Rhus coriaria L. Fruit Extract Prevents UV-A-Induced Genotoxicity and Oxidative Injury in Human Microvascular Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Rhus coriaria L. Extract Preparation and Characterization
2.2. Cell Culture
2.3. Rhus coriaria L. Extract Treatment
2.4. UV-A Radiation Treatment
2.5. ROS Quantification and Lowry Protein Assay
2.6. Quantification of the Total Antioxidant Activity
2.7. Alkaline Comet Assay
2.8. Modified Comet Assay
2.9. Quantification of γ-H2AX and Micronuclei Percentage
2.10. Evaluation of Apoptosis
2.11. Cell Cycle Analysis
2.12. Statistical Analysis
3. Results
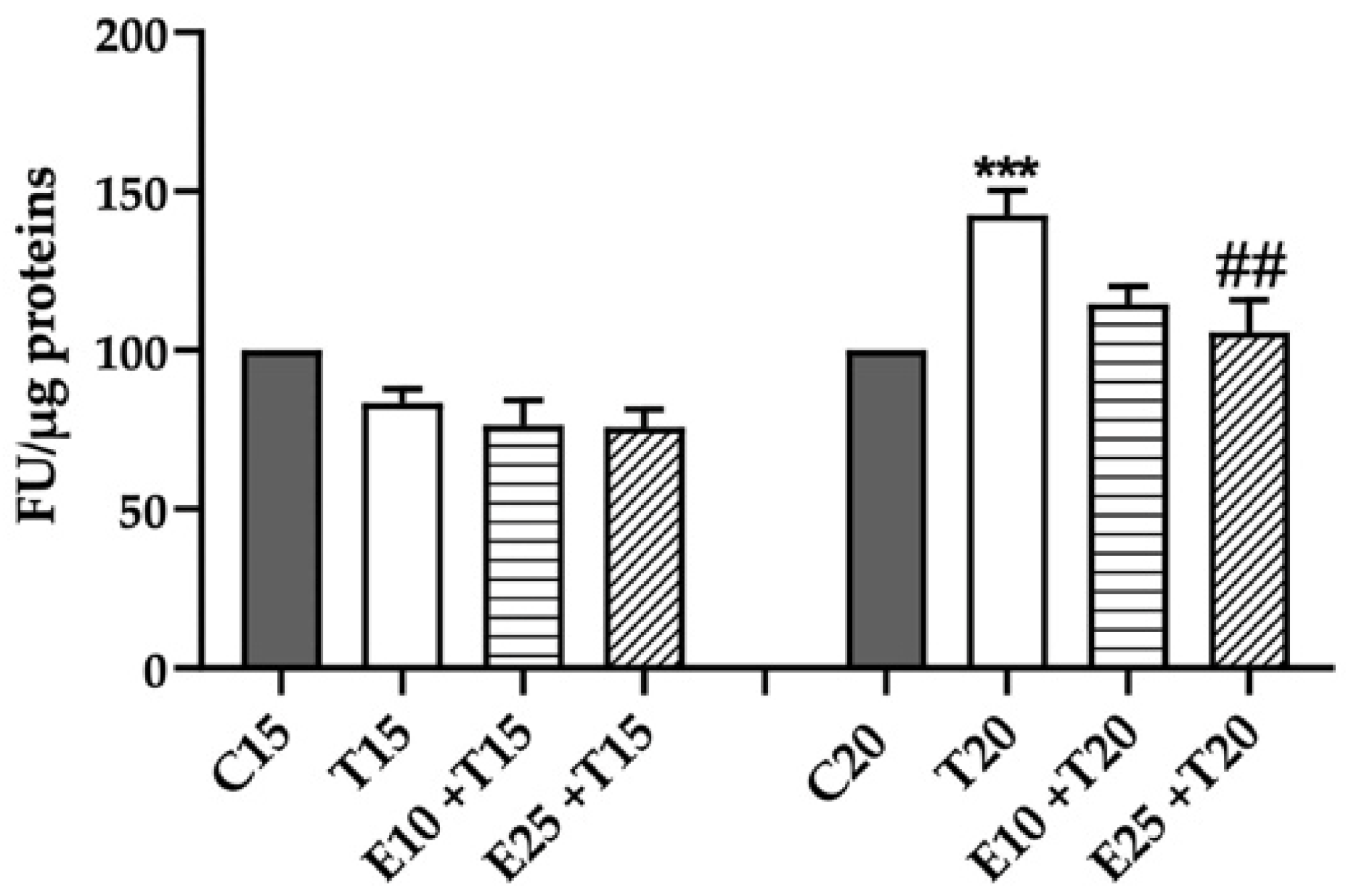
3.1. Rhus coriaria L. Extract Decreases UV-A-induced ROS Production
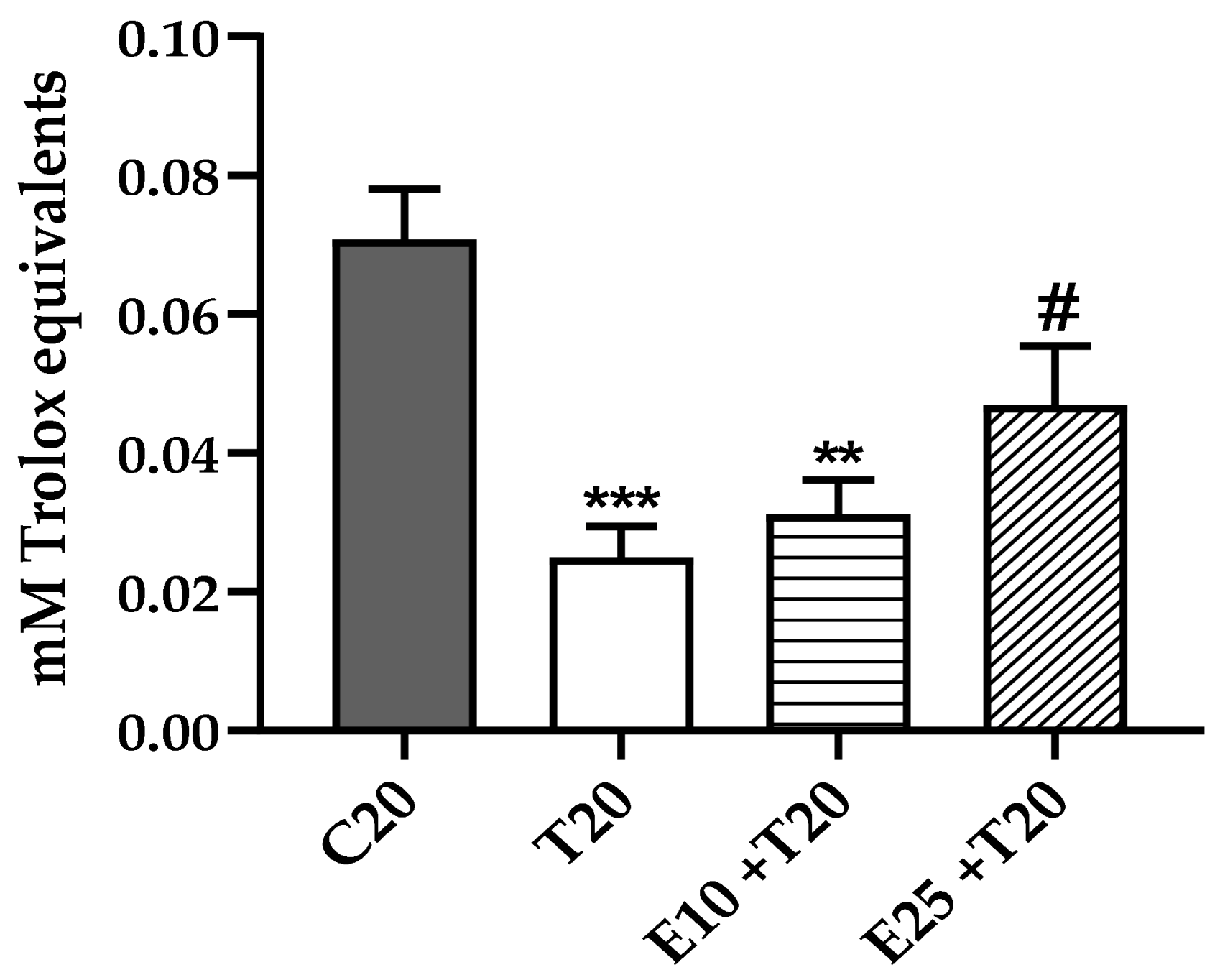
3.2. Total Antioxidant Activity Reduction after UV-A Exposure in the presence of mERC
3.3. Genoprotective Action of mERC Against UV-A Damage
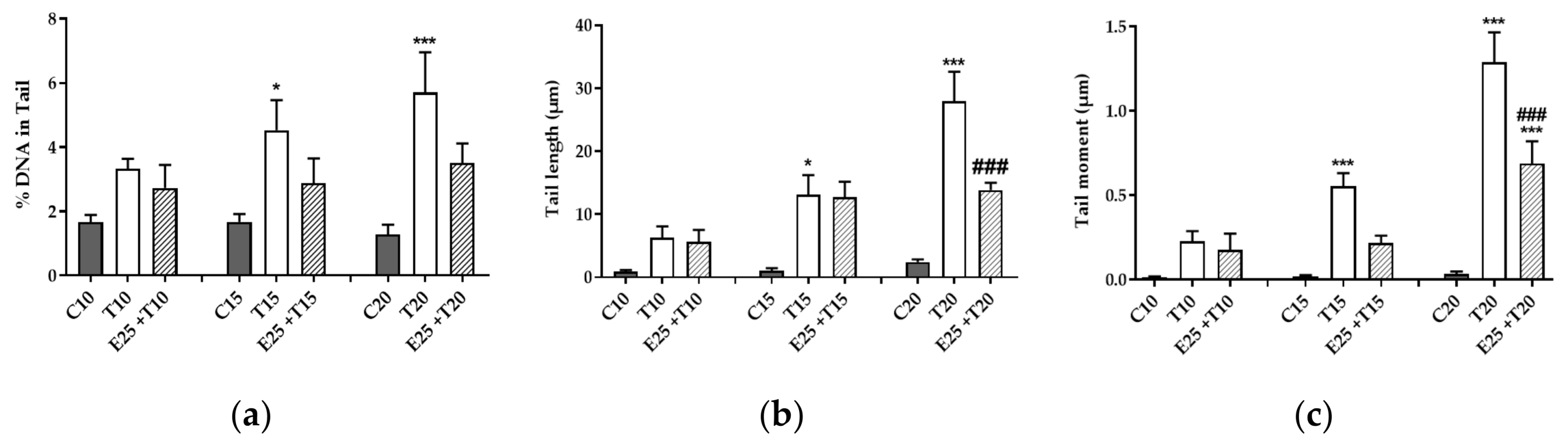
3.3.1. Evaluation of DNA Damage Through Alkaline Comet Assay
3.3.2. Time Course of Genotoxic Damage
3.3.3. DSB Quantification through γ-H2AX Detection
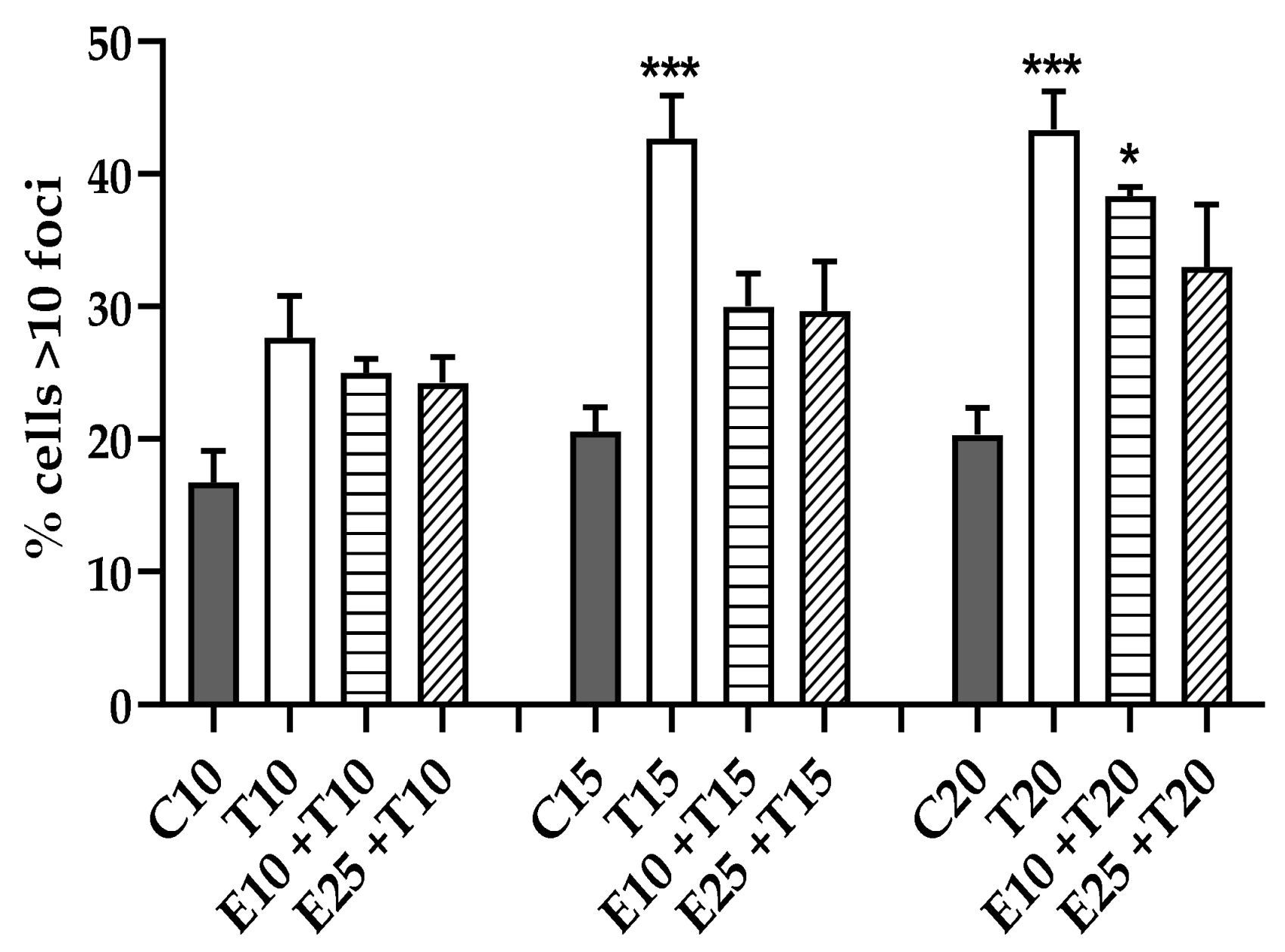
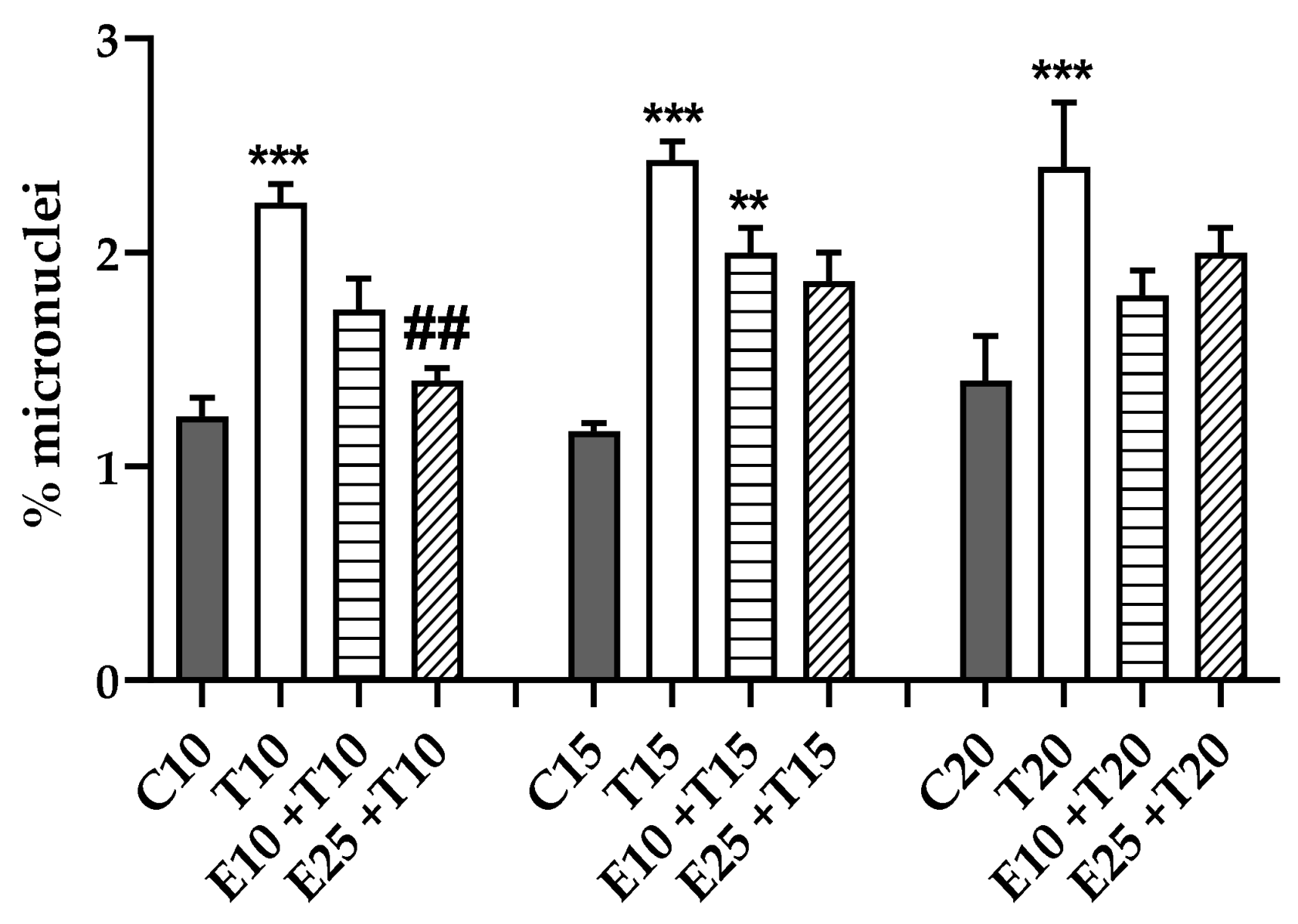
3.3.4. Chromosomal Mis-segregation as Micronuclei Formation
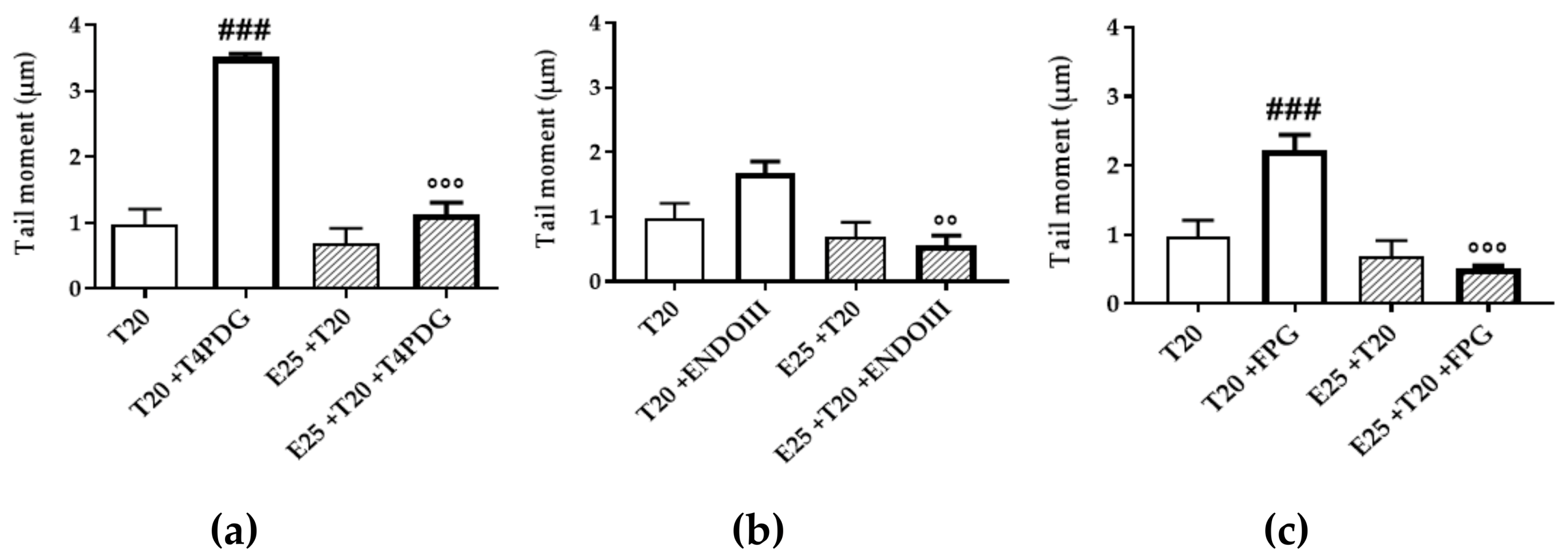
3.4. UV-A Damage Characterization
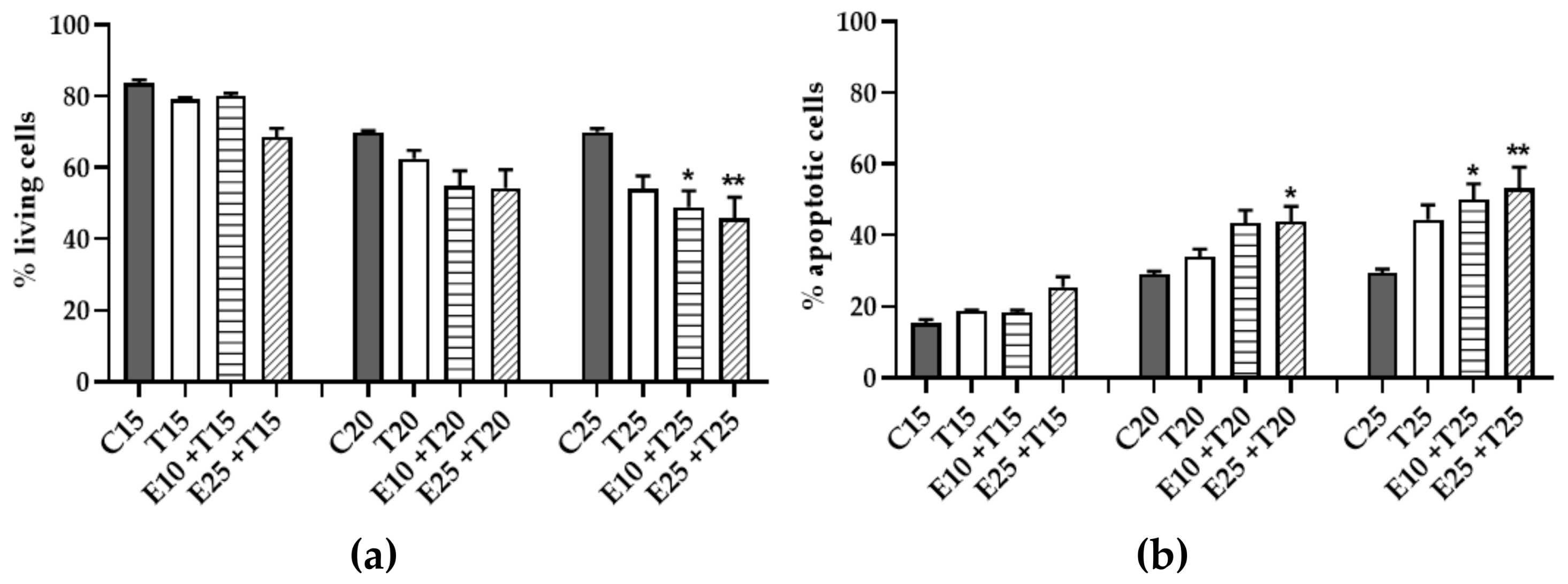
3.5. Assessment of Cytotoxicity
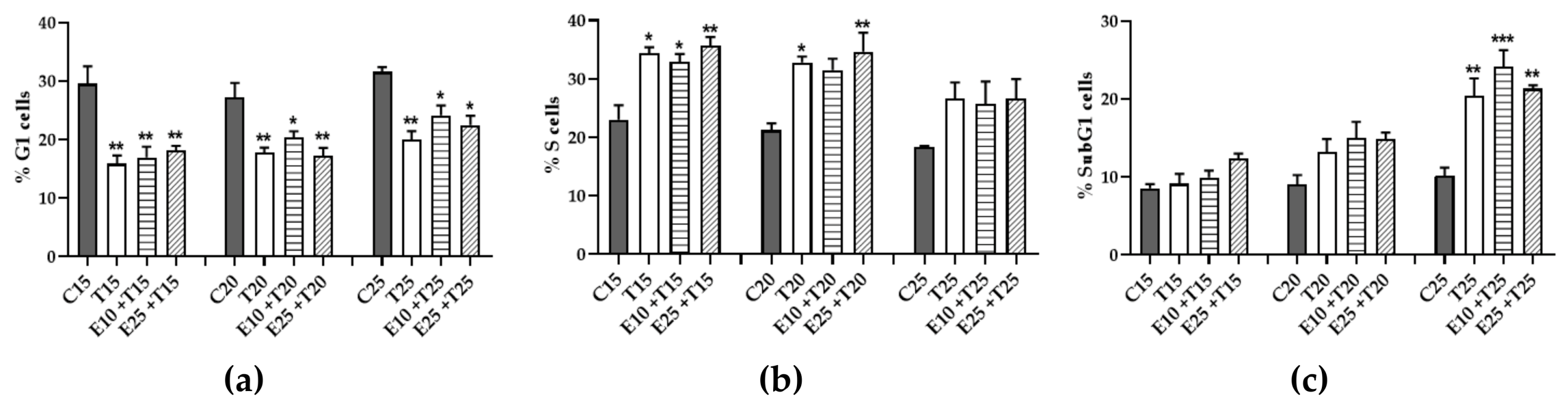
3.6. Role of mERC Pre-treatment and UV-A Exposure on Cell Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tapiero, H.; Tew, K.D.; Nguyen Ba, G.; Mathé, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Saati, S. An overview of phytochemical and pharmacological properties of Rhus coriaria L. Res. J. Pharmacogn. 2014, 1, 47–54. [Google Scholar]
- Behnammanesh, G.; Khalilpour, S.; Majid, A.S.A.; Majid, A.M.S.A. Pharmacological actions and potential neuroprotective effects of Rhus coriaria L. In addition, Echium amoenum L.: A brief review. WebmedCentral Pharmacol. 2015, 6, WMC005008. [Google Scholar]
- Abu-reidah, I.M.; Jamous, R.M.; Ali-shtayeh, M.S. Phytochemistry, Pharmacological Properties and Industrial Applications of Rhus coriaria L.(Sumac). Jordan J. Biol. Sci. 2014, 7, 233–244. [Google Scholar] [CrossRef]
- Kossah, R.; Nsabimana, C.; Zhang, H.; Chen, W. Optimization of extraction of polyphenols from syrian sumac (Rhus Coriaria L.) and chinese sumac (Rhus Typhina L.) fruit. Res. J. Phytochem. 2010, 4, 146–153. [Google Scholar] [CrossRef]
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell’Agli, M. In vitro evidence of the traditional use of Rhus coriaria L. fruit against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829. [Google Scholar] [CrossRef]
- Shabbir, A. Rhus coriaria Linn, a plant of medicinal, nutritional and industrial importance: A review. J. Anim. Plant Sci. 2012, 22, 505–512. [Google Scholar]
- Doğan, A.; Çelik, İ. Healing effects of sumac (Rhus coriaria) in streptozotocin-induced diabetic rats. Pharm. Biol. 2016, 54, 2092–2102. [Google Scholar] [CrossRef]
- Shafiei, M.; Nobakht, M.; Moazzam, A.A. Lipid-lowering effect of Rhus coriaria L. (sumac) fruit extract in hypercholesterolemic rats. Pharmazie 2011, 66, 988–992. [Google Scholar]
- Asgary, S.; Salehizadeh, L.; Keshvari, M.; Taheri, M.; Spence, N.D.; Farvid, M.S.; Rafieian-Kopaei, M.; Sarrafzadegan, N. Potential cardioprotective effects of sumac capsule in patients with hyperlipidemia: A triple-blind randomized, placebo-controlled crossover trial. J. Am. Coll. Nutr. 2018, 37, 286–292. [Google Scholar] [CrossRef]
- Sakhr, K.; El Khatib, S. Physiochemical properties and medicinal, nutritional and industrial applications of Lebanese Sumac (Syrian Sumac-Rhus coriaria): A review. Heliyon 2020, 6, e03207. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Tayman, C.; Cakir, U.; Koyuncu, I.; Taskin Turkmenoglu, T.; Cakir, E. Sumac (Rhus coriaria) for the prevention and treatment of necrotizing enterocolitis. J. Food Biochem. 2019, 43, e13068. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, S.; Behnammanesh, G.; Suede, F.; Ezzat, M.; Muniandy, J.; Tabana, Y.; Ahamed, M.; Tamayol, A.; Majid, A.; Sangiovanni, E.; et al. Neuroprotective and anti-inflammatory effects of rhus coriaria extract in a mouse model of ischemic optic neuropathy. Biomedicines 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, S.; Latifi, S.; Behnammanesh, G.; Majid, A.M.S.A.; Majid, A.S.A.; Tamayol, A. Ischemic optic neuropathy as a model of neurodegenerative disorder: A review of pathogenic mechanism of axonal degeneration and the role of neuroprotection. J. Neurol. Sci. 2017, 375, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, S.; Behnammanesh, G.; Abdul Majid, A.M.S.; Tamayol, A.; Abdul Majid, A.S. Assessment of neuroprotective properties of Rhus coriaria L. ethanol extract in an in vitro model of retinal degeneration. J. Herb. Med. 2017, 10, 45–52. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ferk, F.; Simić, T.; Brantner, A.; Dušinská, M.; Kundi, M.; Hoelzl, C.; Nersesyan, A.; Knasmüller, S. DNA-protective effects of sumach (Rhus coriaria L.), a common spice: Results of human and animal studies. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2009, 661, 10–17. [Google Scholar] [CrossRef]
- Heinrich, U.; Moore, C.E.; De Spirt, S.; Tronnier, H.; Stahl, W. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Afaq, F.; K Katiyar, S. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini-Rev. Med. Chem. 2012, 11, 1200–1215. [Google Scholar]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Metral, E.; Rachidi, W.; Damour, O.; Demarne, F.; Bechetoille, N. Long-term genoprotection effect of sechium edule fruit extract against UVA irradiation in keratinocytes. Photochem. Photobiol. 2018, 94, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection: Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013, 69, 867-e1. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, 171–178. [Google Scholar] [CrossRef]
- Cauchard, J.H.; Robinet, A.; Poitevin, S.; Bobichon, H.; Maziere, J.C.; Bellon, G.; Hornebeck, W. UVA-mediated down-regulation of MMP-2 and MT1-MMP coincides with impaired angiogenic phenotype of human dermal endothelial cells. Biochem. Biophys. Res. Commun. 2006, 345, 681–687. [Google Scholar] [CrossRef]
- Seite, S.; Zucchi, H.; Septier, D.; Igondjo-Tchen, S.; Senni, K.; Godeau, G. Elastin changes during chronological and photo-ageing: The important role of lysozyme. J. Eur. Acad. Dermatol. Venereol. 2006, 30, 980–987. [Google Scholar] [CrossRef]
- Hern, S.; Mortimer, P.S. Visualization of dermal blood vessels-capillaroscopy. Clin. Exp. Dermatol. 1999, 24, 473–478. [Google Scholar] [CrossRef]
- Zheng, P.; Kligman, L.H. UVA-induced ultrastructural changes in hairless mouse skin: A comparison to UVB-induced damage. J. Investig. Dermatol. 1993, 100, 194–199. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Magnavacca, A.; Perego, F.; Fumagalli, M.; Sangiovanni, E.; Prato, M.; Dell’Agli, M.; Basilico, N. Effect of hypoxia on gene expression in cell populations involved in wound healing. BioMed Res. Int. 2019, 2019, 2626374. [Google Scholar] [CrossRef]
- Heckmann, M.; Eberlein-Konig, B.; Wollenberg, A.; Przbylla, B.; Plewig, G. Ultraviolet-A radiation induces adhesion molecule expression on human dermal microvascular endothelial cells. Br. J. Dermatol. 1994, 131, 311–318. [Google Scholar] [CrossRef]
- Meroni, E.; Criscuoli, F.; Casiraghi, M.C.; Erba, D.; Papini, N.; Massaccesi, L.; Basilico, N. Metabolic responses in endothelial cells following exposure to ketone bodies. Nutrients 2018, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Yohn, J.J.; Lyons, M.B.; Norris, D.A. Cultured human melanocytes from black and white donors have different sunlight and ultraviolet a radiation sensitivities. J. Investig. Dermatol. 1992, 99, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, L.; Randall, R. Lowry protein assay. J. Biol. Chem. 1951, 193, 365–375. [Google Scholar]
- Muthusamy, G.; Balupillai, A.; Govindasamy, K.; Ramasamy, K.; Ponniresan, V.; Malla, I.; Nagarajan, R. Modified comet assays for the detection of cyclobutane pyrimidine dimers and oxidative base damages. J. Radiat. Cancer Res. 2017, 8, 82–86. [Google Scholar]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Kosar, M.; Bozan, B.; Temelli, F.; Baser, K.H.C. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007, 103, 952–959. [Google Scholar] [CrossRef]
- Pourahmad, J.; Eskandari, M.R.; Shakibaei, R.; Kamalinejad, M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food Chem. Toxicol. 2010, 48, 854–858. [Google Scholar] [CrossRef]
- Segerbäck, D.; Strozyk, M.; Snellman, E.; Hemminki, K. Repair of UV dimers in skin DNA of patients with basal cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2388–2392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shokrollahi Barough, M.; Hasanzadeh, H.; Barati, M.; Pak, F.; Kokhaei, P.; Rezaei-Tavirani, M. Apoptosis/necrosis induction by ultraviolet, in ER positive and ER negative breast cancer cell lines. Int. J. Cancer Manag. 2015, 8, 41–93. [Google Scholar] [CrossRef] [PubMed]
- Wischermann, K.; Popp, S.; Moshir, S.; Scharfetter-Kochanek, K.; Wlaschek, M.; De Gruijl, F.; Hartschuh, W.; Greinert, R.; Volkmer, B.; Faust, A.; et al. UVA radiation causes DNA strand breaks, chromosomal aberrations and tumorigenic transformation in HaCaT skin keratinocytes. Oncogene 2008, 27, 4269–4280. [Google Scholar] [CrossRef] [PubMed]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1016–1841. [Google Scholar] [CrossRef] [PubMed]
- Mouret, S.; Philippe, C.; Gracia-Chantegrel, J.; Banyasz, A.; Karpati, S.; Markovitsi, D.; Douki, T. UVA-induced cyclobutane pyrimidine dimers in DNA: A direct photochemical mechanism? Org. Biomol. Chem. 2010, 8, 1706–1711. [Google Scholar] [CrossRef]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef]
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E inhibits the UVAI induction of “light” and “dark” cyclobutane pyrimidine dimers, and oxidatively generated DNA damage, in keratinocytes. Sci. Rep. 2018, 8, 423. [Google Scholar] [CrossRef]
- Gong, C.; Yang, Z.; Zhang, L.; Wang, Y.; Gong, W.; Liu, Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. OncoTargets. Ther. 2018, 11, 17–27. [Google Scholar] [CrossRef]
- Lo, H.L.; Nakajima, S.; Ma, L.; Walter, B.; Yasui, A.; Ethell, D.; Owen, L.B. Differential biologic effects of CPD and 6-4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer 2005, 5, 135. [Google Scholar] [CrossRef]
- Timocin, T.; Arslan, M.; Basri Ila, H. Evaluation of in vitro and in vivo genotoxic and antigenotoxic effects of Rhus coriaria. Drug Chem. Toxicol. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 2003, 147, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Olchowik, E.; Sciepuk, A.; Mavlyanov, S.; Abdullajanova, N.; Zamaraeva, M. Antioxidant capacities of polyphenols from Sumac (Rhus typhina L.) leaves in protection of erythrocytes against oxidative damage. Biomed. Prev. Nutr. 2012, 2, 99–105. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in balb/c mice. Drug Des. Dev. Ther. 2015, 9, 3923–3934. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Choi, D.R.; Jeong, J.H.; Yu, K.S.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Na, C.S.; Na, D.S.; Hwang, W.M.; Han, S.Y. Extract of rhus verniciflua stokes protects against renal ischemia-reperfusion injury by enhancing nrf2-mediated induction of antioxidant enzymes. Exp. Ther. Med. 2018, 15, 3827–3835. [Google Scholar] [CrossRef]
- Douki, T.; Sage, E. Dewar valence isomers, the third type of environmentally relevant DNA photoproducts induced by solar radiation. Photochem. Photobiol. Sci. 2016, 15, 24–30. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Pouget, J.P.; Douki, T.; Richard, M.J.; Cadet, J. DNA damage induced in cells by γ and UVA radiation as measured by HPLC/GC-MS and HPLC-EC and comet assay. Chem. Res. Toxicol. 2000, 13, 541–549. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; Mckenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Reboredo-Rodriguez, P.; Cianciosi, D.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M.; Giampieri, F. Strawberry-based cosmetic formulations protect human dermal fibroblasts against UVA-induced damage. Nutrients 2017, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.F.Z.; Kaina, B.; Meneghini, R.; Menck, C.F.M. How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat. Res.-Rev. Mutat. Res. 2009, 681, 197–208. [Google Scholar] [CrossRef]
- D’Angleo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar]
- Armentano, M.F.; Bisaccia, F.; Miglionico, R.; Russo, D.; Nolfi, N.; Carmosino, M.; Andrade, P.B.; Valentão, P.; Diop, M.S.; Milella, L. Antioxidant and proapoptotic activities of sclerocarya birrea [(A. Rich.) Hochst.] methanolic root extract on the hepatocellular carcinoma cell line HepG2. BioMed Res. Int. 2015, 2015, 561589. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.J.; Martin, J.R.; Wulff, B.C.; Stoner, G.D.; Tober, K.L.; Oberyszyn, T.M.; Kusewitt, D.F.; Van Buskirk, A.M. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev. Res. 2009, 2, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.; Syed, D.N.; Chamcheu, J.C.; Vilela, F.M.P.; Pérez, A.M.; Vaillant, F.; Rojas, M.; Mukhtar, H. Protective effect of tropical highland blackberry juice (Rubus adenotrichos Schltdl.) against UVB-mediated damage in human epidermal keratinocytes and in a reconstituted skin equivalent model. Photochem. Photobiol. 2013, 89, 1199–1207. [Google Scholar] [CrossRef]
- Marabini, L.; Melzi, G.; Lolli, F.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E.; Marinovich, M. Effects of Vitis vinifera L. leaves extract on UV radiation damage in human keratinocytes (HaCaT). J. Photochem. Photobiol. B Biol. 2020, 204, 111810. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, R.; Liu, J.; Meng, D.; Zhou, Z.; Zhang, Y.; Blanchard, C. Fabrication, structure, and function evaluation of the ferritin-based nano-carrier for food bioactive compounds. Food Chem. 2019, 299, 125097. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozza, E.; Melzi, G.; Marabini, L.; Marinovich, M.; Piazza, S.; Khalilpour, S.; Dell’Agli, M.; Sangiovanni, E. Rhus coriaria L. Fruit Extract Prevents UV-A-Induced Genotoxicity and Oxidative Injury in Human Microvascular Endothelial Cells. Antioxidants 2020, 9, 292. https://doi.org/10.3390/antiox9040292
Nozza E, Melzi G, Marabini L, Marinovich M, Piazza S, Khalilpour S, Dell’Agli M, Sangiovanni E. Rhus coriaria L. Fruit Extract Prevents UV-A-Induced Genotoxicity and Oxidative Injury in Human Microvascular Endothelial Cells. Antioxidants. 2020; 9(4):292. https://doi.org/10.3390/antiox9040292
Chicago/Turabian StyleNozza, Emma, Gloria Melzi, Laura Marabini, Marina Marinovich, Stefano Piazza, Saba Khalilpour, Mario Dell’Agli, and Enrico Sangiovanni. 2020. "Rhus coriaria L. Fruit Extract Prevents UV-A-Induced Genotoxicity and Oxidative Injury in Human Microvascular Endothelial Cells" Antioxidants 9, no. 4: 292. https://doi.org/10.3390/antiox9040292
APA StyleNozza, E., Melzi, G., Marabini, L., Marinovich, M., Piazza, S., Khalilpour, S., Dell’Agli, M., & Sangiovanni, E. (2020). Rhus coriaria L. Fruit Extract Prevents UV-A-Induced Genotoxicity and Oxidative Injury in Human Microvascular Endothelial Cells. Antioxidants, 9(4), 292. https://doi.org/10.3390/antiox9040292






