Efficacy and Safety of Non-Insulin Antidiabetic Drugs in Cats: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria and Study Selection
2.3. Risk of Bias Assessment
2.4. Declaration on the Use of Artificial Intelligence
3. Results
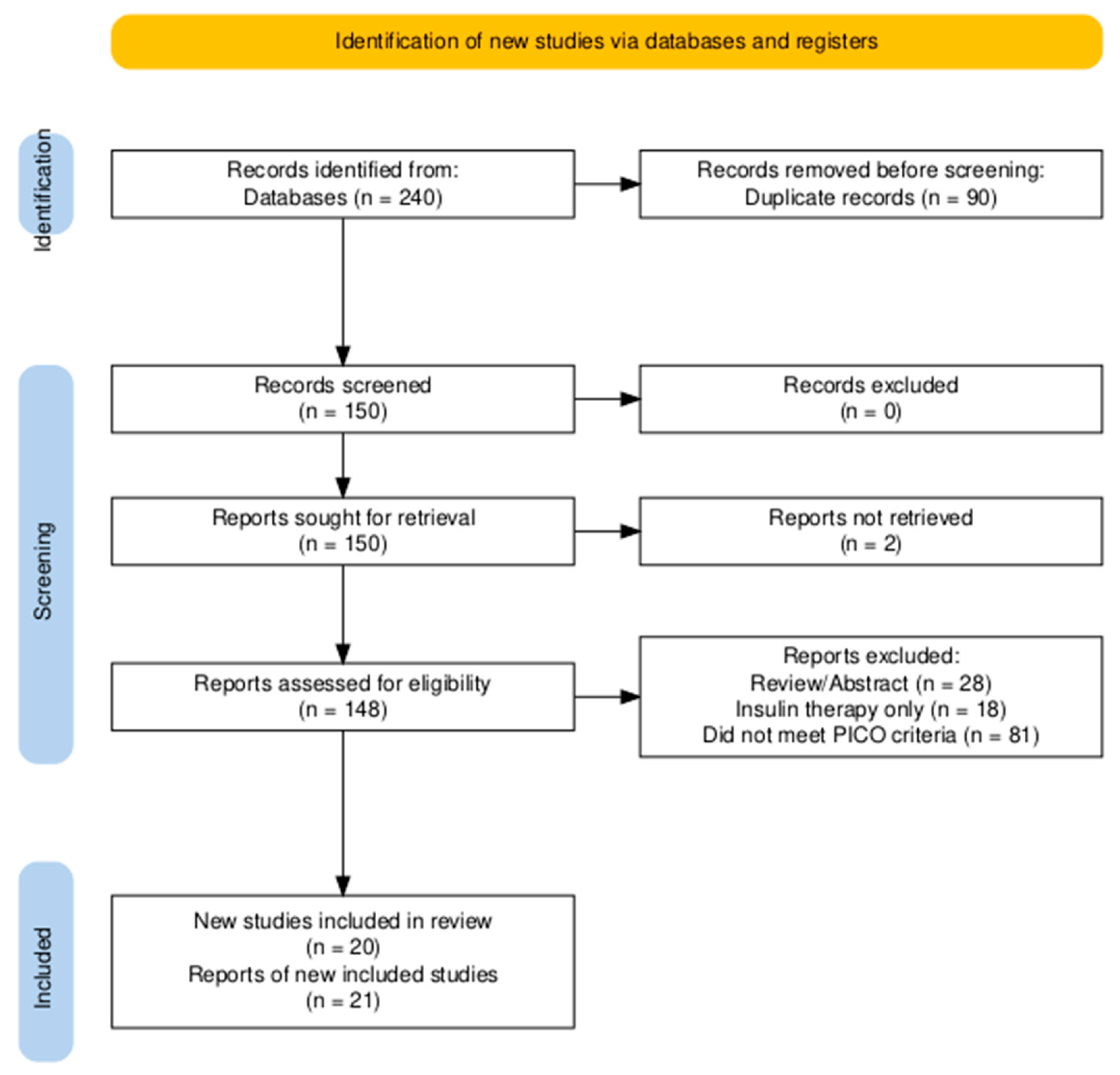
3.1. Study Selection
3.2. Studies in Cats with Diabetes Mellitus
3.2.1. Characteristics of Included Studies
3.2.2. Synthesis of Results
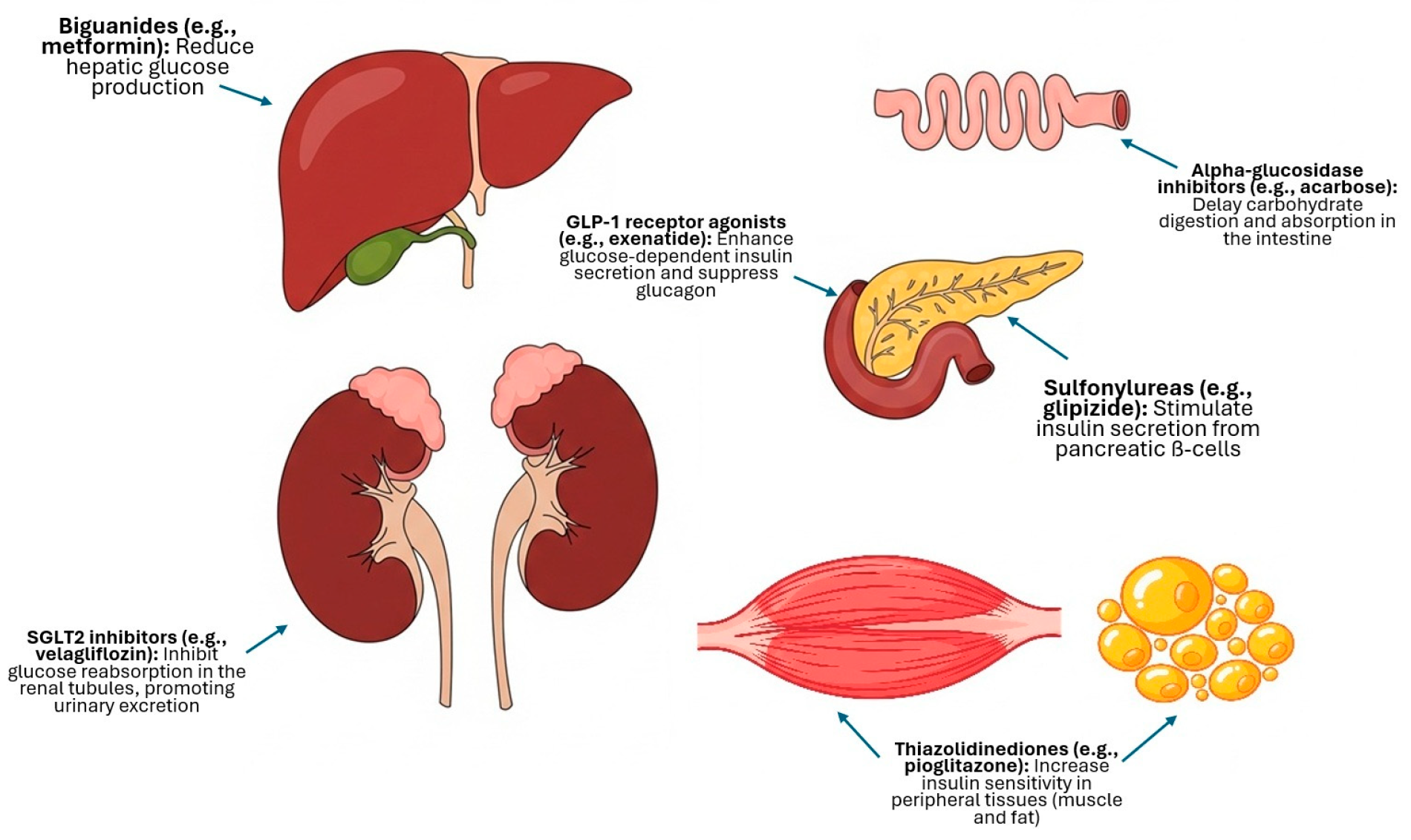
Sulfonylureas (Glipizide)
Alpha-Glucosidase Inhibitors (Acarbose)
Biguanides (Metformin)
Glucagon-like Peptide-1 (GLP-1) Receptor Agonists (Exenatide)
SGLT2 Inhibitors (Bexagliflozin and Velagliflozin)
3.3. Studies in At-Risk or Experimental Feline Models
3.3.1. Characteristics of Included Studies
3.3.2. Synthesis of Results
Effects on Insulin Sensitivity in Obese Cats
Effects in Healthy, Non-Obese, or Mixed-Weight Cats
Effects on Specific Pathophysiological Models
Pharmacokinetic Studies
4. Discussion
4.1. Principal Findings and Overview of the Evidence
4.2. Interpretation of Evidence from Clinical Treatment Studies
4.2.1. Clinical Efficacy: Traditional vs. Newer Agents
4.2.2. Confounding Factors and Effect Modifiers in Clinical Trials
Diet
Age and Chronicity of the Disease
Breed and Genetic Factors
Comorbidities and Concomitant Medications
4.2.3. Safety Profile and Adverse Events
4.2.4. Strengths and Limitations of the Evidence
4.3. Interpretation of Evidence from At-Risk and Experimental Models
4.3.1. Effects on Insulin Sensitivity and Metabolism
4.3.2. Insights into Pathophysiological Mechanisms
4.3.3. Pharmacokinetic Considerations
4.3.4. Strengths and Limitations of the Evidence
4.4. Overall Limitations of the Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behrend, E.N.; Ward, C.R.; Chukwu, V.; Cook, A.K.; Kroh, C.; Lathan, P.; May, J.; Schermerhorn, T.; Scott-Moncrieff, J.C.; Voth, R. Velagliflozin, a Once-Daily, Liquid, Oral SGLT2 Inhibitor, Is Effective as a Stand-Alone Therapy for Feline Diabetes Mellitus: The SENSATION Study. J. Am. Vet. Med. Assoc. 2024, 262, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, M.A.; Ribeiro Petito, M.; Unniappan, S.; Waldner, C.; Mehain, S.; McMillian, C.J.; Snead, E.C. Safety and Efficacy Assessment of a GLP-1 Mimetic: Insulin Glargine Combination for Treatment of Feline Diabetes Mellitus. Domest. Anim. Endocrinol. 2018, 65, 80–89. [Google Scholar] [CrossRef]
- Schneider, E.L.; Reid, R.; Parkes, D.G.; Lutz, T.A.; Ashley, G.W.; Santi, D.V. A Once-Monthly GLP-1 Receptor Agonist for Treatment of Diabetic Cats. Domest. Anim. Endocrinol. 2020, 70, 106373. [Google Scholar] [CrossRef] [PubMed]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. 2018 AAHA Diabetes Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2018, 54, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Leal, K.M.; Rocha, M.B.; Varela, F.V.; Rodrigues, L.; Furtado, P.V.; da Costa, F.V.A.; Pöppl, Á.G. Is Methylprednisolone Acetate-Related Insulin Resistance Preventable in Cats? Top. Companion Anim. Med. 2022, 49, 100648. [Google Scholar] [CrossRef]
- Rosca, M.; Musteata, M.; Solcan, C.; Stanciu, G.D.; Solcan, G. Feline Hypersomatotropism, an Important Cause for the Failure of Insulin Therapy. Bull. UASVM Vet. Med. 2014, 71, 298–304. [Google Scholar] [CrossRef][Green Version]
- Cook, A.K.; Behrend, E. SGLT2 Inhibitor Use in the Management of Feline Diabetes Mellitus. J. Vet. Pharmacol. Ther. 2025, 48 (Suppl. 1), 19–30. [Google Scholar] [CrossRef]
- Bennett, N.; Papich, M.G.; Hoenig, M.; Fettman, M.J.; Lappin, M.R. Evaluation of Transdermal Application of Glipizide in a Pluronic Lecithin Gel to Healthy Cats. Am. J. Vet. Res. 2005, 66, 581–588. [Google Scholar] [CrossRef]
- Hoenig, M.; Hall, G.; Ferguson, D.; Jordan, K.; Henson, M.; Johnson, K.; O’Brien, T. A Feline Model of Experimentally Induced Islet Amyloidosis. Am. J. Pathol. 2000, 157, 2143–2150. [Google Scholar] [CrossRef]
- Feldman, E.C.; Nelson, R.W.; Feldman, M.S. Intensive 50-Week Evaluation of Glipizide Administration in 50 Cats with Previously Untreated Diabetes Mellitus. J. Am. Vet. Med. Assoc. 1997, 210, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W.; Feldman, E.C.; Ford, S.L.; Roemer, O.P. Effect of an Orally Administered Sulfonylurea, Glipizide, for Treatment of Diabetes Mellitus in Cats. J. Am. Vet. Med. Assoc. 1993, 203, 821–827. [Google Scholar] [CrossRef]
- Miller, A.B.; Nelson, R.W.; Kirk, C.A.; Neal, L.; Feldman, E.C. Effect of Glipizide on Serum Insulin and Glucose Concentrations in Healthy Cats. Res. Vet. Sci. 1992, 52, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Lee, P.; Yamashita, T.; Nishimaki, Y.; Oda, H.; Saeki, K.; Miki, Y.; Mizutani, H.; Ishioka, K.; Honjo, T.; et al. Effect of Glimepiride and Nateglinide on Serum Insulin and Glucose Concentration in Healthy Cats. Vet. Res. Commun. 2009, 33, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Spann, D.; Elliott, D.; Brondos, A.; Vulliet, R. Evaluation of the Oral Antihyperglycemic Drug Metformin in Normal and Diabetic Cats. J. Vet. Intern. Med. 2004, 18, 18–24. [Google Scholar] [CrossRef]
- Michels, G.M.; Boudinot, F.D.; Ferguson, D.C.; Hoenig, M. Pharmacokinetics of the Antihyperglycemic Agent Metformin in Cats. Am. J. Vet. Res. 1999, 60, 738–742. [Google Scholar] [CrossRef]
- Singh, R.; Rand, J.S.; Coradini, M.; Morton, J.M. Effect of Acarbose on Postprandial Blood Glucose Concentrations in Healthy Cats Fed Low and High Carbohydrate Diets. J. Feline Med. Surg. 2015, 17, 848–857. [Google Scholar] [CrossRef]
- Mazzaferro, E.M.; Greco, D.S.; Turner, A.S.; Fettman, M.J. Treatment of Feline Diabetes Mellitus Using an α-Glucosidase Inhibitor and a Low-Carbohydrate Diet. J. Feline Med. Surg. 2003, 5, 183–189. [Google Scholar] [CrossRef]
- Singh, R.; Rand, J.S.; Morton, J.M. Switching to an Ultra-Low Carbohydrate Diet Has a Similar Effect on Postprandial Blood Glucose Concentrations to Administering Acarbose to Healthy Cats Fed a High Carbohydrate Diet. In Research Abstract Program of the 24th Annual ACVIM Forum, Proceedings of the 24th Annual ACVIM Forum, Louisville, KY, USA, 31 May–3 June 2006; Wiley-Blackwell: Hoboken, NJ, USA, 2006; Abstract #59; p. 726. [Google Scholar]
- Riederer, A.; Zini, E.; Salesov, E.; Fracassi, F.; Padrutt, I.; Macha, K.; Stöckle, T.M.; Lutz, T.A.; Reusch, C.E. Effect of the Glucagon-like Peptide-1 Analogue Exenatide Extended Release in Cats with Newly Diagnosed Diabetes Mellitus. J. Vet. Intern. Med. 2016, 30, 92–100. [Google Scholar] [CrossRef]
- Padrutt, I.; Lutz, T.A.; Reusch, C.E.; Zini, E. Effects of the Glucagon-like Peptide-1 (GLP-1) Analogues Exenatide, Exenatide Extended-Release, and of the Dipeptidylpeptidase-4 (DPP-4) Inhibitor Sitagliptin on Glucose Metabolism in Healthy Cats. Res. Vet. Sci. 2015, 99, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.L.; Riederer, A.; Fracassi, F.; Boretti, F.S.; Sieber-Ruckstuhl, N.S.; Lutz, T.A.; Contiero, B.; Zini, E.; Reusch, C.E. Glycemic Variability in Newly Diagnosed Diabetic Cats Treated with the Glucagon-like Peptide-1 Analogue Exenatide Extended Release. J. Vet. Intern. Med. 2020, 34, 2287–2295. [Google Scholar] [CrossRef]
- Hall, M.J.; Adin, C.A.; Borin-Crivellenti, S.; Rudinsky, A.J.; Rajala-Schultz, P.; Lakritz, J.; Gilor, C. Pharmacokinetics and Pharmacodynamics of the Glucagon-like Peptide-1 Analog Liraglutide in Healthy Cats. Domest. Anim. Endocrinol. 2015, 51, 114–121. [Google Scholar] [CrossRef]
- Gilor, C.; Graves, T.K.; Gilor, S.; Ridge, T.K.; Rick, M. The GLP-1 Mimetic Exenatide Potentiates Insulin Secretion in Healthy Cats. Domest. Anim. Endocrinol. 2011, 41, 42–49. [Google Scholar] [CrossRef]
- Appleton, D.J.; Rand, J.S.; Sunvold, G.D.; Priest, J. Dietary Chromium Tripicolinate Supplementation Reduces Glucose Concentrations and Improves Glucose Tolerance in Normal-Weight Cats. J. Feline Med. Surg. 2002, 4, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.A.; Dodam, J.R.; McCaw, D.L.; Tate, D.J. Effects of Chromium Supplementation on Glucose Tolerance in Obese and Nonobese Cats. Am. J. Vet. Res. 1999, 60, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M.; Ferguson, D.C. Effect of Darglitazone on Glucose Clearance and Lipid Metabolism in Obese Cats. Am. J. Vet. Res. 2003, 64, 1409–1413. [Google Scholar] [CrossRef]
- Michels, G.M.; Boudinot, F.D.; Ferguson, D.C.; Hoenig, M. Pharmacokinetics of the Insulin-Sensitizing Agent Troglitazone in Cats. Am. J. Vet. Res. 2000, 61, 775–778. [Google Scholar] [CrossRef]
- Clark, M.; Thomaseth, K.; Dirikolu, L.; Ferguson, D.C.; Hoenig, M. Effects of Pioglitazone on Insulin Sensitivity and Serum Lipids in Obese Cats. J. Vet. Intern. Med. 2014, 28, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M.; Clark, M.; Schaeffer, D.J.; Reiche, D. Effects of the Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Velagliflozin, a New Drug with Therapeutic Potential to Treat Diabetes in Cats. J. Vet. Pharmacol. Ther. 2018, 41, 266–273. [Google Scholar] [CrossRef]
- Niessen, S.J.M.; Kooistra, H.S.; Forcada, Y.; Bjørnvad, C.R.; Albrecht, B.; Roessner, F.; Herberich, E.; Kroh, C. Efficacy and Safety of Once Daily Oral Administration of Sodium-Glucose Cotransporter-2 Inhibitor Velagliflozin Compared with Twice Daily Insulin Injection in Diabetic Cats. J. Vet. Intern. Med. 2024, 38, 2099–2119. [Google Scholar] [CrossRef]
- Benedict, S.L.; Mahony, O.M.; McKee, T.S.; Bergman, P.J. Evaluation of Bexagliflozin in Cats with Poorly Regulated Diabetes Mellitus. Can. J. Vet. Res. 2022, 86, 52–58. [Google Scholar]
- Hadd, M.J.; Bienhoff, S.E.; Little, S.E.; Geller, S.; Ogne-Stevenson, J.; Dupree, T.J.; Scott-Moncrieff, J.C. Safety and Effectiveness of the Sodium-Glucose Cotransporter Inhibitor Bexagliflozin in Cats Newly Diagnosed with Diabetes Mellitus. J. Vet. Intern. Med. 2023, 37, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Burton, S.E.; Weidgraaf, K.; Singh, P.; Lopez-Villalobos, N.; Jacob, A.; Malabu, U.; Burchell, R. The Effect of the Sodium-Glucose Cotransporter Type-2 Inhibitor Dapagliflozin on Glomerular Filtration Rate in Healthy Cats. Domest. Anim. Endocrinol. 2020, 70, 106376. [Google Scholar] [CrossRef] [PubMed]
- Nishii, N.; Takashima, S.; Iguchi, A.; Murahata, Y.; Matsuu, A.; Hikasa, Y.; Kitagawa, H. Effects of Sitagliptin on Plasma Incretin Concentrations after Glucose Administration through an Esophagostomy Tube or Feeding in Healthy Cats. Domest. Anim. Endocrinol. 2014, 49, 14–19. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.H.; Hoenig, M.; Ferguson, D.C.; Dirikolu, L. Pharmacokinetics of Pioglitazone in Lean and Obese Cats. J. Vet. Pharmacol. Ther. 2012, 35, 428–436. [Google Scholar] [CrossRef]
- Rudinsky, A.J.; Adin, C.A.; Borin-Crivellenti, S.; Rajala-Schultz, P.; Hall, M.J.; Gilor, C. Pharmacology of the Glucagon-like Peptide-1 Analog Exenatide Extended-Release in Healthy Cats. Domest. Anim. Endocrinol. 2015, 51, 78–85. [Google Scholar] [CrossRef]
- Hoelmkjaer, K.M.; Wewer Albrechtsen, N.J.; Holst, J.J.; Cronin, A.M.; Nielsen, D.H.; Mandrup-Poulsen, T.; Bjornvad, C.R. A Placebo-Controlled Study on the Effects of the Glucagon-Like Peptide-1 Mimetic, Exenatide, on Insulin Secretion, Body Composition and Adipokines in Obese, Client-Owned Cats. PLoS ONE 2016, 11, e0154727. [Google Scholar] [CrossRef]
- Reusch, C.; Kley, S.; Casella, M. Screening diabetic cats for hypersomatotropism: Performance of an enzyme-linked immunosorbent assay for insulin-like growth factor 1. J. Feline Med. Surg. 2014, 16, 82–88. [Google Scholar]
- Haller, N.; Lutz, T.A. Incretin Therapy in Feline Diabetes Mellitus—A Review of the Current State of Research. Domest. Anim. Endocrinol. 2024, 89, 106869. [Google Scholar] [CrossRef]
| Databases | Google Scholar PubMed Scopus |
|---|---|
| Date of Search | 3 March 2025 |
| Date of Dates included | No restrictions |
| Search input | Google Scholar: (“diabetes mellitus felina” OR “feline diabetes”) AND (“antidiabéticos orales” OR “oral hypoglycemic agents” OR “biguanidas” OR “sulfonilureas” OR “meglitinidas” OR “inhibidores de alfa-glucosidasa” OR “inhibidores de DPP-4” OR “agonistas de GLP-1” OR “inhibidores de SGLT2” OR “metformin” OR “metformina” OR “glipizide” OR “glipizida” OR “acarbose” OR “acarbosa” OR “sitagliptin” OR “sitagliptina” OR “exenatide” OR “exenatida” OR “dapagliflozin” OR “dapagliflozina” OR “bexagliflozin” OR “velagliflozin”) AND (“clinical trial” OR “ensayo clínico” OR “retrospective study” OR “estudio retrospectivo” OR “case report” OR “reporte de caso”) PubMed: (“Feline Diabetes Mellitus”[MeSH] OR “feline diabetes” OR “diabetes mellitus felina”) AND (“Hypoglycemic Agents”[MeSH] OR “oral hypoglycemic agents” OR “antidiabéticos orales” OR “metformin” OR “metformina” OR “glipizide” OR “glipizida” OR “acarbose” OR “acarbosa” OR “sitagliptin” OR “sitagliptina” OR “exenatide” OR “exenatida” OR “dapagliflozin” OR “dapagliflozina” OR “bexagliflozin” OR “velagliflozin”) AND (“Clinical Trial”[Publication Type] OR “Retrospective Studies”[MeSH] OR “retrospective study” OR “estudio retrospectivo” OR “Case Reports”[Publication Type] OR “case report” OR “reporte de caso”) Scopus: TITLE-ABS-KEY (“feline diabetes” OR “diabetes mellitus felina”) AND TITLE-ABS-KEY (“oral hypoglycemic agents” OR “antidiabéticos orales” OR “biguanides” OR “biguanidas” OR “sulfonylureas” OR “sulfonilureas” OR “meglitinides” OR “meglitinidas” OR “alpha-glucosidase inhibitors” OR “inhibidores de alfa-glucosidasa” OR “DPP-4 inhibitors” OR “inhibidores de DPP-4” OR “GLP-1 agonists” OR “agonistas de GLP-1” OR “SGLT2 inhibitors” OR “inhibidores de SGLT2” OR “metformin” OR “metformina” OR “glipizide” OR “glipizida” OR “acarbose” OR “acarbosa” OR “sitagliptin” OR “sitagliptina” OR “exenatide” OR “exenatida” OR “dapagliflozin” OR “dapagliflozina” OR “bexagliflozin” OR “velagliflozin”) AND TITLE-ABS-KEY (“clinical trial” OR “ensayo clínico” OR “retrospective study” OR “estudio retrospectivo” OR “case report” OR “reporte de caso”) |
| PICO | PICO A P: Cats with naturally acquired diabetes mellitus (including newly diagnosed and previously treated). I: Treatment with sulfonylureas, alpha-glucosidase inhibitors, biguanides, GLP-1 receptor agonists, or SGLT2 inhibitors. C: Insulin therapy, placebo, or no concurrent control (baseline comparison) O: Glycemic control and adverse effects. PICO B P: Healthy adult cats with risk factors for FDM (eg, obesity), under challenge with diabetogenic drugs (eg, corticosteroids), or in experimental models of FDM. I: Administration of sulfonylureas, thiazolidinediones, biguanides, GLP-1 receptor agonists, SGLT2 inhibitors, or chromium. C: Placebo or an active comparator (insulin). O: Markers of insulin sensitivity, glucose metabolism, pathological changes, and safety. |
| Inclusion criteria | Examines the efficacy, safety profile, and adverse effects of NIADs used in cats with FDM and cats predisposed to diabetes. |
| Exclusion criteria | Not relevant to the PICO question. Not peer-reviewed. Not original research. Abstract only. Healthy cats and cats without risk of developing diabetes. Single case reports unrelated to antidiabetics, or review articles not containing primary data. |
| Author (Year) [Cite] | Study Design | Population (n, Characteristics) | Intervention (Drug, Dose, Duration) | Comparator | Reported Efficacy Outcomes | Reported Adverse Effects | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Nelson et al. (1993) [11] | PCUCT | n = 20 cats with naturally acquired FDM (most non-ketotic). Mean age: 9.5 years. The group was generally overweight/obese. History of prior treatment in some cases. | Glipizide: 5 mg/cat, orally, every 12 h. Duration of follow-up was 12 weeks. | None. Cats served as their own controls. | Three response groups identified: 25% (5/20) had good glycemic control. 35% (7/20) failed to respond. 40% (8/20) had a partial response. | Vomiting (3 cats), hypoglycemia (3 cats), and increased serum hepatic enzyme activities (3 cats) were reported. | Moderate |
| Feldman et al. (1997) [10] | PCUCT | n = 50 cats with recently diagnosed, untreated FDM. Mean age: 10.4 years; mean weight: 5.3 kg. All neutered. Non-ketotic. | Glipizide: 5 mg/cat, orally, every 12 h, administered in treatment phases over 50 weeks. | None. The study included a “no medication” phase as part of its design. | 56% (28/50) were treatment failures and switched to insulin. Of the remaining 22, 13 (26% of total) showed clinical/metabolic improvement, and 6 (12% of total) had their FDM resolved. | Transient anorexia and vomiting (8 cats), transient icterus with elevated liver enzymes (4 cats). | Serious |
| Mazzaferro et al. (2003) [18] | NRCCT | n = 24 client-owned diabetic cats (18 diet/acarbose, 6 diet-only controls). All with a history of obesity. Median age: 10 years. | Acarbose (12.5 mg/cat, orally, BID) combined with a low-carbohydrate diet and concurrent insulin therapy for 4 months. | Low-carbohydrate diet with concurrent insulin therapy. | In the main group (n = 18), 61% (11/18) became ‘responders’ and discontinued insulin. The authors concluded that acarbose likely had a minimal effect compared to the diet alone. | The article does not explicitly report any adverse effects associated with the acarbose treatment. | Serious |
| Nelson et al. (2004) [14] | PUCCS | Phases 1 and 2: n = 8 healthy adult cats. Phase 3: n = 5 cats with newly diagnosed, naturally acquired diabetes mellitus (Mean age: 11 years. Two cats were obese). | Phases 1 and 2: Metformin dose-finding study in healthy cats. Phase 3: Metformin, orally, with a dose escalation protocol up to 50 mg/cat BID for up to 8 weeks in diabetic cats. | None. | Only 20% (1/5) of cats achieved glycemic control. 60% (3/5) failed to respond and were switched to insulin. 1 cat died unexpectedly. | Lethargy, inappetence, vomiting, and weight loss were noted in healthy cats during the safety phase. One diabetic cat died (cause undetermined, but GI hemorrhage was found). | Serious |
| Riederer et al. (2016) [19] | NRPCT | n = 30 client-owned cats with newly diagnosed FDM. Median age: ~9.5 years. Treated with insulin and a low- carbohydrate diet. | Exenatide extended release (200 µg/kg), SC, once weekly for up to 16 weeks, as an adjunct to insulin therapy. | Placebo (0.9% saline), SC, once weekly, as an adjunct to insulin therapy. |
| Decreased appetite (60% vs. 20%) and vomiting (53% vs. 40%) were more frequent in the exenatide group, but the difference was not statistically significant. | Serious |
| Scuderi et al. (2018) [2] | RCT | n = 8 client-owned diabetic cats (Body condition score [BCS] ≥ 5/9). Median age: 12 years. All previously treated with insulin glargine for ≥1 month. | Exenatide (short-acting): 1 µg/kg, SC, twice daily for 6 weeks, as an adjunct to insulin therapy. | Placebo (0.9% saline) injection. | Exenatide significantly lowered the required daily insulin dose and induced greater weight loss compared to placebo. 25% (2/8) of cats achieved remission with exenatide vs. 0% with placebo. | Treatment was well tolerated. 2 cats required a temporary dose reduction due to anorexia and weakness associated with hypoglycemia. | Low |
| Benedict et al. (2022) [31] | PUCCS | n = 5 client-owned cats with poorly regulated FDM on insulin therapy. Median age: 8 years. | Bexagliflozin (10–15 mg/cat), orally, once daily for 4 weeks, as an adjunct to a reduced insulin protocol. | None. Pre-post comparison. | Significant reduction in insulin dose requirement in all cats (p = 0.015), with insulin discontinued in 2/5 cats. Significant decrease in mean BG (p = 0.022). | No significant adverse effects occurred. No episodes of hypoglycemia were documented. | Serious |
| Hadd et al. (2023) [32] | POOLHCT | n = 84 client-owned cats with newly diagnosed FDM. Median age: 10.8 years. | Bexagliflozin: 15 mg/cat, orally, once daily for at least 56 days. | None (historically controlled). | 84% (68/81 evaluable cats) achieved treatment success (defined as glycemic control and improvement of at least one clinical sign) at day 56 | Common: emesis (50%), diarrhea (38%), anorexia (37%). Serious: 8 cats experienced SAEs, including 4 with known or presumed eDKA. 3 deaths/euthanasias occurred. | Serious |
| Niessen et al. (2024) [30] | ROLACT | n = 127 client-owned diabetic cats (n = 116 for efficacy analysis). Included both newly diagnosed and previously insulin-treated cats. Mean age: 11 years. | Velagliflozin: 1 mg/kg, orally, once daily for up to 91 days. | Insulin (Caninsulin), SC, twice daily, with dose adjusted by clinicians. | The study demonstrated non-inferiority. At Day 45, 54% (29/54) of velagliflozin-treated cats were treatment successes, compared to 42% (26/62) of insulin-treated cats. | Adverse effects differed by group. Velagliflozin group: most frequent were diarrhea (38%) and positive urine culture (31%); eDKA occurred in 4/61 cats. Insulin group: most frequent was hypoglycemia (clinical & non-clinical), occurring in 53% of cats. | Some |
| Behrend et al. (2024) [1] | POLBCT | n = 252 client-owned diabetic cats. Included both newly diagnosed (85%) and previously insulin-treated (15%) cats. Median age: 11 years. | Velagliflozin oral solution: 1 mg/kg, orally, once daily for up to 180 days. | None (baseline-controlled). | At day 180, 81% of remaining cats had BG and/or fructosamine within reference ranges. 88.6% and 87.7% showed owner-reported improvement in polyuria and polydipsia, respectively. | Ketoacidosis occurred in 7.1% of cats, with most cases being euglycemic. Ketonuria without acidosis occurred in an additional 6.7%. Most episodes occurred within the first 14 days of treatment. | Serious |
| Author (Year) [Cite] | Reported Breed Composition |
|---|---|
| Nelson et al. (1993) [11] | Not specified |
| Feldman et al. (1997) [10] | Not specified; described as cats from a referral population |
| Mazzaferro et al. (2003) [16] | Not specified |
| Nelson et al. (2004) [14] | Not specified |
| Riederer et al. (2016) [18] | Primarily Domestic Shorthair and Longhair |
| Scuderi et al. (2018) [2] | Not specified |
| Benedict et al. (2022) [31] | Domestic Shorthair (n = 3), Domestic Longhair (n = 2) |
| Hadd et al. (2023) [32] | Primarily domestic mixed-breed cats from North America |
| Niessen et al. (2024) [30] | Primarily Domestic Shorthair and Longhair |
| Behrend et al. (2024) [1] | Primarily Domestic Shorthair/Longhair; purebred cats < 10% |
| Author (Year) [Cite] | Study Design | Population (n, Characteristics) | Intervention (Drug, Dose, Duration) | Comparator | Reported Efficacy Outcomes | Reported Adverse Effects | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Cohn et al. (1999) [25] | NRPCT | n = 19 healthy, non-diabetic cats. Divided into 3 groups: non-obese placebo (n = 6), non-obese Cr (n = 6), and obese Cr (n = 7). | Chromium picolinate: 100 µg/cat, orally, every 24 h for 6 weeks. | Placebo (calcium phosphate). | No significant effect. Chromium supplementation did not alter responses to IV Glucose Tolerance Testing (IVGTT) in either the non-obese or obese groups. | No adverse health effects were observed. A significant decrease in serum potassium was noted in obese cats, but values remained within the reference range. | Moderate |
| Hoenig et al. (2000) [9] | RCT-EM | n = 8 healthy male cats, in FDM was experimentally induced via partial pancreatectomy and hormonal treatment. | Glipizide: 5 mg/cat, orally, 2–3 times daily for 18 months. | Insulin (Humulin N). | The primary outcome was islet amyloid formation. 100% (4/4) of glipizide-treated cats developed islet amyloid, while only 25% (1/4) of insulin-treated cats did. | No clinical adverse effects were reported. The study was terminal. | Some concerns |
| Hoenig & Ferguson (2003) [26] | RCT-PC | n = 22 healthy, non-diabetic adult female cats (4 lean, 18 obese). Obese cats randomized to placebo (n = 9) or darglitazone (n = 9). | Darglitazone: 2 mg/kg, orally, once daily for 42 days. | Placebo capsule. | Darglitazone significantly improved insulin sensitivity (reduced area under the curve (AUC) for glucose and insulin in IVGTT) compared to placebo. Also significantly lowered cholesterol and triglyceride concentrations. | The drug was well tolerated. No negative clinical effects were reported. | Some concerns |
| Clark et al. (2012) [36] | RCT-PK | n = 12 healthy adult cats, divided into lean (n = 6) and obese (n = 6) groups. | Pioglitazone: single oral dose of 3 mg/kg. | IV administration of the same drug (bioavailability study). | This was a pharmacokinetic study. It found that the oral bioavailability of pioglitazone was high (mean 86%) and not significantly different between lean and obese cats. | No adverse effects were observed during the study. | Some concerns |
| Clark et al. (2014) [28] | RCT-3WC | n = 12 obese, healthy, non-diabetic adult cats (6 male, 6 female). Age: 5–7 years. | Pioglitazone: 1 mg/kg or 3 mg/kg, orally, once daily for 7-week periods. | Placebo capsule. | The 3 mg/kg dose significantly improved insulin sensitivity and lowered serum triglyceride and cholesterol concentrations compared to placebo. | No adverse effects attributable to pioglitazone were evident. One cat died during sedation, considered unlikely to be caused by the drug. | Some concerns |
| Hall et al. (2015) [22] | RCT-PCCT | n = 8 healthy adult cats (6 male, 2 female). Three were lean (BCS 5/9) and five were overweight (BCS 6–8/9). | Liraglutide: single SC injection of 3 or 6 nmol/kg. | Placebo (saline) injection. | Liraglutide significantly increased glucose-stimulated insulin secretion and suppressed glucagon secretion compared to placebo. It did not have a significant effect on food intake. | The study did not report any adverse effects. | Some concerns |
| Rudinsky et al. (2015) [37] | RCT-PCCT | n = 6 healthy, lean, adult n = 6 healthy, adult neutered male cats. Three were classified as lean (BCS 5/9) and three as overweight (BCS 6–7/9) male cats. | Exenatide ER: single SC injection of 2 mg/cat. | Placebo (vehicle) injection. | Did not significantly alter glucose or insulin response during an IVGTT. Induced a significant reduction in food intake on days 2–4 post-injection. | Vomiting was observed in 5 of 6 cats within 24 h of exenatide injection. | Some concerns |
| Hoelmkjaer et al. (2016) [38] | RCT-DBPC | n = 11 obese, but otherwise healthy, client-owned cats. | Exenatide extended release: 2 mg/cat, SC, once weekly for 8 weeks. | Placebo injection. | Exenatide significantly decreased food intake and led to weight loss compared to placebo. It did not significantly improve insulin secretion or insulin sensitivity during an IVGTT. | Transient and mild hyporexia and vomiting were the most common adverse effects observed. | Some concerns |
| Hoenig et al. (2018) [29] | NRPCT | n = 12 obese, healthy, non-diabetic adult cats (6 male, 6 female). Median age: 6 years. | Velagliflozin: 1 mg/kg, orally, once daily for 35 days. | Placebo capsule. | Velagliflozin significantly increased urinary glucose excretion but did not significantly alter overall glucose tolerance. It did lower the insulin response, suggesting improved insulin sensitivity. | “All cats tolerated treatment well.” Soft stool was observed in 2 cats in each group. | Moderate |
| Leal et al. (2022) [5] | RCT | n = 28 client-owned, non-diabetic cats receiving a single dose of methylprednisolone acetate (MPA). Mean age: 5.6 years. | Two intervention groups: 1) Metformin (25 mg/cat, orally, once daily) or 2) A commercial obesity/diabetes (O and D) diet. Both for 30 days. | Control group (n = 10) that received only the MPA injection. | No significant protective effect. Neither metformin nor the O and D diet was effective in preventing the insulin resistance induced by the MPA injection. | No side effects associated with metformin or the diet were reported by the owners. | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Vélez, F.; Rejas, J.; Ruiz de Gopegui, R. Efficacy and Safety of Non-Insulin Antidiabetic Drugs in Cats: A Systematic Review. Animals 2025, 15, 2561. https://doi.org/10.3390/ani15172561
Romero-Vélez F, Rejas J, Ruiz de Gopegui R. Efficacy and Safety of Non-Insulin Antidiabetic Drugs in Cats: A Systematic Review. Animals. 2025; 15(17):2561. https://doi.org/10.3390/ani15172561
Chicago/Turabian StyleRomero-Vélez, Félix, Juan Rejas, and Rafael Ruiz de Gopegui. 2025. "Efficacy and Safety of Non-Insulin Antidiabetic Drugs in Cats: A Systematic Review" Animals 15, no. 17: 2561. https://doi.org/10.3390/ani15172561
APA StyleRomero-Vélez, F., Rejas, J., & Ruiz de Gopegui, R. (2025). Efficacy and Safety of Non-Insulin Antidiabetic Drugs in Cats: A Systematic Review. Animals, 15(17), 2561. https://doi.org/10.3390/ani15172561






