Simple Summary
In modern animal husbandry production, rabbits are readily affected by heat stress in summer. A critical issue in rabbit production is to determine the mechanism by which heat stress prevents muscle growth. In this study, we found that heat stress reduced the carcass yield of meat rabbits, changed the physical characteristics of the skeletal muscle, and influenced protein metabolism by changing blood indices, potentially through the PI3K/Akt signalling pathway.
Abstract
The purpose of this experiment was to study the effects of heat stress on the performance and protein metabolism of skeletal muscle in meat rabbits. A total of 160 New Zealand White rabbits aged 80 days with mean initial body weights of 2359 ± 200 g were randomly divided into a control group and a heat stress group. The experiment duration was 20 days. Heat stress treatment reduced the growth performance and slaughter performance of the rabbits (p < 0.05) and increased muscle yellowness (b*, p < 0.05). In addition, heat stress treatment increased the concentrations of leptin, cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol in serum (p < 0.05), and decreased the serum total protein and immunoglobulin (IgG, IgM, and IgA) contents of rabbits. Under the criteria fold-change ≥ 1.20 or ≤0.84 and p-value ≤ 0.05, 7 up-regulated proteins and 122 down-regulated proteins were screened. A gene ontology (GO) enrichment analysis of the differentially expressed proteins was performed. The most enriched specific GO terms among the differential proteins were response to stress, extracellular region, and protein binding in the biological process (BP), cellular component (CC), and molecular function (MF) categories, respectively, and the most enriched pathway was the PI3K/Akt signalling pathway. In conclusion, heat stress could reduce the carcass yield of meat rabbits, change the physical characteristics of the skeletal muscle, and influence protein metabolism by changing blood indices, potentially through the PI3K/Akt signalling pathway.
1. Introduction
In modern animal husbandry production, livestock and poultry are readily affected by many abiotic stress factors that negatively impact animal performance, especially heat stress in summer [1]. The entire body of the meat rabbit is coated, the sweat gland function is underdeveloped, body temperature regulation is poor, and the animal is readily subject to heat stress, all of which affect its production performance and product quality [2,3]. One crucial issue in rabbit production is to elucidate the mechanism by which heat stress prevents muscle growth. Meat is the most important product in animal production, and the reduced growth caused by heat stress is an impediment to overall animal performance and farm profitability [4,5]. Short-term heat stress may also lead to a decline in performance and weight loss in pigs [6]. Skeletal muscle catabolism increases during heat stress and leads to an increase in plasma markers of muscle catabolism in several species [7,8,9,10]. Li et al. [11] reported that heat stress has no direct effect on cow insulin and leptin secretion. However, heat stress leads to lower dry matter feed intake, which increases insulin and leptin concentrations on top of the same dry matter feed intake. Due to changes in muscle metabolism, effective muscle growth and function may be countered. Increasing evidence shows that heat stress changes intracellular metabolism, causing increased glycolysis and incomplete oxidative phosphorylation [12]. With the rapid development of genomics, proteomics, and other technologies, the use of tandem mass tag (TMT)-based proteomics has become a powerful tool to explore the biological processes of animal growth and development and is widely used in the research on the regulatory mechanisms of animal production traits [13]. Zheng et al. [14] used a TMT-based proteomic technique to study differentially expressed proteins in bovine mammary glands at peak lactation and late lactation. A total of 179 differentially expressed proteins were screened, of which 14 were associated with lactation performance and mammary gland morphology. Wang et al. [15] studied differentially expressed proteins during the development of skeletal muscle in sheep embryos using a TMT-labelling proteomic technique, as well as identified 1316 differentially expressed proteins and further analyzed the functions of these proteins. However, the application of TMT-based proteomics technology in rabbits is rare. Moreover, in the Fujian province of China, people like to use rabbits weighing more than 3 kg to make soup. Based on the above requirements, in this experimental study, we chose to use purebred New Zealand rabbits as experimental animals, and the purebred New Zealand rabbits can reach a weight of 3 kg at the age of 100 days, at which time the slaughter achieves a better yield. Therefore, the effect of heat stress on the growth performance, slaughter performance, and meat quality of New Zealand White rabbits was investigated from 80 days to 100 days, and the protein metabolism of skeletal muscle was evaluated using a TMT proteomics approach.
2. Materials and Methods
2.1. Experimental Design
In this experiment, 160 New Zealand White rabbits aged 80 days with a mean initial body weight of 2359 ± 200 g and in good health were purchased by the Qingdao Kangda Rabbit Industry Development Co., Ltd., Qingdao, China. The rabbits were randomly divided into two groups, namely a control group and a heat stress treatment group, with 10 replicates in each group and eight rabbits in each replicate. The experimental animals were fed in two completely identical closed rabbit houses. In the control group, the air temperature was maintained at 25–28 °C with adequate ventilation, and the relative humidity was 50–60%. For the heat stress treatment group, the air temperature was maintained above 32 °C, and a humidifier maintained the relative humidity at 70–80%. The treatment period was 20 days. The rabbits were fed the same diet (the feed ingredients and nutritional levels are shown in Table 1), and feed and water were provided ad libitum. During the experiment, the temperature and humidity near the door, window, and in the centre of the house in the feeding room of the control group and the heat stress group were measured with a digital thermometer every day, and the daily average temperature and humidity were calculated. The temperature–humidity index (THI) formula proposed by Marai et al. [16] was used to calculate the environmental THI: THI = db − [(0.31 − 0.31RH)/(db − 14.4)], where db and RH are the thermometer value (°C) and relative humidity (%), respectively.

Table 1.
Composition and nutritional level of the experimental diets (dry matter basis, %).
2.2. Sample Collection and Preparation
At the end of the experiment (100 days of age), ten experimental rabbits in each treatment (one rabbit whose weight was close to the average weight of this repetition was selected) were selected for sample collection. A 10 mL blood sample was collected from the heart and then centrifuged at a centrifugal force of 1500 g for 10 min. The isolated serum samples were stored at −80 °C for the determination of serum biochemical indices. In addition, the longissimus thoracis et lumborum (LTL) muscles from both sides of each carcass were collected to determine their physical properties. A sample (1 g) of the LTL muscle was placed in a freezing tube and stored in liquid nitrogen. Samples of four rabbits randomly selected from the control group and the treatment group were selected for inspection, and a TMT-based quantitative proteomic analysis was conducted.
2.3. Determination of Indicators and Methods
2.3.1. Growth Performance
At the beginning and end of the experiment, the weight of each rabbit was measured, and the average daily gain was calculated. The average daily feed intake was calculated by dividing the total feed intake of each repetition by the total number of days of the experiment and the number of test rabbits. The feed–gain ratio was calculated as feed intake/weight gain.
2.3.2. Slaughter Performance
Twelve hours prior to slaughter, the rabbits were fasted and weighed to determine the preslaughter body weight. The slaughter procedure and carcass analysis were performed as described by Blasco and Ouhayoun [17]. Before slaughter, the animals were stunned by electric shock and then slaughtered by bloodletting. After bleeding, the pelts, paws, and full gastrointestinal tract were removed, and the semiclean carcass weight was the carcass weight after removing the head at the first cervical vertebra, removing the trachea and esophagus, and retaining the heart, liver, and kidney. The full clean carcass weight is the semiclean carcass weight after removing the heart, liver, and kidney. The head, heart, liver, and kidney were also weighed. The semiclean slaughter ratio and full clean slaughter ratio were calculated by dividing their weights by the live weight before slaughter.
2.3.3. Blood Indices
Commercial radioactive immune assay kits supplied by Tianjin Jiuding Company (Tianjin, China) were used to analyze insulin, glucagon, and leptin contents in serum, and radioactivity was determined in DFM-96 10 tubes with a radioactive immune gamma counter (Hefei Zhongcheng Electromechanical Technology Development Co., Ltd., Hefei, China). Serum glucose, cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were determined with a sequential multiple analyzer (Hitachi 7020, Tokyo, Japan) following the manual of commercial protocols (Wako, Japan). The IgG, IgM, and IgA concentrations were measured by immunoturbidimetry using enzyme-linked immunosorbent assay kits (CEA544Rb, CEA543Rb, and SEA641Rb, respectively; Cloud-Clone Corp., Wuhan, China).
2.3.4. Meat Physical Characteristics
The pHu values (pH measured at 24 h postmortem), muscle colour (L*, a*, and b*), drip loss ratio, cooking loss ratio, and shear force were measured following a previous report [18].
2.3.5. Identification and Bioinformatic Analysis of Protein
After retrieving the original data from the database, blank values were removed. Based on credible proteins, the differentially expressed proteins were screened based on different screening conditions. A gene ontology (GO) analysis of the identified differentially expressed proteins was performed with Blast2GO software(Version 4.1.9). The attributes of these proteins were described in relation to the biological process, molecular function, and cellular component. The identified differential proteins were uploaded to the UniProt database (http://www.uniprot.org/uploadlists/ (accessed on 28 August 2025)), exported as a fasta file, and the enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were ascertained to determine the most important metabolic pathways involving the differentially expressed proteins.
2.4. Statistical Analysis
The data were analyzed by analysis of variance (ANOVA) followed by Duncan’s multiple range test. The general linear model (GLM) procedure of SAS 9.4 statistical software (SAS Institute Inc., Cary, NC, USA) was used. The data are expressed as the mean ± standard deviation, and p < 0.05 was considered to be significant.
3. Results and Analysis
3.1. Temperature—Humidity Index
- Heat stress in rabbits can be divided into four grades: no heat stress at THI < 27.8; moderate heat stress at 27.8 < THI < 28.9; severe heat stress at 28.9 < THI < 30.0; and especially severe heat stress at THI > 30. The average daily THI of the control group was lower than 27.8 (Figure 1), indicating that the experimental rabbits in this group were in a state of no heat stress throughout the experiment. The average daily THI of the heat stress group was greater than 28.9, and thus, the rabbits were in a state of heat stress.
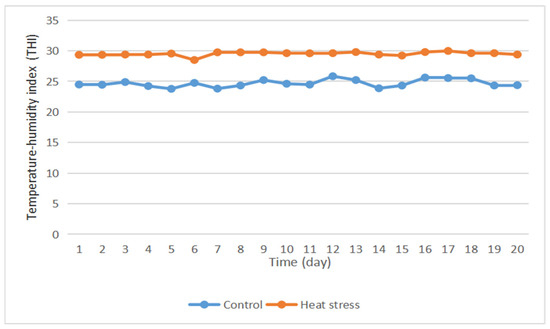 Figure 1. Diurnal variation curve of temperature and humidity index during the experiment.
Figure 1. Diurnal variation curve of temperature and humidity index during the experiment.
3.2. Growth Performance
Heat stress treatment reduced the average daily feed intake and average daily gain of the rabbits (p < 0.05), but there was no observed difference in the feed–gain ratio (p > 0.05; Table 2).

Table 2.
Effects of heat stress on growth performance of meat rabbits.
3.3. Slaughter Performance
The preslaughter body weight, semiclean carcass weight, full clean carcass weight, semiclean slaughter ratio, full clean slaughter ratio, and liver weight of the experimental group treated with heat stress were lower than those of the control group (p < 0.05; Table 3). No differences in head weight, heart weight, and kidney weight were observed in this experiment (p > 0.05).

Table 3.
Effects of heat stress on slaughter performance of meat rabbits.
3.4. Blood Indices
Heat stress treatment increased the concentrations of leptin, cholesterol, HDL, and LDL in serum (p < 0.05), and decreased the serum total protein and immunoglobulin (IgG, IgM, and IgA) contents of the rabbits (Table 4). No effects on serum insulin, glucagon, and glucose contents were observed (p > 0.05).

Table 4.
Effects of heat stress on blood indices of meat rabbits.
3.5. Physical Characteristics of the LTL Meat
Heat stress changed the muscle colour and increased muscle yellowness (b*, p < 0.05). However, no differences in the pHu value, shear force, drip loss ratio, and cooking loss ratio were observed (p > 0.05; Table 5).

Table 5.
Effects of different ambient temperatures on meat quality of meat rabbits.
3.6. Protein Metabolism of Skeletal Muscle
3.6.1. Data Quality Control and Identification
The mass spectrometry data were searched against the uniprot-oryctolagus-cuniculus-filtered-organism-Oryctolagus-cuniculus protein database. The obtained fasta file contained 23,058 sequences. To improve the quality of the analysis results and reduce the false positive rate, Proteome Discoverer 2.2 software was used to filter the search results. Peptide spectrum matches (PSMs) with more than 99% confidence were trusted PSMs, and proteins containing at least one unique peptide segment were trusted proteins. Only the trusted peptides and proteins were retained, and those with a false discovery rate > 1% were removed. In this study, there were a total of 324,622 spectra, 79,888 matched spectra, 18,941 identified peptides, 2463 identified proteins, and 2460 quantifiable proteins.
3.6.2. Protein Function Annotation
To explore the functional characteristics of the differential proteins, the identified proteins were annotated based on information in the GO, KEGG, COG, and IPR databases. In total, 1147 proteins were annotated from these databases (Figure 2).

Figure 2.
Results of function annotation.
3.6.3. Protein Quantitative Analysis
The coefficient of variation (CV), which is the ratio of the standard deviation to the mean, was used to measure the degree of variation in each observed value of a sample, which reflects the dispersion degree of the data, and thus, is a measure of repeatability. The smaller the CV value, the better the repeatability (Figure 3).
Figure 3.
Coefficient of variation analysis, hs: heat stress.
3.6.4. Differential Protein Analysis
Applying the criteria of fold-change (FC) ≥ 1.20 or ≤0.84 and p-value ≤ 0.05, 7 up-regulated proteins and 122 down-regulated proteins were screened (Table 6). With FC ≥ 1.30 or ≤0.77 and p-value ≤ 0.05, 4 up-regulated proteins and 64 down-regulated proteins were screened. With FC ≥ 1.50 or ≤0.67 and p-value ≤ 0.05, 2 up-regulated proteins (G1SUJ3, Histone H2A, and B7NZF9, Nucleophosmin 1 isoform 1, predicted) and 19 down-regulated proteins were screened (Table 7). Finally, with FC ≥ 2.00 or ≤0.50 and p-value ≤ 0.05, no up-regulated proteins and three down-regulated proteins (P01870, Ig gamma chain C region; A0A1Y1B8B3 and A0A1Y1BG72, IgG heavy chain VDJ region, fragment) were screened. For each protein multiple of difference, the log2-transformed FC and log10-transformed absolute p-value were plotted to generate a volcano map (Figure 4).

Table 6.
The quantity of differential proteins.

Table 7.
The information on differential proteins (fold-change > 1.5 or ≤0.67).

Figure 4.
Volcano map of differential proteins, hs: heat stress.
3.6.5. Enrichment Analysis
The most enriched GO terms among the differential proteins were response to stress, extracellular region, and protein binding in the biological process, cellular component, and molecular function categories, respectively (Figure 5). The target genes were identified in the KEGG database to determine the pathways involving the differential proteins. The most enriched pathway was the PI3K/Akt signalling pathway (Figure 6).
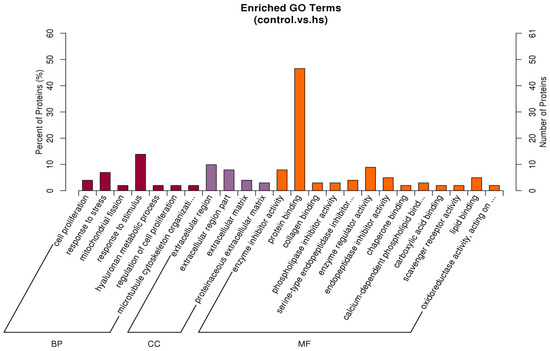
Figure 5.
The histogram of GO enrichment for three categories, each of which shows up to 20 kinds (p-value ≤ 0.05). The percentage of ordinate represents x/n in the table. BP: biological process; CC: cellular component; MF: molecular function, hs: heat stress.

Figure 6.
The bubble diagram of KEGG enrichment, hs: heat stress.
4. Discussion
Growth involves a series of complex metabolic events, which are controlled by heredity and environment. Heat stress can reduce the productivity of almost all livestock breeds [19]. As warm-blooded animals, rabbits use physical, morphological, biochemical, and behavioural processes to regulate their body’s heat input and output to maintain a constant body temperature [20]. The temperature of a rabbit’s thermal neutral zone is approximately 18–21 °C [21]. Therefore, under exposure to high ambient temperature, the body temperature of a rabbit will be out of balance, which has adverse effects on growth and reproduction traits [22,23,24]. Previous studies have reported a reduction in rabbit feed consumption under heat stress [25] because high ambient temperature stimulates the peripheral heat receptors to transmit inhibitory nerve impulses to the appetite centre of the hypothalamus. In this study, total and daily weight gains in growing rabbits were suppressed. The reduction in daily gain was due to a decrease in rabbit feed intake (215.31 vs. 185.76 g/d; Table 2), which might have led to decreased protein biosynthesis and less fat deposition [26,27].
Owing to changes in muscle metabolism, effective muscle growth and function may be countered [28]. Increasing evidence shows that heat stress changes intracellular metabolism and that these changes indicate an increase in glycolysis and incomplete oxidative phosphorylation [5]. Lactic acid production and pyruvate kinase activity in the muscle of broilers increase under chronic heat stress, which provides evidence of increased glycolytic ability [29]. Similarly, when exercising at high temperatures, the plasma lactic acid concentration will increase [30]. With an increase in temperature, the decrease in serum total protein seems to be due to the dilution of serum total protein caused by the increase in water consumption, and/or it may be due to the increase in protein utilization and amino acid transamination in heat-stressed rabbits. Heat stress will affect the lipid metabolism of broilers, and the high cholesterol and triglyceride contents in the blood reflect enhanced lipid catabolism [31]. Leptin is a hormone secreted by adipose tissue and involved in promoting fat breakdown and utilization, and its content in serum is proportional to the size of the animal’s adipose tissue [32,33]. Leptin acts on receptors located in the central nervous system to regulate the behaviour and metabolism of organisms [34]. Leptin regulates the energy balance and weight of organisms through a negative feedback mechanism [35]. Leptin has the effect of suppressing appetite, increasing protein degradation, and inhibiting fat synthesis, affecting many physiological systems and metabolic pathways of the body [36]. The HDL receptor mediates the selective uptake of cholesterol [37]. Rinaldo and Le [38] showed that heat stress may reduce the activity of glucose-6-phosphate dehydrogenase, thus reducing the activity of reduced nicotinamide adenine dinucleotide dehydrogenase and inhibiting energy metabolism. In addition, we observed that heat stress reduced the content of immunoglobulin in the blood, which may lead to a decrease in animal immunity and an increase in mortality during a hot summer.
Heat stress also affected the amount and type of volatile substances in muscle, which affected muscle odour [39], dietary supplementations of vitamin C, organic selenium, betaine, and the effect of pomegranate peel on alleviating the effect of heat stress on growing rabbits [40]. Our findings show that heat stress increased the expression of the proteins G1SUJ3 (Histone H2A) and B7NZF9 (Nucleophosmin 1 isoform 1, predicted), and decreased the expression of the proteins P01870 (Ig gamma chain C region), A0A1Y1B8B3, and A0A1Y1BG72 (IgG heavy chain VDJ region, fragment). Moreover, the most enriched specific GO terms for the differential proteins were response to stress, extracellular region, and protein binding in the biological process, cellular component, and molecular function categories (Figure 5). As the central link in the insulin pathway, the PI3K/AKT axis regulates hepatic glycogen synthesis, gluconeogenesis, and lipid synthesis [41,42]. In addition, the PI3K/AKT axis regulates lipogenesis by inhibiting a sterol regulatory element-binding transcription factor (SREBP-1c), subsequently increasing hepatic LDL receptor protein expression [43,44].
5. Conclusions
Heat stress disrupts the growth and carcass yield of growing meat rabbits, alters some physical characteristics of the skeletal muscle, and influences protein metabolism and lipid metabolism by changing blood indices. These responses may be mediated through the PI3K/Akt signalling pathway.
Author Contributions
G.L., C.L. and S.G. performed the experiments; H.S. and Y.Z. conceived the project idea, designed the study, and conducted the animal experiments; L.Y. and L.B. performed the laboratory work; and G.L. and S.G. analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Shandong Province Modern Agricultural Industry Technology System (SDAIT-21-09); the Key Research and Development Program of Shandong Province (Action Plan for Rural Revitalization of Science and Technology Innovation, 2023TZXD044); the Natural Science Foundation of Shandong Province (ZR2023QC017); and the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-43-G-7).
Institutional Review Board Statement
The experimental procedures were approved by the Shandong Academy of Agricultural Sciences Animal Care and Use Committee (SAAS-2019-03, 1 September 2019) and were conducted in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). The experimental procedures in this test are compliant with the ARRIVE guidelines, in accordance with the U.K. Animals (Scientific Procedures) Act, 1986, and associated guidelines, EU Directive 2010/63/EU for animal experiments, or with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets obtained and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the staff of Liaocheng Huifu Agriculture and Animal Husbandry Co., Ltd., for the feeding experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chang-Fung-Martel, J.; Harrison, M.T.; Brown, J.N.; Rawnsley, R.; Smith, A.P.; Meinke, H. Negative relationship between dry matter intake and the temperature-humidity index with increasing heat stress in cattle: A global meta-analysis. Int. J. Biometeorol. 2021, 65, 2099–2109. [Google Scholar] [CrossRef]
- Asemota, O.D.; Aduba, P.; Mello, G.; Orheruata, A.M. Effect of temperature humidity index (THI) on the performance of rabbits (Oryctolagus cuniculus) in the humid tropics. Arch. De Zootec. 2017, 66, 257–261. [Google Scholar]
- Marco-Jiménez, F.; García-Diego, F.J.; Vicente, J.S. Effect of gestational and lactational exposure to heat stress on performance in rabbits. World Rabbit Sci. 2017, 25, 17–25. [Google Scholar] [CrossRef][Green Version]
- Brown-Brandl, T.M.; Nienaber, J.A.; Xin, H.; Gates, R.S. A literature review of swine heat production. Trans. ASABE 2004, 47, 259–270. [Google Scholar] [CrossRef]
- Jing, J.; Wang, J.; Wu, Q.; Yin, S.; He, Z.; Tang, J.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; et al. Nano-Se exhibits limited protective effect against heat stress induced poor breast muscle meat quality of broilers compared with other selenium sources. J. Anim. Sci. Biotechno. 2024, 15, 2571–2588. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Pontalti, E.; Pirrone, F.; Zotte, D.A.; Eleonora, N.; Rina, V.; Simona, M.; Marco, B. Impact of heat stress on growth performance and carcass traits of fast- medium- and slow-growing broiler chicken genotypes. Poult. Sci. 2025, 104, 105509. [Google Scholar] [CrossRef]
- Genz, J.; West, C. Effects of rearing temperature on growth, energy reserves, and thermal plasticity of juvenile lake sturgeon. Fish Physiol. Biochem. 2025, 51, 124. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.B.; Shi, B.L.; Yang, H.J.; Zheng, N.; Wang, J.Q. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J. Zhejiang Univ.-Sci. Biotech. 2015, 16, 541–548. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Zheng, X.; Ning, C.; Dong, Y.; Zhao, P.; Li, J.; Fan, Z.; Li, J.; Yu, Y.; Mrode, R.; Liu, J. Quantitative proteome analysis of bovine mammary gland reveals protein dynamic changes involved in peak and late lactation stages. Biochem. Biophys. Res. Commun. 2017, 494, 292–297. [Google Scholar] [CrossRef]
- Wang, X.; Shi, T.; Zhao, Z.; Hou, H.; Zhang, L. Proteomic analyses of sheep (ovis aries) embryonic skeletal muscle. Sci. Rep. 2020, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.M.; Habeeb, A.A.M.; Gad, A.E. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: A review. Livest. Prod. Sci. 2002, 78, 71–90. [Google Scholar] [CrossRef]
- Blasco, A.; Ouhayoun, J. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1996, 4, 93–99. [Google Scholar] [CrossRef]
- Liu, G.; Bai, L.; Sun, H.; Liu, C.; Yang, L.; Jiang, W.; Zhang, Y.; Gao, S. The effect of conjugated linoleic acids on the growth performance, carcase composition and meat quality of fattening rabbits. Ital. J. Anim. Sci. 2022, 21, 1074–1083. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Ruminant production and metabolic responses to heat stress. J. Anim. Sci. 2012, 90, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Ratel, E.T.I.; Mekawy, A.; Hassab, H.S.; Abdelnour, S. Enhancing growing rabbit heat stress resilience through dietary supplementation with natural antioxidants. BMC Vet. Res. 2025, 21, 28. [Google Scholar]
- El-Ratel, I.T.; Al-Samarai, E.A.; El Basuini, M.F.; El-Kholy, K.H.; Gomaa, A.M.; Abdel-Khalek, A.M.; Fouda, S.F.; Hassan, M.A.E.; El-Raghi, A.A.; Momenah, M.A.; et al. Investigating the dose-response relationship of gum Arabic (Acacia senegal) in ameliorating heat stress responses in rabbits. J. Agricul. Food Res. 2025, 21, 101936. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Ayyat, M.S.; Abd El-Monem, U.M. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001, 33, 451–462. [Google Scholar] [CrossRef]
- Okab, A.B.; El-Banna, S.G. Physiological and biochemical parameters in New-Zealand white male rabbits during spring and summer seasons. Egypt. J. Basic Appl. Physiol. 2003, 2, 289–300. [Google Scholar]
- Okab, A.B.; El-Banna, S.G.; Koriem, A.A. Influence of environmental temperatures on some physiological and biochemical parameters of male New-Zealand rabbits. Slovak J. Anim. Sci. 2008, 41, 12–19. [Google Scholar]
- Sirotkin, A.; Parkanyi, V.; Pivko, J. High temperature impairs rabbit viability, feed consumption, growth and fecundity: Examination of endocrine mechanisms. Domest. Anim. Endocrin. 2021, 74, 106478. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Marai, I.F.M. Effects of heat stress on growth, carcass traits and blood components of New Zealand White rabbits fed various dietary energy-fiber levels, under Egyptian conditions. J. Arid Environ. 1997, 37, 557–568. [Google Scholar] [CrossRef]
- Ogunjimi, L.A.O.; Ogunwande, G.A.; Osunade, J.A. Rabbit weight gain, feed efficiency, rectal temperature and respiration rate as affected by building thermal environment in the humid tropical climate of Southwestern Nigeria. Agric. Int. CIGR E-J. 2008, 10, 1–14. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.J.; Febbraio, M.A.; Lasini, D.; Hargreaves, M. Effect of carbohydrate ingestion on glucose kinetics during exercise in the heat. J. Appl. Physiol. 2001, 90, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Bai, X.; Xu, K.; Zhang, C.; Chen, L. Effect of phloretin ongrowth performance, serum biochemical parameters and antioxidant profile in heat-stressed broilers. Poult. Sci. 2021, 100, 101217. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Balthasar, N.; Coppari, R.; Mc, M.J.; Liu, S.M.; Lee, C.E.; Tang, V.; Kenny, C.D.; Robert, A.M.; Streamson, C.J.; Joel, K.E.; et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004, 42, 983–991. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef]
- Norton, P.A. Affect of serum leptin on nutritional status in renal disease. J. Am. Diet. Assoc. 2002, 102, 1119–1125. [Google Scholar] [CrossRef]
- Thuahnai, S.T.; Lund-Katz, S.; Williams, D.L.; Phillips, M.C. Scavenger receptor class B, type I-mediated uptake of various lipids into cells. Influence of the nature of the donor particle interaction with the receptor. J. Biol. Chem. 2001, 276, 43801–43808. [Google Scholar] [CrossRef]
- Rinaldo, D.; Le, D.J. Effects of warm exposure on adipose tissue and muscle metabolism in growing pigs. Comp. Biochem. Phys. A 1991, 100, 995–1002. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Zhang, Y.; Zhao, X. Effects of heat stress on the muscle meat quality of Rainbow Trout. Fishes 2024, 9, 459. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Centoducati, G.; Hassan, A.A.; Maggiolino, A.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Effects of dietary supplementations of Vitamin C, organic selenium, betaine, and pomegranate peel on alleviating the effect of heat stress on growing rabbits. Animals 2024, 14, 950. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, Z.; Wu, D.; Wang, L.; Wang, C.; Liu, H.; Yang, Y.; Han, S. Effects of arginine supplementation in high-carbohydrate diets on the growth, hematological parameters, and hepatic and skeletal muscle glucose metabolism of juvenile mirror carp (Cyprinus carpio) based on PI3K/Akt signaling pathway. Aquac. Rep. 2024, 39, 102409. [Google Scholar] [CrossRef]
- Qu, Y.; Xiong, W.; Zhou, R.; Song, N.; Qian, J. Dexmedetomidine mitigates oxidative stress in H9C2 cardiac myoblasts under a high-glucose environment via the PI3K/AKT signaling pathway. Mol. Med. Rep. 2025, 32, 251. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Yang, W.; Xiao, C.; Fu, S.; Deng, Q.; Ding, H.; Wang, Z.; Liu, Q.; Li, X. SREBP-1c overexpression induces triglycerides accumulation through increasing lipid synthesis and decreasing lipid oxidation and VLDL assembly in bovine hepatocytes. J. Steroid Biochem. 2014, 143, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R.P.S.; Das, A.; Ramamurthy, K.; Pasupuleti, M.; Rajagopal, R.; Arockiaraj, J. Exposure to bisphenol A and sodium nitrate found in processed meat induces endocrine disruption and dyslipidemia through PI3K/AKT/SREBP pathway in zebrafish larvae. J. Nutr. Biochem. 2025, 140, 109887. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).