Epidemiology, Outcomes and Tolerability of Protracted Treatment of Nontuberculous Mycobacterial Infections at a Community Teaching Hospital in the Southeastern United States
Abstract
1. Introduction
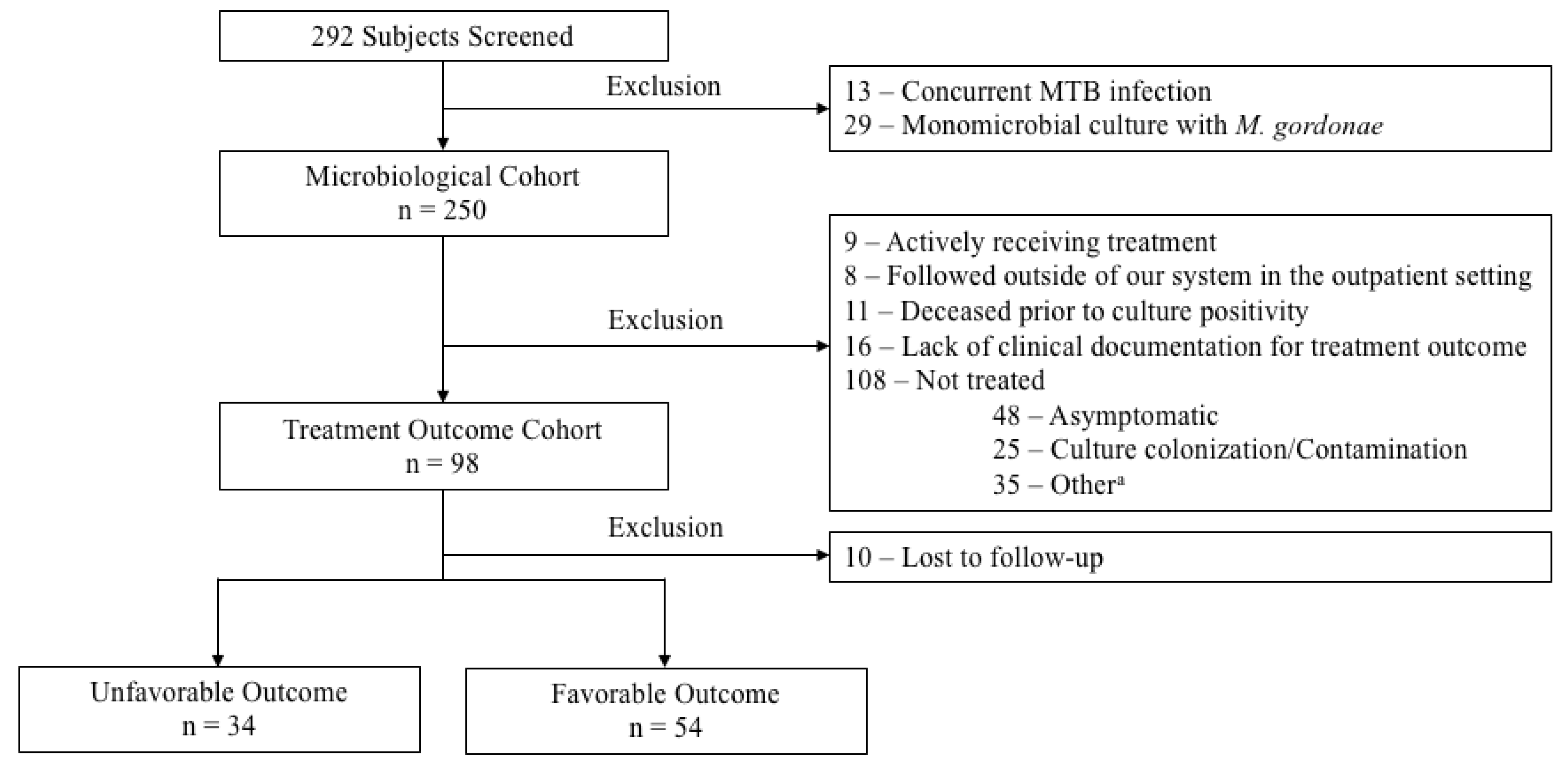
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falkinham, J.O., III. Environmental sources of nontuberculous mycobacteria. Clin. Chest Med. 2015, 36, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, C.R., Jr. Epidemiology of disease caused by nontuberculous mycobacteria. Semin. Respir. Infect. 1996, 11, 244. [Google Scholar] [PubMed]
- American Thoracic Society. Mycobacterioses and the acquired immunodeficiency syndrome. Joint Position Paper of the American Thoracic Society and the Centers for Disease Control. Am. Rev. Respir. Dis. 1987, 136, 492. [Google Scholar] [CrossRef]
- Wu, U.-I.; Holland, S.M. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect. Dis. 2015, 15, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Greenberg, D.E.; Ehrmantraut, M.E.; Guide, S.V.; Ding, L.; Shea, Y.; Brown, M.R.; Chernick, M.; Steagall, W.K.; Glasgow, C.G.; et al. Pulmonary nontuberculous mycobacterial disease: Prospective study of a distinct preexisting syndrome. Am. J. Respir. Crit. Care Med. 2008, 178, 1066–1074. [Google Scholar] [CrossRef]
- Iseman, M.D.; Buschman, D.L.; Ackerson, L.M. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am. Rev. Respir. Dis. 1991, 144, 914–916. [Google Scholar] [CrossRef]
- Gonzalez-Santiago, T.M.; Drage, L.A. Nontuberculous Mycobacteria Skin and Soft Tissue Infections. Dermatol. Clin. 2015, 33, 563–577. [Google Scholar] [CrossRef]
- Jones, M.M.; Winthrop, K.L.; Nelson, S.D.; Duvall, S.L.; Patterson, O.V.; Nechodom, K.E.; Findley, K.E.; Radonovich, L.J., Jr.; Samore, M.H.; Fennelly, K.P. Epidemiology of nontuberculous mycobacterial infections in the U.S. Veterans Health Administration. PLoS ONE 2018, 13, e0197976. [Google Scholar] [CrossRef]
- Abate, G.; Stapleton, J.T.; Rouphael, N.; Creech, B.; Stout, J.E.; El Sahly, H.M.; Jackson, L.; Leyva, F.J.; Tomashek, K.M.; Tibbals, M.; et al. Variability in the Management of Adults with Pulmonary Nontuberculous Mycobacterial Disease. Clin. Infect. Dis. 2021, 72, 1127–1137. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Ohama, Y.; Okazaki, M.; Nukui, Y.; Moriya, K. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect. Dis. 2017, 17, 197. [Google Scholar] [CrossRef]
- Cho, E.H.; Huh, H.J.; Song, D.J.; Lee, S.H.; Kim, C.K.; Shin, S.Y.; Ki, C.S.; Jhun, B.W.; Moon, S.M.; Kwon, O.J.; et al. Drug susceptibility patterns of Mycobacterium abscessus and Mycobacterium massiliense isolated from respiratory specimens. Diagn. Microbiol. Infect. Dis. 2019, 93, 107–111. [Google Scholar] [CrossRef]
- Tang, S.S.; Lye, D.C.; Jureen, R.; Sng, L.H.; Hsu, L.Y. Rapidly growing mycobacteria in Singapore, 2006–2011. Clin. Microbiol. Infect. 2015, 21, 236–241. [Google Scholar] [CrossRef]
- Mata-Jardín, D.; Angulo, A.; Rodríguez, M.; Fernández-Figueiras, S.; de Waard, J.H. Drug susceptibility patterns of rapidly growing mycobacteria isolated from skin and soft tissue infections in Venezuela. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 433–441. [Google Scholar] [CrossRef]
- Peloquin, C.A. Controversies in the Mangement of Mycobacterium avium Complex Infection in AIDS Patients. Ann. Pharmacother. 1993, 27, 928–937. [Google Scholar] [CrossRef]
- Peloquin, C.A. The Role of Therapeutic drug monitoring in Mycobacterial infection. Microbiol. Spectr. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Peloquin, C.A. Pharmacokinetic and pharmacodynamic considerations that may improve clinical outcomes. Clin. Pharmacokinet. 2017, 32, 132–165. [Google Scholar] [CrossRef]
- Kim, H.O.; Lee, K.; Choi, H.K.; Ha, S.; Lee, S.M.; Seo, G.H. Incidence, comorbidities, and treatment patterns of nontuberculous mycobacterial infection in South Korea. Medicine 2019, 98, e17869. [Google Scholar] [CrossRef]
- Koh, W.J.; Jeong, B.H.; Kim, S.Y.; Jeon, K.; Park, K.U.; Jhun, B.W.; Lee, H.; Park, H.Y.; Kim, D.H.; Huh, H.J.; et al. Mycobacterial Characteristics and Treatment Outcomes in Mycobacterium abscessus Lung Disease. Clin. Infect. Dis. 2017, 64, 309–316. [Google Scholar] [CrossRef]
- Rawson, T.M.; Abbara, A.; Kranzer, K.; Ritchie, A.; Milburn, J.; Brown, T.; Adeboyeku, D.; Buckley, J.; Davidson, R.N.; Berry, M.; et al. Factors which influence treatment initiation for pulmonary non-tuberculous mycobacterium infection in HIV negative patients; a multicentre observational study. Respir. Med. 2016, 120, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.A.; Kim, S.; Jo, K.W.; Shim, T.S. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur. Respir. J. 2017, 49, 1600537. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Takayanagi, N.; Kanauchi, T.; Miyahara, Y.; Yanagisawa, T.; Sugita, Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Abe, M.; Mouri, K.; Obase, Y.; Kato, S.; Oka, M. Relationship between clinical efficacy for pulmonary MAC and drug-sensitivity test for isolated MAC in a recent 6-year period. J. Infect. Dis. Chemother. 2012, 18, 436–443. [Google Scholar] [CrossRef]
- Aliberti, S.; Sotgiu, G.; Castellotti, P.; Ferrarese, M.; Pancini, L.; Pasat, A.; Vanoni, N.; Spotti, M.; Mazzola, E.; Gramegna, A.; et al. Real-life evaluation of clinical outcomes in patients undergoing treatment for non-tuberculous mycobacteria lung disease: A ten-year cohort study. Respir. Med. 2020, 164, 105899. [Google Scholar] [CrossRef]
- Brizzi, M.B.; Burgos, R.M.; Chiampas, T.D.; Michienzi, S.M.; Smith, R.; Yanful, P.K.; Badowski, M.E. Impact of Pharmacist-Driven Antiretroviral Stewardship and Transitions of Care Interventions on Persons with Human Immunodeficiency Virus. Open Forum Infect. Dis. 2020, 7, ofaa073. [Google Scholar] [CrossRef]
- Brozek, J.; Falavigna, M. What determines the width of the confidence interval. In Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice; McGraw-Hill: New York, NY, USA, 2008; pp. 225–231. [Google Scholar]
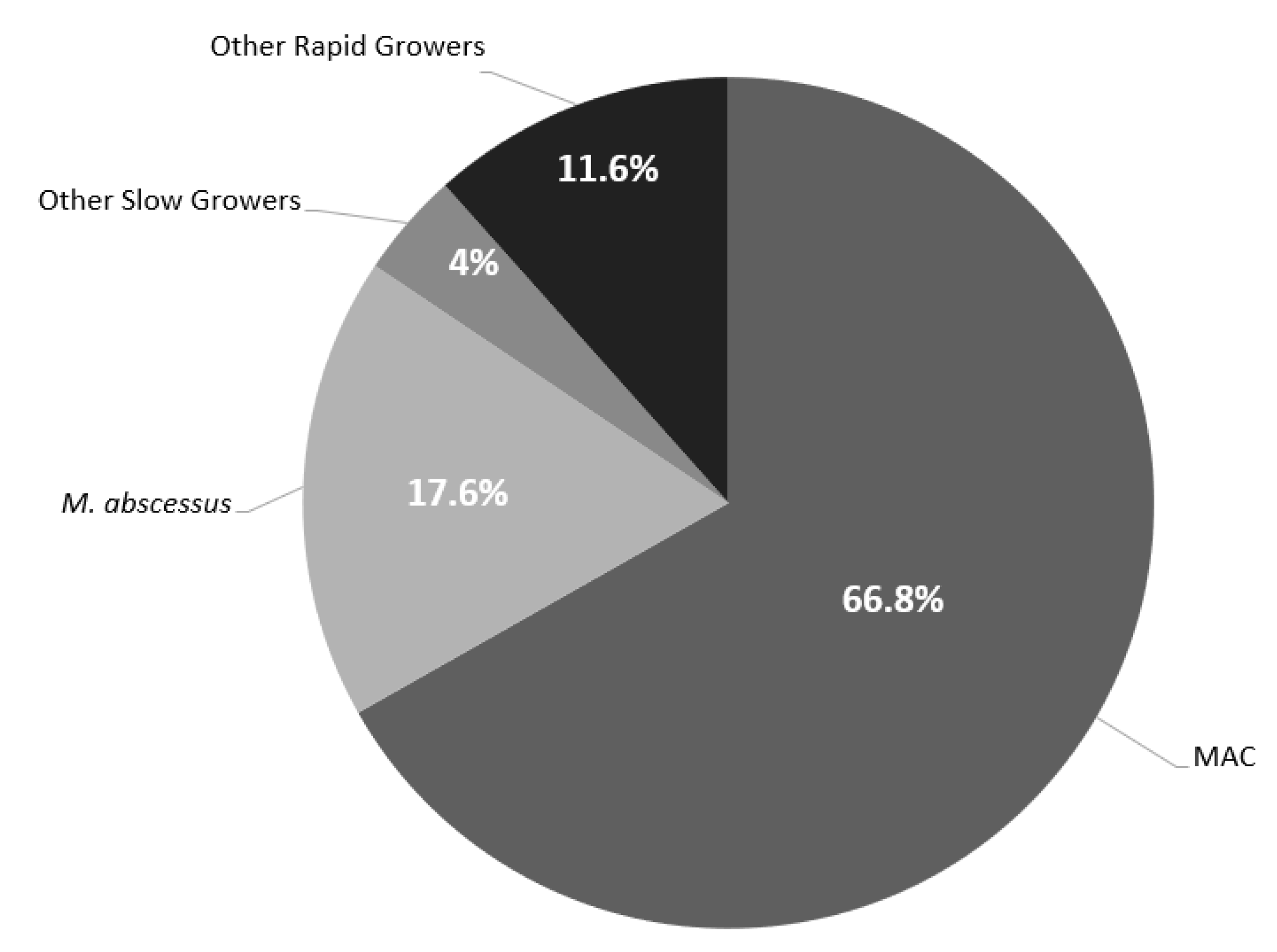
| All (n = 250) | MAC (n = 167) | M. abscessus (n = 44) | Other Slow Growers a (n = 10) | Other Rapid Growers b (n = 29) | |
|---|---|---|---|---|---|
| Age (y), median (IQR) | 67.4 (24.1) | 68.4 (21.9) | 65.7 (17.8) | 56.5 (21.9) | 66 (29.5) |
| Male, n (%) | 108/250 (43.2) | 70/167 (41.9) | 21/44 (47.7) | 8/10 (80) | 9/29 (31) |
| BMI (kg/m2), n (%) | |||||
| Underweight, (<18.5) | 29/250 (11.6) | 18/167 (10.8) | 7/44 (15.9) | 2/10 (20) | 2/29 (6.9) |
| Normal weight, (18.5–24.9) | 92/250 (36.8) | 67/167 (40.1) | 15/44 (34.1) | 5/10 (50) | 5/29 (17.2) |
| Overweight, (25–29.9) | 44/250 (17.6) | 27/167 (16.2) | 8/44 (18.2) | 3/10 (30) | 6/29 (20.7) |
| Obese, (30–39.9) | 34/250 (13.6) | 20/167 (12) | 7/44 (15.9) | 0 (0) | 7/29 (24.1) |
| Severely obese, (≥40) | 13/250 (5.2) | 9/167 (5.4) | 1/44 (2.3) | 0 (0) | 3/29 (10.3) |
| Race/Ethnicity, n (%) | |||||
| Non-Hispanic white | 165/250 (66) | 114/167 (68.3) | 28/44 (63.6) | 4/10 (40) | 19/29 (65.5) |
| Non-Hispanic black | 80/250 (32) | 51/167 (30.5) | 13/44 (29.5) | 6/10 (60) | 10/29 (34.5) |
| Hispanic | 3/250 (1.2) | 2/167 (1.2) | 1/44 (2.3) | 0 (0) | 0 (0) |
| Unknown | 2/250 (0.8) | 0 (0) | 2/44 (4.5) | 0 (0) | 0 (0) |
| Insurance, n (%) | |||||
| Private | 33/250 (17.6) | 24/167 (14.4) | 12/44 (27.3) | 3/10 (30) | 5/29 (17.2) |
| Medicare | 122/250 (48.8) | 84/167 (50.3) | 19/44 (43.2) | 4/10 (40) | 15/29 (51.7) |
| Medicaid | 25/250 (10) | 18/167 (10.8) | 2/44 (4.5) | 2/10 (20) | 3/29 (10.3) |
| Uninsured | 59/250 (23.6) | 41/167 (24.6) | 11/44 (25) | 1/10 (10) | 6/29 (20.7) |
| Type of NTM, n (%) | |||||
| Pulmonary | 197/250 (79.2) | 150/167 (89.8) | 26/44 (59.1) | 6/10 (50) | 15/29 (51.7) |
| Extrapulmonary | 50/250 (20) | 14/167 (8.4) | 18/44 (40.9) | 4/10 (40) | 14/29 (48.3) |
| Tissue | 30/50 (60) | 7/14 (50) | 8/18 (44.4) | 4/4 (100) | 11/14 (78.6) |
| Blood | 11/50 (22) | 7/14 (50) | 4/18 (22.2) | 0 (0) | 0 (0) |
| Other | 9/50 (18) | 0 (0) | 6/18 (33.3) | 0 (0) | 3/14 (21.4) |
| Mixed | 3/250 (1.2) | 3/167 (1.8) | 0 (0) | 1/10 (10) | 0 (0) |
| Co-infection, n (%) | 52/250 (20.8) | 36/167 (21.6) | 8/44 (18.2) | 3/10 (30) | 5/29 (17.2) |
| Immunosuppressant, n (%) | 34/250 (13.6) | 23/167 (13.8) | 6/44 (13.6) | 2/10 (20) | 3/29 (10.3) |
| Chronic Comorbidities, n (%) c | 194/249 (77.9) | 134/167 (80.2) | 33/43 (76.7) | 5/10 (50) | 22/29 (75.9) |
| DM, n (%) | 35/249 (14.1) | 19/167 (11.4) | 8/43 (18.6) | 1/10 (10) | 7/29 (24.1) |
| CKD, n (%) | 19/249 (7.6) | 13/167 (7.8) | 2/43 (4.7) | 1/10 (10) | 3/29 (10.3) |
| Liver disease, n (%) | 6/249 (2.4) | 3/167 (1.8) | 3/43 (7.0) | 0 (0) | 0 (0) |
| Pulmonary disease, n (%) | 125/249 (50.2) | 88/167 (52.4) | 22/43 (51.2) | 1/10 (10) | 14/29 (48.3) |
| COPD | 37/125 (29.6) | 24/88 (27.3) | 7/22 (31.8) | 0 (0) | 6/14 (42.9) |
| Bronchitis | 13/125 (10.4) | 12/88 (13.6) | 2/22 (9.1) | 0 (0) | 0 (0) |
| Emphysema | 6/125 (4.8) | 2/88 (2.3) | 2/22 (9.1) | 0 (0) | 2/14 (14.3) |
| CF | 4/125 (3.2) | 1/88 (1.1) | 3/22 (13.6) | 0 (0) | 0 (0) |
| Lung cancer | 3/125 (2.4) | 2/88 (2.3) | 1/22 (4.5) | 0 (0) | 0 (0) |
| Bronchiectasis | 33/125 (26.4) | 20/88 (22.7) | 6/22 (27.3) | 1/1 (100) | 6/14 (42.9) |
| Asthma | 23/125 (18.4) | 15/88 (17) | 4/22 (18.2) | 1/1 (100) | 3/14 (21.4) |
| Interstitial lung disease | 7/125 (5.6) | 6/88 (6.8) | 0 (0) | 0 (0) | 1/14 (7.1) |
| Nodules/Masses | 15/125 (12) | 15/88 (17) | 0 (0) | 0 (0) | 0 (0) |
| Empyema | 2/125 (1.6) | 2/88 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| Fibrosis | 4/125 (3.2) | 3/88 (3.4) | 0 (0) | 0 (0) | 1/14 (7.1) |
| Immunocompromised, n (%) d | 68/249 (27.3) | 50/167 (29.9) | 8/43 (18.6) | 4/10 (40) | 6/29 (20.7) |
| Prior NTM infection, n (%) | 33/249 (13.3) | 21/167 (12.6) | 6/43 (14.0) | 1/10 (10 | 5/29(17.2) |
| Treated | 19/33 (57.6) | 12/21 (57.1) | 5/6 (83.3) | 0 (0) | 2/5 (40) |
| Not treated | 14/33 (42.4) | 9/21 (42.9) | 1/6 (16.7) | 1/1 (100) | 3/5 (60) |
| Prior MTB treatment, n (%) | 6/249 (2.4) | 4/167 (2.4) | 1/43 (2.3) | 1/10 (10) | 0 (0) |
| Smoking, n (%) | 133/249 (53.4) | 97/167 (58) | 18/43 (41.9) | 4/10 (40) | 14/29 (48.3) |
| Antimicrobials (No. Isolates Tested) | No. of Isolates and Cumulative % of Inhibited at MIC of: | MIC (mg/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.016 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 50% | 90% | |
| Ciprofloxacin | |||||||||||||||||
| M. abscessus (44) | 4 | 40 | 8 | 8 | |||||||||||||
| 9.1% | 100% | ||||||||||||||||
| Other (27) | 5 | 7 | 7 | 1 | 1 | 4 | 2 | ≤1 | ≥4 | ||||||||
| 18.5% | 44% | 70.4% | 74.1% | 75% | 92.6% | 100% | |||||||||||
| Total (71) | 5 | 7 | 7 | 1 | 1 | 8 | 42 | 8 | 8 | ||||||||
| 7.0% | 16.9% | 26.8% | 28.2% | 29.6% | 40.8% | 100% | |||||||||||
| Moxifloxacin | |||||||||||||||||
| M. abscess (44) | 2 | 4 | 37 | 1 | 16 | 16 | |||||||||||
| 4.5% | 13.6% | 97.7% | 100% | ||||||||||||||
| Other (27) | 18 | 1 | 2 | 3 | 2 | 1 | 0.25 | 8 | |||||||||
| 66.7% | 70.4% | 77.8% | 88.9% | 96.3% | 100% | ||||||||||||
| Total (71) | 18 | 1 | 2 | 5 | 6 | 38 | 1 | 16 | 16 | ||||||||
| 25.4% | 26.8% | 29.6% | 36.6% | 45.1% | 98.6% | 100% | |||||||||||
| Cefoxitin | |||||||||||||||||
| M. abscessus (44) | 1 | 5 | 29 | 7 | 2 | 32 | ≥64 | ||||||||||
| 2.3% | 13.6% | 79.5% | 95.5% | 100% | |||||||||||||
| Other (27) | 1 | 3 | 8 | 7 | 2 | 6 | ≥64 | 256 | |||||||||
| 3.7 | 18.5 | 44.4 | 70.4 | 77.8 | 100% | ||||||||||||
| Total (71) | 2 | 8 | 37 | 14 | 2 | 8 | 32 | 256 | |||||||||
| 2.8% | 14.1% | 66.2% | 65.9% | 88.7 | 100% | ||||||||||||
| Doxycycline | |||||||||||||||||
| M. abscessus (44) | 2 | 2 | 40 | 32 | 32 | ||||||||||||
| 4.5% | 9.1% | 100% | |||||||||||||||
| Other (27) | 5 | 1 | 2 | 2 | 3 | 4 | 10 | 16 | 32 | ||||||||
| 18.5% | 22.2% | 29.6% | 37% | 48.1% | 63% | 100% | |||||||||||
| Total (71) | 5 | 1 | 2 | 2 | 5 | 6 | 50 | 32 | 32 | ||||||||
| 7.0% | 8.5% | 11.3% | 14.1% | 21.1% | 29.6% | 100% | |||||||||||
| Tigecycline a | |||||||||||||||||
| M. abscessus (27) | 5 | 11 | 9 | 1 | 1 | 0.12 | 0.25 | ||||||||||
| 18.5% | 59.35 | 92.6% | 96.3% | 100% | |||||||||||||
| Other (19) | 7 | 4 | 6 | 2 | 0.03 | 0.12 | |||||||||||
| 36.8% | 57.9% | 89.5% | 100% | ||||||||||||||
| Total (46) | 7 | 4 | 11 | 13 | 9 | 1 | 1 | 0.12 | 0.25 | ||||||||
| 15.2% | 23.9% | 47.8% | 76.1% | 95.7% | 97.8% | 100% | |||||||||||
| Clarithromycin | |||||||||||||||||
| M. abscessus (44) | 1 | 3 | 4 | 7 | 5 | 9 | 15 | 16 | 32 | ||||||||
| 2.3% | 9.1% | 18.2% | 34.1% | 45.5% | 65.9% | 100% | |||||||||||
| Other (25) | 1 | 3 | 2 | 3 | 2 | 6 | 5 | 3 | ≥8 | 32 | |||||||
| 4% | 16% | 24% | 36% | 56% | 64% | 84% | 100% | ||||||||||
| Total (69) | 1 | 4 | 5 | 7 | 9 | 11 | 14 | 18 | ≥8 | 32 | |||||||
| 1.4% | 7.2% | 14.5% | 24.6% | 37.7% | 53.6% | 73.9% | 100% | ||||||||||
| Linezolid | |||||||||||||||||
| M. abscessus (44) | 4 | 15 | 18 | 7 | 16 | 32 | |||||||||||
| 9.1% | 43.2% | 84.1% | 100% | ||||||||||||||
| Other (27) | 5 | 5 | 6 | 10 | 1 | 4 | ≤8 | ||||||||||
| 18.5% | 37% | 59.3% | 96.3% | 100% | |||||||||||||
| Total (71) | 5 | 5 | 10 | 25 | 19 | 7 | ≤8 | 16 | |||||||||
| 7% | 14.1 | 28.2% | 63.4% | 90.1% | 100% | ||||||||||||
| Imipenem | |||||||||||||||||
| M. abscessus (41) | 12 | 24 | 5 | 16 | 32 | ||||||||||||
| 29.3% | 87.8% | 100% | |||||||||||||||
| Other (27) | 1 | 4 | 9 | 6 | 2 | 5 | ≤4 | 32 | |||||||||
| 3.7% | 18.5% | 51.9% | 74.1% | 81.5% | 100% | ||||||||||||
| Total (68) | 1 | 4 | 9 | 18 | 26 | 10 | 16 | 32 | |||||||||
| 1.5% | 7.4% | 20.6% | 47.1% | 85.3% | 100% | ||||||||||||
| Amikacin | |||||||||||||||||
| M. abscessus (44) | 1 | 37 | 5 | 1 | ≤16 | 32 | |||||||||||
| 2.3% | 86.4% | 97.7% | 100% | ||||||||||||||
| Other (22) | 17 | 3 | 1 | 1 | 1 | 2 | |||||||||||
| 77.3% | 90.9% | 95.5% | 100% | ||||||||||||||
| Total (66) | 17 | 3 | 2 | 38 | 5 | 1 | ≤16 | ≤16 | |||||||||
| 25.8% | 30.3% | 33.3% | 90.9% | 98.5% | 100% | ||||||||||||
| Tobramycin | |||||||||||||||||
| M. chelonae (5) | 2 | 3 | NA | NA | |||||||||||||
| 40% | 100% | ||||||||||||||||
| Minocycline b | |||||||||||||||||
| M. abscessus (43) | 4 | 21 | 18 | 8 | 16 | ||||||||||||
| 9.3% | 58.1% | 100% | |||||||||||||||
| Other (26) | 8 | 2 | 2 | 7 | 7 | 4 | 16 | ||||||||||
| 30.8% | 38.5% | 84.6% | 73.1% | 100% | |||||||||||||
| Total (69) | 8 | 2 | 6 | 28 | 25 | 8 | 16 | ||||||||||
| 11.6% | 14.5% | 23.2% | 63.8% | 100% | |||||||||||||
| SXT | |||||||||||||||||
| M. abscessus (44) | 2 | 13 | 29 | 8 | 16 | ||||||||||||
| 4.5% | 34.1% | 100% | |||||||||||||||
| Other (27) | 7 | 4 | 2 | 6 | 2 | 2 | 3 | 1 | ≥4 | ||||||||
| 25.9% | 40.7% | 48.1% | 70.4% | 77.8% | 88.9% | 100% | |||||||||||
| Total (71) | 7 | 4 | 2 | 6 | 4 | 15 | 32 | 16 | 16 | ||||||||
| 9.9% | 15.5% | 18.3% | 26.8% | 32.4% | 53.5% | 100% | |||||||||||
| Antimicrobial (No. Isolates Tested) | No. of Isolates and Cumulative % of Inhibited at MIC of: | MIC (mg/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 50% | 90% | |
| Ciprofloxacin | ||||||||||||||
| MAC (11) a | 1 | 1 | 1 | 3 | 2 | 3 | 2 | 8 | ||||||
| 9.1% | 18.2% | 27.3% | 54.5% | 72.7% | 100% | |||||||||
| Other (3) | 1 | 1 | 1 | NA | NA | |||||||||
| 33.3% | 66.7% | 100% | ||||||||||||
| Moxifloxacin | ||||||||||||||
| MAC (53) | 7 | 14 | 26 | 4 | 1 | 1 | 0.5 | ≤1 | ||||||
| 13.2% | 39.6% | 88.7% | 96.2% | 98.1% | 100% | |||||||||
| Other (3) | 1 | 1 | 1 | NA | NA | |||||||||
| 33.3% | 66.7% | 100% | ||||||||||||
| Clarithromycin | ||||||||||||||
| MAC (53) | 9 | 10 | 15 | 9 | 7 | 2 | 1 | 0.25 | 1 | |||||
| 17% | 35.8% | 64.2% | 81.1% | 94.3% | 98.1% | 100% | ||||||||
| M. kansasii (2) | 1 | 1 | NA | NA | ||||||||||
| 50% | 100% | |||||||||||||
| Other (5) | 3 | 1 | 1 | NA | NA | |||||||||
| 60% | 80% | 100% | ||||||||||||
| Linezolid | ||||||||||||||
| MAC (53) | 6 | 10 | 10 | 15 | 9 | 2 | 1 | ≤8 | 16 | |||||
| 11.3% | 30.2% | 49.1% | 77.4% | 94.3% | 96.2% | 100% | ||||||||
| Other (3) | 3 | NA | NA | |||||||||||
| 100% | ||||||||||||||
| Amikacin intravenous | ||||||||||||||
| MAC (53) | 7 | 12 | 19 | 11 | 2 | 1 | 1 | 4 | 8 | |||||
| 13.2% | 35.8% | 71.7% | 92.5% | 96.2% | 98.1% | 100% | ||||||||
| Other (5) | 5 | NA | NA | |||||||||||
| 100% | ||||||||||||||
| Amikacin liposomal or inhaled | ||||||||||||||
| MAC (53) | 7 | 12 | 19 | 11 | 2 | 1 | 1 | 4 | 8 | |||||
| 13.2% | 35.8% | 71.7% | 92.5% | 96.2% | 98.1% | 100% | ||||||||
| Doxycycline | ||||||||||||||
| Other (3) | 2 | 1 | NA | NA | ||||||||||
| 66.7% | 100% | |||||||||||||
| Rifampin | ||||||||||||||
| M.kansasii (2) | 2 | NA | NA | |||||||||||
| 100% | ||||||||||||||
| Other (3) | 3 | NA | NA | |||||||||||
| 100% | ||||||||||||||
| Ethambutol b | ||||||||||||||
| Other (3) | 2 | 1 | NA | NA | ||||||||||
| 66.7% | 100% | |||||||||||||
| SXT | ||||||||||||||
| Other (4) | 1 | 1 | 1 | 1 | NA | NA | ||||||||
| 25% | 50% | 75% | 100% | |||||||||||
| All (n = 88) | Unfavorable (n = 34) | Favorable (n = 54) | p-Value | |
|---|---|---|---|---|
| Age (y) median (IQR) | 66.8 ± 27 | 66.9 ± 32.7 | 66.8 ± 15.7 | 0.48 |
| Male, n (%) | 35/88 (39.8) | 11/34 (32.4) | 24/54 (44.4) | 0.26 |
| Body Mass Index (kg/m2), n (%) | ||||
| Underweight, (<18.5) | 14/88 (15.9) | 10/34 (29.4) | 4/54 (7.4) | 0.006 |
| Normal weight, (18.5–24.9) | 32/88 (36.4) | 11/34 (32.4) | 21/54 (38.9) | 0.54 |
| Overweight, (25–29.9) | 14/88 (15.9) | 3/34 (8.8) | 11/54 (20.4) | 0.15 |
| Obese, (30–39.9) | 10/88 (11.4) | 5/34 (14.7) | 5/54 (9.3) | 0.50 |
| Severely obese, (≥40) | 4/88 (4.5) | 1/34 (2.9) | 3/54 (5.6) | 1.00 |
| Race/Ethnicity, n (%) | ||||
| Non-Hispanic white | 64/88 (72.7) | 24/34 (70.6) | 40/54 (74.1) | 0.72 |
| Non-Hispanic black | 23/88 (26.1) | 10/34 (29.4) | 13/54 (24.1) | 0.58 |
| Hispanic | 1/88 (1.1) | 0 (0) | 1/54 (1.9) | 1.00 |
| Insurance, n (%) | ||||
| Private | 17/88 (19.3) | 2/34 (5.9) | 15/54 (27.8) | 0.011 |
| Medicare | 48/88 (54.5) | 18/34 (52.9) | 30/54 (55.6) | 0.81 |
| Medicaid | 6/88(6.8) | 4/34 (11.8) | 2/54 (3.7) | 0.20 |
| Uninsured | 17/88 (19.3) | 10/34 (29.4) | 7/54 (13) | 0.057 |
| Type of NTM, n (%) | ||||
| Pulmonary | 61/88 (69.3) | 24/34 (70.6) | 37/54 (68.5) | 0.84 |
| Extrapulmonary | 25/88 (28.4) | 9/34 (26.5) | 16/54 (29.6) | 0.75 |
| Mixed | 2/88 (2.3) | 1/34 (2.9) | 1/54 (2.9) | 1.00 |
| Co-infection, n (%) | 15/88 (17) | 5/34 (14.7) | 10/54 (18.5) | 0.64 |
| On immunosuppressants, n (%) | 13/88 (14.8) | 7/34 (20.6) | 6/54 (11.1) | 0.22 |
| Comorbidities | ||||
| Diabetes mellitus | 13/88 (14.9) | 4/34 (11.8) | 9/54 (16.7) | 0.53 |
| Chronic kidney disease | 9/88 (10.2) | 4/34 (11.8) | 5/54 (9.3) | 0.73 |
| Liver disease | 2/88 (2.3) | 1/34 (2.9) | 1/54 (1.9) | 1.00 |
| Pulmonary disease | 44/88 (50) | 19/34 (55.9) | 25/54 (46.3) | 0.38 |
| COPD | 11/44 (25) | 5/19 (26.3) | 6/25 (24) | 1.00 |
| Bronchitis | 6/44 (13.6) | 1/19 (5.3) | 5/25 (20) | 0.21 |
| Emphysema | 1/44 (2.3) | 0 (0) | 1/25 (4) | 1.00 |
| CF | 2/44 (4.5) | 1/19 (5.3) | 1/25 (4) | 1.00 |
| Lung cancer | 2/44 (4.5) | 1/19 (5.3) | 1/25 (4) | 1.00 |
| Non-CF Bronchiectasis | 14/44 (31.8) | 5/19 (26.3) | 9/25 (36) | 0.50 |
| Asthma | 9/44 (20.5) | 7/19 (36.8) | 2/25 (8) | 0.02 |
| Interstitial lung disease | 1/44 (2.3) | 1/19 (5.3) | 0 (0) | 0.43 |
| Nodules/Masses | 3/44 (6.8) | 2/19 (10.5) | 1/25 (4) | 0.57 |
| Empyema | 1/44 (2.3) | 1/19 (5.3) | 0 (0) | 0.43 |
| Fibrosis | 1/44 (2.3) | 0 (0) | 1/25 (4) | 1.00 |
| Immunocompromised a | 20/88 (22.7) | 11/34 (32.4) | 9/54 (16.7) | 0.09 |
| Prior NTM infection | 15/88 (17) | 5/34 (14.7) | 10/54 (18.5) | 0.64 |
| Treated | 9/15 (60) | 4/5 (80) | 5/10 (50) | 0.58 |
| Not treated | 6/15 (40) | 1/5 (20) | 5/10 (50) | 0.58 |
| Prior MTB treatment | 4/88 (4.5) | 4/34 (11.8) | 0 (0) | 0.02 |
| Smoking | 42/88 (47.7) | 20/34 (58.8) | 22/54 (40.7) | 0.10 |
| All (n = 88) | Unfavorable (n = 34) | Favorable (n = 54) | p-Value (95% Cl) | |
|---|---|---|---|---|
| Antimicrobial Change, n (%) | 53/88 (60.2) | 25/34 (73.5) | 28/54 (51.9) | 0.043 |
| 1 only | 15/53 (28.3) | 5/25 (20) | 10/28 (35.7) | 0.21 |
| ≥2 | 38/53 (71.7) | 20/25 (80) | 18/28 (64.3) | 0.21 |
| ≥3 | 29/53 (54.7) | 15/25 (60) | 14/28 (50) | 0.47 |
| ≥4 | 21/53 (39.6) | 13/25 (52) | 8/28 (28.6) | 0.082 |
| ≥5 | 14/53 (26.4) | 10/25 (40) | 4/28 (14.3) | 0.034 |
| Reasons for Change | ||||
| Drug-Drug Interaction | 2/53 (3.8) | 1/25 (4) | 1/28 (3.6) | 1.00 |
| Susceptibility | 13/53 (24.5) | 5/25 (20) | 8/28 (28.6) | 0.47 |
| Disease Status | 14/53 (26.4) | 10/25 (40) | 4/28 (14.3) | 0.034 |
| Escalation | 12/53 (22.6) | 9/25 (36) | 3/28 (10.7) | 0.51 |
| De-escalation | 5/53 (9.4) | 3/25 (12) | 2/28 (7.1) | 0.58 |
| Treatment Optimization | 9/53 (17) | 4/25 (16) | 5/28 (17.9) | 1.00 |
| ADEs | 36/53 (67.9) | 16/25 (64) | 20/28 (71.4) | 0.56 |
| Drug Allergy | 6/36 (16.7) | 3/16 (18.8) | 3/20 (15) | 1.00 |
| AKI | 1/36 (2.8) | 1/16 (6.3) | 0 (0) | 0.44 |
| DILI | 3/36 (8.3) | 3/16 (18.8) | 0 (0) | 0.08 |
| GI Intolerance | 14/36 (38.9) | 5/16 (31.3) | 9/20 (45) | 0.40 |
| General Intolerance | 10/36 (27.8) | 7/16 (43.8) | 3/20 (15) | 0.07 |
| Other | 18/36 (50) | 9/16 (56.3) a | 9/20 (45) b | 0.50 |
| Drug Access | 6/53 (11.3) | 3/25 (12) | 3/28 (10.7) | 1.00 |
| Insurance | 5/6 (83.3) | 3/3 (100) | 2/3 (66.7) | 1.00 |
| National back order | 1/6 (16.7) | 0 (0) | 1/3 (33.3) | 1.00 |
| Administration Issue | 7/53 (13.2) | 5/25 (20) | 2/28 (7.1) | 0.23 |
| NPO | 2/7 (28.6) | 2/5 (40) | 0 (0) | 1.00 |
| No IV access | 3/7 (42.9) | 2/5 (40) | 1/2 (50) | 1.00 |
| PO regimen only | 2/7 (28.6) | 1/5 (20) | 1/2 (50) | 1.00 |
| Dialysis access | 1/7 (14.3) | 1/5 (20) | 0 (0) | 1.00 |
| Other | 8/53 (15.1) | 6/25 (24) | 2/28 (7.1) | 0.13 |
| Nonspecific/Unclear | 12/53 (22.6) | 7/25 (28) | 5/28 (17.9) | 0.38 |
| Univariable Analysis | Odds Ratio | 95% Cl | p-Value |
|---|---|---|---|
| Greater than 5 Antimicrobial Changes | 0.192 | 0.055–0.675 | 0.010 |
| Private Insurance | 6.150 | 1.310–28.930 | 0.021 |
| Underweight | 0.192 | 0.055–0.675 | 0.010 |
| History of Asthma | 0.148 | 0.029–0.764 | 0.022 |
| Antimicrobial Change, Intolerance | 0.249 | 0.065–0.962 | 0.044 |
| Multivariable Analysis a | |||
| Greater than 5 Antimicrobial Changes | 0.187 | 0.049–0.714 | 0.014 |
| Private Insurance | 6.112 | 1.12–33.29 | 0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.V.; Derrick, C.; Yunusa, I.; Weissman, S.; Al-Hasan, M.N.; Justo, J.A.; Bookstaver, P.B. Epidemiology, Outcomes and Tolerability of Protracted Treatment of Nontuberculous Mycobacterial Infections at a Community Teaching Hospital in the Southeastern United States. Antibiotics 2022, 11, 1720. https://doi.org/10.3390/antibiotics11121720
Tsai YV, Derrick C, Yunusa I, Weissman S, Al-Hasan MN, Justo JA, Bookstaver PB. Epidemiology, Outcomes and Tolerability of Protracted Treatment of Nontuberculous Mycobacterial Infections at a Community Teaching Hospital in the Southeastern United States. Antibiotics. 2022; 11(12):1720. https://doi.org/10.3390/antibiotics11121720
Chicago/Turabian StyleTsai, Yuwei Vivian, Caroline Derrick, Ismaeel Yunusa, Sharon Weissman, Majdi N. Al-Hasan, Julie Ann Justo, and Paul Brandon Bookstaver. 2022. "Epidemiology, Outcomes and Tolerability of Protracted Treatment of Nontuberculous Mycobacterial Infections at a Community Teaching Hospital in the Southeastern United States" Antibiotics 11, no. 12: 1720. https://doi.org/10.3390/antibiotics11121720
APA StyleTsai, Y. V., Derrick, C., Yunusa, I., Weissman, S., Al-Hasan, M. N., Justo, J. A., & Bookstaver, P. B. (2022). Epidemiology, Outcomes and Tolerability of Protracted Treatment of Nontuberculous Mycobacterial Infections at a Community Teaching Hospital in the Southeastern United States. Antibiotics, 11(12), 1720. https://doi.org/10.3390/antibiotics11121720








