Santos, M.J.; Giles, J.; Labarta, E.; Domingo, J.; et al. Preimplantation Genetic Screening Using Fluorescence in Situ Hybridization in Patients with Repetitive Implantation Failure and Advanced Maternal Age: Two Randomized Trials. Fertil. Steril. 2013, 99, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-K.; Chen, H.-H.; Huang, C.-C.; Lee, C.-I.; Lin, P.-Y.; Lee, M.-S.; Lee, T.-H. Peripheral CD56(+)CD16(+) NK Cell Populations in the Early Follicular Phase Are Associated With Successful Clinical Outcomes of Intravenous Immunoglobulin Treatment in Women With Repeated Implantation Failure. Front. Endocrinol. 2019, 10, 937. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, Y.; Liu, C.; Hu, Y.; Li, L. Intravenous Immunoglobulin Treatment for Repeated IVF/ICSI Failure and Unexplained Infertility: A Systematic Review and a Meta-Analysis. Am. J. Reprod. Immunol. 2013, 70, 434–447. [Google Scholar] [CrossRef]
- Li, J.; Mo, S.; Chen, Y. The Effect of G-CSF on Infertile Women Undergoing IVF Treatment: A Meta-Analysis. Syst. Biol. Reprod. Med. 2017, 63, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, J.; Cheng, Y.; Zhou, D.; Yin, T.; Xu, W.; Yu, N.; Yang, J. Intrauterine Administration of HCG-Activated Autologous Human Peripheral Blood Mononuclear Cells (PBMC) Promotes Live Birth Rates in Frozen/Thawed Embryo Transfer Cycles of Patients with Repeated Implantation Failure. J. Reprod. Immunol. 2017, 119, 15–22. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kwak-Kim, J.; Ota, K.; Kuroda, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Immunosuppression with Tacrolimus Improved Reproductive Outcome of Women with Repeated Implantation Failure and Elevated Peripheral Blood TH1/TH2 Cell Ratios. Am. J. Reprod. Immunol. 2015, 73, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, F.; Esmaeilzadeh, S.; Basirat, Z.; Mahouti, T.; Heidari, F.N.; Golsorkhtabaramiri, M. Using Autologous Intrauterine Platelet-Rich Plasma to Improve the Reproductive Outcomes of Women with Recurrent Implantation Failure. JBRA Assist. Reprod. 2020, 24, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Zebeidi, J.; Agdi, M.; Lary, S.; Al-Obaid, S.; Salim, G.; Al-Jaroudi, D. Effect of Empiric Intravenous Intralipid Therapy on Pregnancy Outcome in Women with Unexplained Recurrent Implantation Failure Undergoing Intracytoplasmic Sperm Injection-Embryo Transfer Cycle: A Randomized Controlled Trial. Gynecol. Endocrinol. 2020, 36, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wei, L.; Li, X.; Qin, A. Effects of Intrauterine Perfusion of Human Chorionic Gonadotropin in Women with Different Implantation Failure Numbers. Am. J. Reprod. Immunol. 2018, 79, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Eljabu, H.; Hopkisson, J.; Raine-Fenning, N.; Quenby, S.; Jayaprakasan, K. Aspirin and Heparin as Adjuvants during IVF Do Not Improve Live Birth Rates in Unexplained Implantation Failure. Reprod. Biomed. Online 2013, 26, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.A.; Eljabu, H.; Hopkisson, J.; Raine-Fenning, N.; Quenby, S.; Jayaprakasan, K.; Altmäe, S.; Mendoza-Tesarik, R.; Mendoza, C.; Mendoza, N.; et al. Effect of Growth Hormone on Uterine Receptivity in Women With Repeated Implantation Failure in an Oocyte Donation Program: A Randomized Controlled Trial. Fertil. Steril. 2018, 100, 818–824. [Google Scholar] [CrossRef]
- Berker, B.; Taşkin, S.; Kahraman, K.; Taşkin, E.A.; Atabekoğlu, C.; Sönmezer, M. The Role of Low-Molecular-Weight Heparin in Recurrent Implantation Failure: A Prospective, Quasi-Randomized, Controlled Study. Fertil. Steril. 2011, 95, 2499–2502. [Google Scholar] [CrossRef]
- Ruiz-Alonso, M.; Blesa, D.; Díaz-Gimeno, P.; Gómez, E.; Fernández-Sánchez, M.; Carranza, F.; Carrera, J.; Vilella, F.; Pellicer, A.; Simón, C. The Endometrial Receptivity Array for Diagnosis and Personalized Embryo Transfer as a Treatment for Patients with Repeated Implantation Failure. Fertil. Steril. 2013, 100, 818–824. [Google Scholar] [CrossRef]
- Siristatidis, C.; Dafopoulos, K.; El-Khayat, W.; Salamalekis, G.; Anifandis, G.; Vrantza, T.; Elsadek, M.; Papantoniou, N. Administration of Prednisolone and Low Molecular Weight Heparin in Patients with Repeated Implantation Failures: A Cohort Study. Gynecol. Endocrinol. 2018, 34, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.; Martínez-Escribano, J.; Roo, E.; Pino, A.; Anitua, E. Plasma Rich in Growth Factor Gel as an Autologous Filler for Facial Volume Restoration. J. Cosmet. Dermatol. 2020, 19, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H.; Orive, G. Clinical, Radiographical, and Histological Outcomes of Plasma Rich in Growth Factors in Extraction Socket: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2015, 19, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Fernández-de-Retana, S.; Alkhraisat, M.H. Platelet Rich Plasma in Oral and Maxillofacial Surgery from the Perspective of Composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Azofra, J.; Anitua, E.; Andía, I.; Padilla, S.; Santisteban, J.; Mujika, I. Plasma Rich in Growth Factors to Treat an Articular Cartilage Avulsion: A Case Report. Med. Sci. Sports Exerc. 2003, 35, 1648–1652. [Google Scholar] [CrossRef] [Green Version]
- Padilla, S.; Sánchez, M.; Vaquerizo, V.; Malanga, G.A.; Fiz, N.; Azofra, J.; Rogers, C.J.; Samitier, G.; Sampson, S.; Seijas, R.; et al. Platelet-Rich Plasma Applications for Achilles Tendon Repair: A Bridge between Biology and Surgery. Int. J. Mol. Sci. 2021, 22, 824. [Google Scholar] [CrossRef]
- Sharara, F.I.; Lelea, L.-L.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. A Narrative Review of Platelet-Rich Plasma (PRP) in Reproductive Medicine. J. Assist. Reprod. Genet. 2021, 38, 1003–1012. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; de Melo Bezerra Cavalcante, C.T.; Sarno, M.; Barini, R. Intrauterine Perfusion Immunotherapies in Recurrent Implantation Failures: Systematic Review. Am. J. Reprod. Immunol. 2020, 83, e13242. [Google Scholar] [CrossRef]
- Ferrari, A.R.; Cortrezzi, S.; Borges, E.J.; Braga, D.; do Carmo Borges de Souza, M.; de Azevedo Antunes, R. Evaluation of the Effects of Platelet-Rich Plasma on Follicular and Endometrial Growth: A Literature Review. JBRA Assist. Reprod. 2021, 25, 601–607. [Google Scholar] [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rouholamin, S.; Rezaeinejad, M.; Maroufizadeh, S.; Sepidarkish, M. Intrauterine Infusion of Autologous Platelet-Rich Plasma in Women Undergoing Assisted Reproduction: A Systematic Review and Meta-Analysis. J. Reprod. Immunol. 2020, 137, 103078. [Google Scholar] [CrossRef]
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of Therapies and Interventions for Repeated Embryo Implantation Failure: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, Y.-B.; Ha, C.-W.; Roh, Y.J.; Park, J.-G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2021, 9, 23259671211011948. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Cui, X.; Li, S.; Ding, P.; Zhang, S.; Zhang, Y. Endometrial Thickness and IVF Cycle Outcomes: A Meta-Analysis. Reprod. Biomed. Online 2020, 40, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasius, A.; Smit, J.; Torrance, H.; Eijkemans, M.; Mol, B.; Opmeer, B.; Broekmans, F. Endometrial Thickness and Pregnancy Rates after IVF: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, V.C.; Santos-Ribeiro, S.; de Munck, N.; Drakopoulos, P.; Polyzos, N.P.; Schutyser, V.; Verheyen, G.; Tournaye, H.; Blockeel, C. Should We Continue to Measure Endometrial Thickness in Modern-Day Medicine? The Effect on Live Birth Rates and Birth Weight. Reprod. Biomed. Online 2018, 36, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Saravelos, S.H.; Wang, Q.; Xu, Y.; Li, T.-C.; Zhou, C. Endometrial Thickness as a Predictor of Pregnancy Outcomes in 10787 Fresh IVF-ICSI Cycles. Reprod. Biomed. Online 2016, 33, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Kirshenbaum, M.; Orvieto, R. Should We Offer In Vitro Fertilization to Couples with Unexplained Recurrent Pregnancy Loss? J. Clin. Med. 2019, 8, 2001. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Qi, J.; Sun, Y. Platelet-Rich Plasma as a Potential New Strategy in the Endometrium Treatment in Assisted Reproductive Technology. Front. Endocrinol. 2021, 12, 707584. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Cazzaniga, N.; Garlappi, R.; Spelta, C.; Pollera, C.; Perrini, C.; Cremonesi, F. Platelet Concentrate in Bovine Reproduction: Effects on in Vitro Embryo Production and after Intrauterine Administration in Repeat Breeder Cows. Reprod. Biol. Endocrinol. 2015, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- de Miguel-Gómez, L.; López-Martínez, S.; Campo, H.; Francés-Herrero, E.; Faus, A.; Díaz, A.; Pellicer, A.; Domínguez, F.; Cervelló, I. Comparison of Different Sources of Platelet-Rich Plasma as Treatment Option for Infertility-Causing Endometrial Pathologies. Fertil. Steril. 2021, 115, 490–500. [Google Scholar] [CrossRef]
- Marini, M.G.; Perrini, C.; Esposti, P.; Corradetti, B.; Bizzaro, D.; Riccaboni, P.; Fantinato, E.; Urbani, G.; Gelati, G.; Cremonesi, F.; et al. Effects of Platelet-Rich Plasma in a Model of Bovine Endometrial Inflammation in Vitro. Reprod. Biol. Endocrinol. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segabinazzi, L.G.; Friso, A.M.; Correal, S.B.; Crespilho, A.M.; Dell’Aqua, J.A.J.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Uterine Clinical Findings, Fertility Rate, Leucocyte Migration, and COX-2 Protein Levels in the Endometrial Tissue of Susceptible Mares Treated with Platelet-Rich Plasma before and after AI. Theriogenology 2017, 104, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.Y.; Myoung, S.M.; Choe, J.M.; Kim, T.; Cheon, Y.P.; Kim, Y.M.; Park, H. Effects of Autologous Platelet-Rich Plasma on Regeneration of Damaged Endometrium in Female Rats. Yonsei Med. J. 2017, 58, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Reghini, M.F.S.; Ramires Neto, C.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; de Paula, F. Dell’Aqua, C.; Bussiere, M.C.C.; Dell’Aqua, J.A.J.; Papa, F.O.; Alvarenga, M.A. Inflammatory Response in Chronic Degenerative Endometritis Mares Treated with Platelet-Rich Plasma. Theriogenology 2016, 86, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Park, M.; Paek, J.Y.; Lee, W.S.; Song, H.; Lyu, S.W. Intrauterine Infusion of Human Platelet-Rich Plasma Improves Endometrial Regeneration and Pregnancy Outcomes in a Murine Model of Asherman’s Syndrome. Front. Physiol. 2020, 11, 105. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. The Role of Platelets in Inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Herter, J.M.; Rossaint, J.; Zarbock, A. Platelets in Inflammation and Immunity. J. Thromb. Haemost. 2014, 12, 1764–1775. [Google Scholar] [CrossRef]
- Assinger, A.; Laky, M.; Schabbauer, G.; Hirschl, A.M.; Buchberger, E.; Binder, B.R.; Volf, I. Efficient Phagocytosis of Periodontopathogens by Neutrophils Requires Plasma Factors, Platelets and TLR2. J. Thromb. Haemost. 2011, 9, 799–809. [Google Scholar] [CrossRef]
- Page, C.; Pitchford, S. Neutrophil and Platelet Complexes and Their Relevance to Neutrophil Recruitment and Activation. Int. Immunopharmacol. 2013, 17, 1176–1184. [Google Scholar] [CrossRef]
- van Gils, J.M.; Zwaginga, J.J.; Hordijk, P.L. Molecular and Functional Interactions among Monocytes, Platelets, and Endothelial Cells and Their Relevance for Cardiovascular Diseases. J. Leukoc. Biol. 2009, 85, 195–204. [Google Scholar] [CrossRef]
- Wetterslev, J.; Jakobsen, J.C.; Gluud, C. Trial Sequential Analysis in Systematic Reviews with Meta-Analysis. BMC Med. Res. Methodol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandulwadkar, S.R.; Naralkar, M.V.; Surana, A.D.; Selvakarthick, M.; Kharat, A.H. Autologous Intrauterine Platelet-Rich Plasma Instillation for Suboptimal Endometrium in Frozen Embryo Transfer Cycles: A Pilot Study. J. Hum. Reprod. Sci. 2017, 10, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Zadehmodarres, S.; Salehpour, S.; Saharkhiz, N.; Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. JBRA Assist. Reprod. 2017, 21, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.V.L.; Fanton, V.; Sosa, D.; Criado Scholz, E.; Lotti, J.; Aragona, S.E.; Lotti, T. Use of platelet rich plasma in human infertility. J. Biol. Regul. Homeost. Agents 2017, 31, 179–182. [Google Scholar]
- Molina, A.; Sánchez, J.; Sánchez, W.; Vielma, V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist. Reprod. 2018, 22, 42–48. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.E.; Koo, H.S.; Kwon, H.; Choi, D.H.; Kim, J.H. Effect of Autologous Platelet-Rich Plasma Treatment on Refractory Thin Endometrium During the Frozen Embryo Transfer Cycle: A Pilot Study. Front. Endocrinol. 2019, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Li, J.; Wei, L.N.; Pang, J.; Chen, J.; Liang, X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine 2019, 98, e14062. [Google Scholar] [CrossRef]
- Frantz, N.; Ferreira, M.; Kulmann, M.I.; Frantz, G.; Bos-Mikich, A.; Oliveira, R. Platelet-Rich plasma as an effective alternative approach for improving endometrial receptivity—A clinical retrospective study. J. Bras. Reprod. Assist 2020, 24, 442–446. [Google Scholar] [CrossRef]
- Tehraninejad, E.S.; Kashani, N.G.; Hosseini, A.; Tarafdari, A. Autologous platelet-rich plasma infusion does not improve pregnancy outcomes in frozen embryo transfer cycles in women with history of repeated implantation failure without thin endometrium. J. Obstet. Gynaecol. Res. 2021, 47, 147–151. [Google Scholar] [CrossRef]
- Godha, Z.; Nayar, K.D.; Gupta, S.; Singh, M.; Gupta, M.; Kant, G.; Nayar, K. Randomized controlled trial of intrauterine infusion of autologous platelet rich plasma (PRP) versus granulocyte colony stimulating factor (G-CSF) in thin endometrium in frozen embryo transfer. In Human Reproduction; Oxford University Press: Oxford, UK, 2019; Volume 34, p. 277. [Google Scholar]
| Study | Sample Size | Age (Years) | Cause of Failed Embryo Transfer | PRP Obtention Protocol | Anticoagulant | Leukocytes | Platelet Concentration | Intervention (Volume of PRP) | PRP Activation | Application Method | Embryo Transfer (Time after PRP) | Second PRP Instillation | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PRP | Control | PRP | |||||||||||
| Allahveisi et al., 2020 | 25 | 25 | 33.8 ± 0.54 | 33 ± 0.9 | RIF | 1700 g 12 min; 3300 g 7 min | ACD-A | Y | 411 × 103–1067 × 103/uL | 0.5 mL | NR | Intrauterine infusion | 48 h | No |
| Bakhsh et al., 2021 | 50 | 50 | 32.7 | 35 | RIF (≥4) | 1400 rpm 10 min; 3500 rpm 6 min | ACD | N | 4–5 times | 0.5 mL | NR | Intrauterine infusion under ultrasound guidance | 48 h | NR |
| Eftekhar et al., 2018 | 43 | 40 | 32.4 ± 2.63 | 31.98 ± 2.26 | Poor endometrial response (ET < 7 mm) to HRT | 1600 g 10 min; 3500 g 5 min | ACD-A | Y | 4–5 times, 2000 lymphocyte | 0.5–1 mL | NR | Intrauterine infusion | When ET ≥ 7 mm | Yes (if ET < 7 mm) |
| Ershadi et al., 2022 | 45 | 40 | 31.2 ± 4.8 | 31.3 ± 4.3 | RIF | 1200 rpm 12 min; 3300 rpm 7 min | Citrate acid | Y | 4–5 times | 0.5 mL | NR | Intrauterine infusion under ultrasound guidance | 48 h | NR |
| Ghasemi et al., 2020 | 85 | NR | NR | RIF | NR | NR | NR | NR | NR | NR | Intrauterine infusion | 48 h | NR | |
| Nazari et al., 2020 | 48 | 49 | 34.95 ± 4.23 | 35.73 ± 3.49 | RIF (≥3) | 1200 rpm 10 min; 3300 rpm 5 min | ACD-A | Y | 4–5 times | 0.5 mL | Not clear | Intrauterine infusion under ultrasound guidance | 48 h | No |
| Nazari et al., 2019 | 30 | 30 | 32.33 ± 4.79 | 33.93 ± 2.76 | Thin endometrium (ET < 7 mm) | 1200 rpm 12 min; 3300 rpm 7 min | ACD-A | Y | NR | 0.5 mL | NR | Intrauterine infusion under ultrasound guidance | 48 h | Yes (if ET < 7 mm) |
| Nazari et al. 2021 | 197 | 196 | 33.61 ± 4.06 | 34.11 ± 3.75 | RIF (≥3) | 1200 rpm 12 min; 3300 rpm 7 min | NR | Y | 4–5 times | 0.5 mL | NR | Intrauterine infusion | 48 h | No |
| Nazari et al. 2022 | 20 | 20 | 34.75 ± 4.57 | 35. 70 ± 5.10 | RPL | 1200 rpm 12 min; 3300 rpm 7 min | ACD-A | Y | 4–5 times | 0.5 mL | NR | Intrauterine infusion under ultrasound guidance | 48 h | NR |
| Obidniak et al., 2017 | 45 | 45 | 28–39 | RIF | Plasmolifting technology | NR | NR | NR | 2 mL | NR | Intrauterine infusion | NR | NR | |
| Zamaniyan et al., 2020 | 43 | 55 | 33.13 ± 5.00 | 33.88 ± 6.32 | RIF (≥3) | 1200 rpm 12 min; 3300 rpm 7 min | ACD-A | Y | 4–7 times | 0.5 mL | NR | Intrauterine infusion | 48 h | No |
| Zargar et al., 2021 | 40 | 40 | 32.82 ± 5.18 | 34.15 ± 5.14 | RIF (≥2) | 12,000 g 10 min; 12,000 g 10 min | ACD | N | NR | 1.5 mL | NR | Intrauterine infusion | 48 h | Yes (if ET < 7 mm) |
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | PRP | Conventional Treatment | Relative (95% CI) | Absolute (95% CI) | ||
| Implantation rate | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 48/120 (40.0%) | 26/108 (24.1%) | RR 1.57 (1.07 to 2.30) | 137 more per 1000 (from 17 more to 313 more) | ⨁⨁◯◯ Low | IMPORTANT |
| Biochemical pregnancy rate | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 163/340 (47.9%) | 84/333 (25.2%) | RR 1.91 (1.54 to 2.37) | 230 more per 1000 (from 136 more to 346 more) | ⨁⨁⨁⨁ High | CRITICAL |
| Clinical pregnancy rate | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Not serious | Strong association | 196/450 (43.6%) | 88/443 (19.9%) | RR 2.18 (1.76 to 2.70) | 234 more per 1000 (from 51 more to 338 more) | ⨁⨁⨁⨁ High | CRITICAL |
| Live birth rate | ||||||||||||
| 3 | Randomized trials | Not serious | Serious b | Not serious | Very serious c | Strong association | 89/261 (34.1%) | 18/262 (6.9%) | RR 3.36 (0.84 to 13.45) | 162 more per 1000 (from 11 more to 855 more) | ⨁⨁◯◯ Low | CRITICAL |
| Miscarriage rate | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Very serious d | None | 7/135 (5.2%) | 4/128 (3.1%) | RR 1.71 (0.54 to 5.48) | 22 more per 1000 (from 14 fewer to 140 more) | ⨁⨁◯◯ Low | CRITICAL |
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | PRP | Conventional Treatment | Relative (95% CI) | Absolute (95% CI) | ||
| Biochemical pregnancy rate | ||||||||||||
| 1 | Randomized trials | Serious a | Not serious | Not serious | Very serious b | None | 26/70 (37.1%) | 10/73 (13.7%) | RR 1.97 (1.57 to 2.48) | 133 more per 1000 (from 78 more to 203 more) | ⨁⨁◯◯ Low | CRITICAL |
| Clinical pregnancy rate | ||||||||||||
| 1 | Randomized trials | Serious a | Not serious | Not serious | Very serious b | Strong association | 23/70 (32.9%) | 7/73 (9.6%) | RR 3.46 (1.58 to 7.59) | 236 more per 1000 (from 56 more to 632 more) | ⨁⨁◯◯ Low | CRITICAL |
| Live birth rate | ||||||||||||
| 1 | Randomized trials | Serious a | Not serious | Not serious | Very serious b | None | 11/40 (27.5%) | 6/43 (14.0%) | RR 1.97 (0.80 to 4.83) | 135 more per 1000 (from 28 fewer to 534 more) | ⨁◯◯◯ Very low | CRITICAL |
| Miscarriage rate | ||||||||||||
| 1 | Randomized trials | Serious a | Not serious | Not serious | Very serious c | None | 3/40 (7.5%) | 2/43 (4.7%) | RR 1.61 (0.28 to 9.16) | 28 more per 1000 (from 33 fewer to 380 more) | ⨁◯◯◯ Very low | CRITICAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Allende, M.; de la Fuente, M.; Del Fabbro, M.; Alkhraisat, M.H. Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Bioengineering 2023, 10, 303. https://doi.org/10.3390/bioengineering10030303
Anitua E, Allende M, de la Fuente M, Del Fabbro M, Alkhraisat MH. Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Bioengineering. 2023; 10(3):303. https://doi.org/10.3390/bioengineering10030303
Chicago/Turabian StyleAnitua, Eduardo, Mikel Allende, María de la Fuente, Massimo Del Fabbro, and Mohammad Hamdan Alkhraisat. 2023. "Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis" Bioengineering 10, no. 3: 303. https://doi.org/10.3390/bioengineering10030303
APA StyleAnitua, E., Allende, M., de la Fuente, M., Del Fabbro, M., & Alkhraisat, M. H. (2023). Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Bioengineering, 10(3), 303. https://doi.org/10.3390/bioengineering10030303








