Identifying Infectious Agents in Snakes (Boidae and Pythonidae) with and Without Respiratory Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Extraction and PCR
2.2. Sequence Analysis
2.3. Third-Generation Nanopore Metagenomic Sequencing
2.4. Mycoplasma Isolation and Matrix-Assisted Desorption/Ionization-Time of Flight Mass Spectrometry (MALDI-TOF)
3. Results
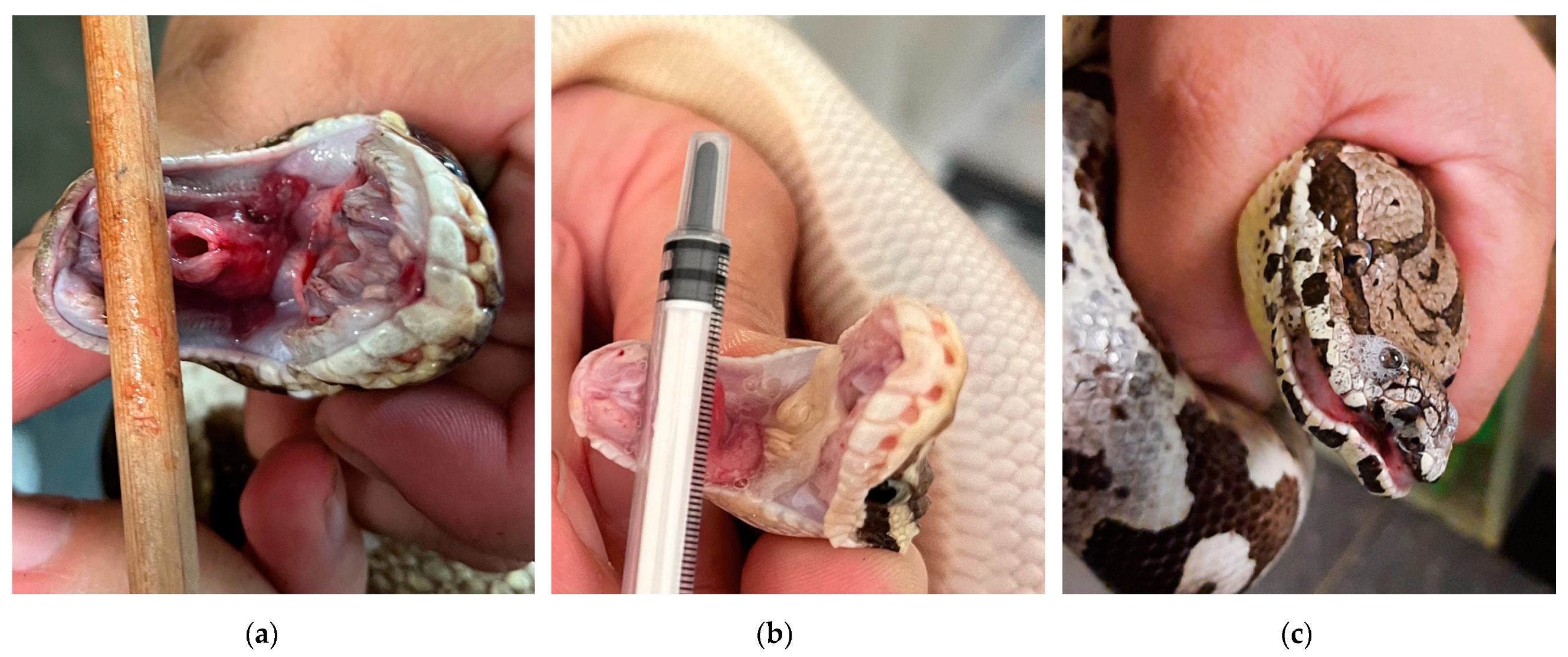
3.1. Clinical Signs
3.2. Detected Pathogens
3.2.1. Overview
3.2.2. Identification of Mycoplasmas
3.2.3. Mycoplasma Cultures and MALDI-TOF
3.2.4. Identification of Serpentoviruses
3.2.5. Detection of Chlamydia sp.
3.3. Clinical Signs and Pathogens
4. Discussion
4.1. Prevalence and Characteristics of Mycoplasma Infections
4.2. Detection and Diversity of Serpentoviruses in Snakes
4.3. Chlamydia spp. and Other Bacteria Species Identified
4.4. Influence of Husbandry and Stress
4.5. Treatment
4.6. Challenges in Interpreting Bacterial Findings in Reptile Diagnostics
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCR | Polymerase chain reaction |
| TGS | Third-generation sequencing |
| ICTV | International Committee on Taxonomy of Viruses |
| BPNV-1 | Ball Python Nidovirus 1 |
| URT | Upper respiratory tract |
| LPSN | list of prokaryotic names with standing in nomenclature |
| MBA | Mycoplasmology, bacteriology, and antimicrobial resistance unit |
| BLAST | Basic Local Alignment Search Tool |
| MALDI-TOF | Matrix-assisted laser desorption/ionization-time of flight mass spectrometry |
| DNA | Deoxyribonucleic acid |
References
- Schmidt, V.; Marschang, R.E.; Abbas, M.D.; Ball, I.; Szabo, I.; Helmuth, R.; Plenz, B.; Spergser, J.; Pees, M. Detection of pathogens in Boidae and Pythonidae with and without respiratory disease. Vet. Rec. 2013, 172, 236. [Google Scholar] [CrossRef] [PubMed]
- Hoon-Hanks, L.L.; Ossiboff, R.J.; Bartolini, P.; Fogelson, S.B.; Perry, S.M.; Stöhr, A.C.; Cross, S.T.; Wellehan, J.F.X.; Jacobson, E.R.; Dubovi, E.J.; et al. Longitudinal and Cross-Sectional Sampling of Serpentovirus (Nidovirus) Infection in Captive Snakes Reveals High Prevalence, Persistent Infection, and Increased Mortality in Pythons and Divergent Serpentovirus Infection in Boas and Colubrids. Front. Vet. Sci. 2019, 6, 338. [Google Scholar] [CrossRef] [PubMed]
- Comolli, J.R.; Divers, S.J. Respiratory Diseases of Snakes. Vet. Clin. Exot. Anim. 2021, 24, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Salzmann, E.; Pees, M. Diagnostics of Infectious Respiratory Pathogens in Reptiles. Vet. Clin. N. Am. Exot. Anim. Pract. 2021, 24, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Hilf, M.; Wagner, R.A.; Yu, V.L. A Prospective Study of Upper Airway Flora in Healthy Boid Snakes and Snakes with Pneumonia. J. Zoo Wildl. Med. 1990, 21, 318–325. [Google Scholar]
- Orós, J.; Rodríguez, J.L.; Herráez, P.; Santana, P.; Fernández, A. Respiratory and digestive lesions caused by Salmonella arizonae in two snakes. J. Comp. Pathol. 1996, 115, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Govendan, P.N.; Purbantoro, S.; Erika, E.; Rumbay, Y.; Rompis, A. Clinical Findings and Bacterial Identification in Eight Pythons with Respiratory Disorders in Bali. J. Vet. 2022, 23, 211–216. [Google Scholar] [CrossRef]
- Lamirande, E.W.; Nichols, D.K.; Owens, J.W.; Gaskin, J.M.; Jacobson, E.R. Isolation and experimental transmission of a reovirus pathogenic in ratsnakes (Elaphe species). Virus Res. 1999, 63, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, T.H.; Marschang, R.E.; Wellehan, J.F., Jr.; Nicholls, P.K. Isolation and molecular identification of Sunshine virus, a novel paramyxovirus found in Australian snakes. Infect. Genet. Evol. 2012, 12, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, T.H.; Shilton, C.M.; Marschang, R.E. Paramyxoviruses in reptiles: A review. Vet. Microbiol. 2013, 165, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Crossland, N.A.; DiGeronimo, P.M.; Sokolova, Y.; Childress, A.L.; Wellehan, J.F.X.; Nevarez, J.; Paulsen, D. Pneumonia in a Captive Central Bearded Dragon with Concurrent Detection of Helodermatid Adenovirus 2 and a Novel Mycoplasma Species. Vet. Pathol. 2018, 55, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Hoon-Hanks, L.L.; Layton, M.L.; Ossiboff, R.J.; Parker, J.S.L.; Dubovi, E.J.; Stenglein, M.D. Respiratory disease in ball pythons (Python regius) experimentally infected with ball python nidovirus. Virology 2018, 517, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Enjuanes, L.; Ziebuhr, J.; Snijder, E.J. Nidovirales: Evolving the largest RNA virus genome. Virus Res. 2006, 117, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Parrish, K.; Kirkland, P.D.; Skerratt, L.F.; Ariel, E. Nidoviruses in Reptiles: A Review. Front. Vet. Sci. 2021, 8, 733404. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.; Iredale, M.; Tillis, S.; Ossiboff, R. Ophidian Serpentoviruses: A Review and Perspective. J. Herpetol. Med. Surg. 2023, 33, 205. [Google Scholar] [CrossRef]
- Tillis, S.B.; Josimovich, J.M.; Miller, M.A.; Hoon-Hanks, L.L.; Hartmann, A.M.; Claunch, N.M.; Iredale, M.E.; Logan, T.D.; Yackel Adams, A.A.; Bartoszek, I.A.; et al. Divergent Serpentoviruses in Free-Ranging Invasive Pythons and Native Colubrids in Southern Florida, United States. Viruses 2022, 14, 2726. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, R.; Lempp, C.; Schürch, A.C.; Habierski, A.; Hahn, K.; Lamers, M.; von Dörnberg, K.; Wohlsein, P.; Drexler, J.F.; Haagmans, B.L.; et al. Novel divergent nidovirus in a python with pneumonia. J. Gen. Virol. 2014, 95, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Jacobson, E.R.; Wozniak, E.J.; Wellehan, J.F.; Kincaid, A.; Gordon, M.; Porter, B.F.; Baumgartner, W.; Stahl, S.; Kelley, K.; et al. Ball python nidovirus: A candidate etiologic agent for severe respiratory disease in Python regius. mBio 2014, 5, e01484-14. [Google Scholar] [CrossRef] [PubMed]
- Uccellini, L.; Ossiboff, R.J.; de Matos, R.E.C.; Morrisey, J.K.; Petrosov, A.; Navarrete-Macias, I.; Jain, K.; Hicks, A.L.; Buckles, E.L.; Tokarz, R.; et al. Identification of a novel nidovirus in an outbreak of fatal respiratory disease in ball pythons (Python regius). Virol. J. 2014, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, M.; Jackson, B.; Jackson, C.; Xavier, P.; Warren, K. Discovery and Partial Genomic Characterisation of a Novel Nidovirus Associated with Respiratory Disease in Wild Shingleback Lizards (Tiliqua rugosa). PLoS ONE 2016, 11, e0165209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Finlaison, D.S.; Frost, M.J.; Gestier, S.; Gu, X.; Hall, J.; Jenkins, C.; Parrish, K.; Read, A.J.; Srivastava, M.; et al. Identification of a novel nidovirus as a potential cause of large scale mortalities in the endangered Bellinger River snapping turtle (Myuchelys georgesi). PLoS ONE 2018, 13, e0205209. [Google Scholar] [CrossRef] [PubMed]
- Hoon-Hanks, L.L.; Stöhr, A.C.; Anderson, A.J.; Evans, D.E.; Nevarez, J.G.; Díaz, R.E.; Rodgers, C.P.; Cross, S.T.; Steiner, H.R.; Parker, R.R.; et al. Serpentovirus (Nidovirus) and Orthoreovirus Coinfection in Captive Veiled Chameleons (Chamaeleo calyptratus) with Respiratory Disease. Viruses 2020, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Blahak, S.; Jenckel, M.; Höper, D.; Beer, M.; Hoffmann, B.; Schlottau, K. Investigations into the presence of nidoviruses in pythons. Virol. J. 2020, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Dervas, E.; Hepojoki, J.; Laimbacher, A.; Romero-Palomo, F.; Jelinek, C.; Keller, S.; Smura, T.; Hepojoki, S.; Kipar, A.; Hetzel, U. Nidovirus-Associated Proliferative Pneumonia in the Green Tree Python (Morelia viridis). J. Virol. 2017, 91, e00718-17. [Google Scholar] [CrossRef] [PubMed]
- Dervas, E.; Hepojoki, J.; Smura, T.; Prähauser, B.; Windbichler, K.; Blümich, S.; Ramis, A.; Hetzel, U.; Kipar, A. Serpentoviruses: More than Respiratory Pathogens. J. Virol. 2020, 94, e00649-20. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Brown, D.R.; Klein, P.A.; McLaughlin, G.S.; Schumacher, I.M.; Jacobson, E.R.; Adams, H.P.; Tully, J.G. Mycoplasma agassizii sp. nov., isolated from the upper respiratory tract of the desert tortoise (Gopherus agassizii) and the gopher tortoise (Gopherus polyphemus). Int. J. Syst. Evol. Microbiol. 2001, 51, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Merritt, J.L.; Jacobson, E.R.; Klein, P.A.; Tully, J.G.; Brown, M.B. Mycoplasma testudineum sp. nov., from a desert tortoise (Gopherus agassizii) with upper respiratory tract disease. Int. J. Syst. Evol. Microbiol. 2004, 54, 1527–1529. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.R.; Brown, M.B.; Wendland, L.D.; Brown, D.R.; Klein, P.A.; Christopher, M.M.; Berry, K.H. Mycoplasmosis and upper respiratory tract disease of tortoises: A review and update. Vet. J. 2014, 201, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Penner, J.D.; Jacobson, E.R.; Brown, D.R.; Adams, H.P.; Besch-Williford, C.L. A novel Mycoplasma sp. associated with proliferative tracheitis and pneumonia in a Burmese python (Python molurus bivittatus). J. Comp. Pathol. 1997, 117, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.; Heckers, K.; Dietz, J.; Kolesnik, E. Detection of a mycoplasma in a python (Morelia spilota) with stomatitis. J. Herpetol. Med. Surg. 2016, 26, 90–93. [Google Scholar] [CrossRef]
- Magalhães, B.; Machado, L.; Figueira, A.; Dias, T.; Feijó, T.; Barreto, M.; Tuffanelli, G.; Cunha, N.; Nascimento, E.; Pereira, V.; et al. Mycoplasma spp. in captive snakes (Boa constrictor and Bothrops atrox) from Brazil. Ciênc. Rural 2021, 51, e20200583. [Google Scholar] [CrossRef]
- Faulhaber, M.M.; Tardy, F.; Saul, F.; Müller, E.; Pees, M.; Marschang, R.E. Detection of Mycoplasma spp. from snakes from five different families. BMC Vet. Res. 2025, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Sawnani, S.; Adeolu, M.; Alnajar, S.; Oren, A. Correction to: Phylogenetic framework for the phylum Tenericutes based on genome sequence data: Proposal for the creation of a new order Mycoplasmoidales ord. nov., containing two new families Mycoplasmoidaceae fam. nov. and Metamycoplasmataceae fam. nov. harbouring Eperythrozoon, Ureaplasma and five novel genera. Antonie Van Leeuwenhoek 2018, 111, 2485–2486. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Oren, A. Necessity and rationale for the proposed name changes in the classification of Mollicutes species. Reply to: ’Recommended rejection of the names Malacoplasma gen. nov., Mesomycoplasma gen. nov., Metamycoplasma gen. nov., Metamycoplasmataceae fam. nov., Mycoplasmoidaceae fam. nov., Mycoplasmoidales ord. nov., Mycoplasmoides gen. nov., Mycoplasmopsis gen. nov. [Gupta, Sawnani, Adeolu, Alnajar and Oren 2018] and all proposed species comb. nov. placed therein’, by M. Balish et al. (Int J Syst Evol Microbiol, 2019;69:3650–3653). Int. J. Syst. Evol. Microbiol. 2020, 70, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-H.; Pei, S.-C.; Yen, H.-C.; Blanchard, A.; Sirand-Pugnet, P.; Baby, V.; Gasparich, G.; Kuo, C.-H. Delineating bacterial genera based on gene content analysis: A case study of the Mycoplasmatales-Entomoplasmatales clade within the class Mollicutes. Microb. Genom. 2024, 10, 001321. [Google Scholar] [CrossRef] [PubMed]
- Wellehan, J.F.; Johnson, A.J.; Harrach, B.; Benkö, M.; Pessier, A.P.; Johnson, C.M.; Garner, M.M.; Childress, A.; Jacobson, E.R. Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J. Virol. 2004, 78, 13366–13369. [Google Scholar] [CrossRef] [PubMed]
- VanDevanter, D.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Wellehan, J.; Johnson, A.; Latimer, K.; Whiteside, D.; Crawshaw, G.; Detrisac, C.; Terrell, S.; Heard, D.; Childress, A.; Jacobson, E. Varanid herpesvirus 1: A novel herpesvirus associated with proliferative stomatitis in green tree monitors (Varanus prasinus). Vet. Microbiol. 2005, 105, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Catoi, C.; Gal, A.F.; Taulescu, M.A.; Palmieri, C.; Catoi, A.F. Lethal herpesvirosis in 16 captive horned vipers (Vipera ammodytes ammodytes): Pathological and ultrastructural findings. J. Comp. Pathol. 2014, 150, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Hetterich, J.; Mirolo, M.; Kaiser, F.; Ludlow, M.; Reineking, W.; Zdora, I.; Hewicker-Trautwein, M.; Osterhaus, A.D.M.E.; Pees, M. Concurrent Detection of a Papillomatous Lesion and Sequence Reads Corresponding to a Member of the Family Adintoviridae in a Bell’s Hinge-Back Tortoise (Kinixys belliana). Animals 2024, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.; Baggio, F.; Prähauser, B.; Ruiz Subira, A.; Michalopoulou, E.; Kipar, A.; Hetzel, U.; Hepojoki, J. Reptarenavirus S Segment RNA Levels Correlate with the Presence of Inclusion Bodies and the Number of L Segments in Snakes with Reptarenavirus Infection-Lessons Learned from a Large Breeding Colony. Microbiol. Spectr. 2023, 11, e0506522. [Google Scholar] [CrossRef] [PubMed]
- Argenta, F.F.; Hepojoki, J.; Smura, T.; Szirovicza, L.; Hammerschmitt, M.E.; Driemeier, D.; Kipar, A.; Hetzel, U. Identification of Reptarenaviruses, Hartmaniviruses, and a Novel Chuvirus in Captive Native Brazilian Boa Constrictors with Boid Inclusion Body Disease. J. Virol. 2020, 94, e00001-20. [Google Scholar] [CrossRef] [PubMed]
- Houpikian, P.; Raoult, D. Traditional and molecular techniques for the study of emerging bacterial diseases: One laboratory’s perspective. Emerg. Infect. Dis. 2002, 8, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Sanders, C.; Kistler, A.L.; Ruby, J.G.; Franco, J.Y.; Reavill, D.R.; Dunker, F.; Derisi, J.L. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: Candidate etiological agents for snake inclusion body disease. mBio 2012, 3, e00180-12. [Google Scholar] [CrossRef] [PubMed]
- Wellehan, J.F., Jr.; Childress, A.L.; Marschang, R.E.; Johnson, A.J.; Lamirande, E.W.; Roberts, J.F.; Vickers, M.L.; Gaskin, J.M.; Jacobson, E.R. Consensus nested PCR amplification and sequencing of diverse reptilian, avian, and mammalian orthoreoviruses. Vet. Microbiol. 2009, 133, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sachse, K.; Hotzel, H.; Slickers, P.; Ehricht, R. The use of DNA microarray technology for detection and genetic characterisation of Chlamydiae. Dev. Biol. 2006, 126, 203–210, discussion 326. [Google Scholar]
- Geneious Prime 2025.0.3. Available online: http://www.geneious.com/ (accessed on 1 December 2024).
- National Center for Biotechnology Information (NCBI) [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 1988. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 9 May 2024).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Flandrois, J.P.; Perrière, G.; Gouy, M. leBIBIQBPP: A set of databases and a webtool for automatic phylogenetic analysis of prokaryotic sequences. BMC Bioinform. 2015, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Theuns, S.; Vanmechelen, B.; Bernaert, Q.; Deboutte, W.; Vandenhole, M.; Beller, L.; Matthijnssens, J.; Maes, P.; Nauwynck, H.J. Nanopore sequencing as a revolutionary diagnostic tool for porcine viral enteric disease complexes identifies porcine kobuvirus as an important enteric virus. Sci. Rep. 2018, 8, 9830. [Google Scholar] [CrossRef] [PubMed]
- Vereecke, N.; Zwickl, S.; Gumbert, S.; Graaf, A.; Harder, T.; Ritzmann, M.; Lillie-Jaschniski, K.; Theuns, S.; Stadler, J. Viral and Bacterial Profiles in Endemic Influenza A Virus Infected Swine Herds Using Nanopore Metagenomic Sequencing on Tracheobronchial Swabs. Microbiol. Spectr. 2023, 11, e0009823. [Google Scholar] [CrossRef] [PubMed]
- Bokma, J.; Vereecke, N.; Pas, M.L.; Chantillon, L.; Vahl, M.; Weesendorp, E.; Deurenberg, R.H.; Nauwynck, H.; Haesebrouck, F.; Theuns, S.; et al. Evaluation of Nanopore Sequencing as a Diagnostic Tool for the Rapid Identification of Mycoplasma bovis from Individual and Pooled Respiratory Tract Samples. J. Clin. Microbiol. 2021, 59, e0111021. [Google Scholar] [CrossRef] [PubMed]
- Van Herzele, C.; Coppens, S.; Vereecke, N.; Theuns, S.; de Graaf, D.C.; Nauwynck, H. New insights into honey bee viral and bacterial seasonal infection patterns using third-generation nanopore sequencing on honey bee haemolymph. Vet. Res. 2024, 55, 118. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.L.; Hanson, R.P.; Andrson, D.P. A medium for the isolation of avian mycoplasmas. Am. J. Vet. Res. 1968, 29, 2163–2171. [Google Scholar] [PubMed]
- Freundt, E.A. C7-Culture Media for Classic Mycoplasmas. In Methods in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press: Cambridge, MA, USA, 1983; pp. 127–135. [Google Scholar]
- Friis, N.F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord. Vet. Med. 1975, 27, 337–339. [Google Scholar] [PubMed]
- Cisneros-Tamayo, M.; Kempf, I.; Coton, J.; Michel, V.; Bougeard, S.; de Boisséson, C.; Lucas, P.; Bäyon-Auboyer, M.H.; Chiron, G.; Mindus, C.; et al. Investigation on eggshell apex abnormality (EAA) syndrome in France: Isolation of Mycoplasma synoviae is frequently associated with Mycoplasma pullorum. BMC Vet. Res. 2020, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Racz, K.; Salzmann, E.; Müller, E.; Marschang, R.E. Detection of Mycoplasma and Chlamydia in Pythons With and Without Serpentovirus Infection. J. Zoo Wildl. Med. 2021, 52, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1,000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Pees, M.; Schmidt, V.; Marschang, R.E.; Heckers, K.O.; Krautwald-Junghanns, M.E. Prevalence of viral infections in captive collections of boid snakes in Germany. Vet. Rec. 2010, 166, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Wellehan, J.F.X.; Divers, S.J. 29-Bacteriology. In Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed.; Divers, S.J., Stahl, S.J., Eds.; W.B. Saunders: St. Louis, MO, USA, 2019; pp. 235–246.e4. [Google Scholar]
- Brown, M.; Schumacher, I.; Klein, P.; Harris, K.; Correll, T.; Jacobson, E. Mycoplasma agassizii causes upper respiratory tract disease in the desert tortoise. Infect. Immun. 1994, 62, 4580–4586. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; McLaughlin, G.; Klein, P.; Crenshaw, B.; Schumacher, I.; Brown, D.; Jacobson, E. Upper respiratory tract disease in the gopher tortoise is caused by Mycoplasma agassizii. J. Clin. Microbiol. 1999, 37, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.R.; Berry, K.H. Mycoplasma testudineum in free-ranging desert tortoises, Gopherus agassizii. J. Wildl. Dis. 2012, 48, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Plenz, B.; Schmidt, V.; Grosse-Herrenthey, A.; Krüger, M.; Pees, M. Characterisation of the aerobic bacterial flora of boid snakes: Application of MALDI-TOF mass spectrometry. Vet. Rec. 2015, 176, 285. [Google Scholar] [CrossRef] [PubMed]
- Zancolli, G.; Mahsberg, D.; Sickel, W.; Keller, A. Reptiles as Reservoirs of Bacterial Infections: Real Threat or Methodological Bias? Microbial. Ecol. 2015, 70, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Blaylock, R.S. Normal oral bacterial flora from some southern African snakes. Onderstepoort J. Vet. Res. 2001, 68, 175–182. [Google Scholar] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; et al. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020). Arch. Virol. 2020, 165, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Tillis, S.B.; Ossiboff, R.J.; Wellehan, J.F.X., Jr. Serpentoviruses Exhibit Diverse Organization and ORF Composition with Evidence of Recombination. Viruses 2024, 16, 310. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Padhi, L.; Sahoo, G. Oral bacterial flora of Indian cobra (Naja naja) and their antibiotic susceptibilities. Heliyon 2018, 4, e01008. [Google Scholar] [CrossRef] [PubMed]
- Busse, H.J.; Huptas, C.; Baumgardt, S.; Loncaric, I.; Spergser, J.; Scherer, S.; Wenning, M.; Kämpfer, P. Proposal of Lysobacter pythonis sp. nov. isolated from royal pythons (Python regius). Syst. Appl. Microbiol. 2019, 42, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Artavia-León, A.; Romero-Guerrero, A.; Sancho-Blanco, C.; Rojas, N.; Umaña-Castro, R. Diversity of Aerobic Bacteria Isolated from Oral and Cloacal Cavities from Free-Living Snakes Species in Costa Rica Rainforest. Int. Sch. Res. Not. 2017, 2017, 8934285. [Google Scholar] [CrossRef] [PubMed]
- Parrish, K.; Kirkland, P.; Horwood, P.; Chessman, B.; Ruming, S.; McGilvray, G.; Rose, K.; Hall, J.; Skerratt, L. Delving into the Aftermath of a Disease-Associated Near-Extinction Event: A Five-Year Study of a Serpentovirus (Nidovirus) in a Critically Endangered Turtle Population. Viruses 2024, 16, 653. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Moreira, W.M.Q.; da Cunha, K.; Ribeiro, A.; Almeida, M. Oral microbiota of Brazilian captive snakes. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 54–60. [Google Scholar] [CrossRef]
- Taylor-Brown, A.; Bachmann, N.L.; Borel, N.; Polkinghorne, A. Culture-independent genomic characterisation of Candidatus Chlamydia sanzinia, a novel uncultivated bacterium infecting snakes. BMC Genom. 2016, 17, 710. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Brown, A.; Rüegg, S.; Polkinghorne, A.; Borel, N. Characterisation of Chlamydia pneumoniae and other novel chlamydial infections in captive snakes. Vet. Microbiol. 2015, 178, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.S.; Mathews, S.A.; Eppinger, M.; Mitchell, C.; O’Brien, K.K.; White, O.R.; Benahmed, F.; Brunham, R.C.; Read, T.D.; Ravel, J.; et al. Evidence that human Chlamydia pneumoniae was zoonotically acquired. J. Bacteriol. 2009, 191, 7225–7233. [Google Scholar] [CrossRef] [PubMed]
- Bodetti, T.J.; Jacobson, E.; Wan, C.; Hafner, L.; Pospischil, A.; Rose, K.; Timms, P. Molecular evidence to support the expansion of the hostrange of Chlamydophila pneumoniae to include reptiles as well as humans, horses, koalas and amphibians. Syst. Appl. Microbiol. 2002, 25, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.R.; Heard, D.; Andersen, A. Identification of Chlamydophila pneumoniae in an emerald tree boa, Corallus caninus. J. Vet. Diagn. Invest. 2004, 16, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Lu, Z.H.; Vaughan, L.; Polkinghorne, A.; Zimmermann, D.R.; Huder, J.B.; Pospischil, A. Detection of Mycobacteria and Chlamydiae in Granulomatous Inflammation of Reptiles: A Retrospective Study. Vet. Pathol. 2004, 41, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Staub, E.; Marti, H.; Biondi, R.; Levi, A.; Donati, M.; Leonard, C.A.; Ley, S.D.; Pillonel, T.; Greub, G.; Seth-Smith, H.M.B.; et al. Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci. Rep. 2018, 8, 5660. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.R.; Gaskin, J.M.; Mansell, J. Chlamydial Infection in Puff Adders, Bitis arietans. J. Zoo Wildl. Med. 1989, 20, 364–369. [Google Scholar]
- Lock, B.; Heard, D.; Detrisac, C.; Jacobson, E. An epizootic of chronic regurgitation associated with Chlamydophilosis in recently imported emerald tree boas (Corallus caninus). J. Zoo Wildl. Med. 2003, 34, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.; Origgi, F.; Heard, D.; Detrisac, C. Immunohistochemical Staining of Chlamydial Antigen in Emerald Tree Boas (Corallus Caninus). J. Vet. Diagn. Invest. 2002, 14, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, S.R.; Regenscheit, N.; Origgi, F.C.; Kaiser, C.; Borel, N. Detection of Chlamydia pneumoniae in a collection of captive snakes and response to treatment with marbofloxacin. Vet. J. 2015, 205, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Pembroke, J.T. The Genus Ochrobactrum as Major Opportunistic Pathogens. Microorganisms 2020, 8, 1797. [Google Scholar] [CrossRef] [PubMed]
- Wernick, M.; Novo Matos, J.; Ebling, A.; Kühn Campbell, K.; Ruetten, M.; Hilbe, M.; Howard, J.; Chang, R.; Prohaska, S.; Hatt, J.-M. Valvulopathy consistent with endocarditis in an Argentine boa (Boa constrictor occidentalis). J. Zoo Wildl. Med. 2015, 46, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Romero, C.; López-Goñi, I.; Leiva, J.; Díaz, R.; Moriyón, I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int. J. Syst. Bacteriol. 1998, 48 Pt 3, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Pasterny, J.; Skomorucha, Ł.; Stanicki, K.; Marschang, R.E. Detection of Infectious Agents in Samples from Reptiles Presented at Veterinary Clinics in Poland. J. Herpetol. Med. Surg. 2021, 31, 64–72. [Google Scholar] [CrossRef]
- Flanders, A.J.; Ossiboff, R.J.; Wellehan, J.F.X., Jr.; Alexander, A.B.; Fredholm, D.V.E.; Desiderio, T.M.; Stacy, N.I. Presumptive heterophil extracellular traps recognized cytologically in nine reptile patients with inflammatory conditions. Vet. Q. 2021, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Meddings, J.I.; Ariel, E. Viruses of reptiles. In Studies in Viral Ecology, 2nd ed.; Hurst, C.J., Ed.; Wiley: New York, NY, USA, 2021; pp. 449–510. [Google Scholar] [CrossRef]
- Kischinovsky, M.; Raftery, A.; Sawmy, S. Husbandry and nutrition. In Reptile Medicine and Surgery in Clinical Practice; Doneley, D., Monks, D., Johnson, R., Carmel, B., Eds.; Wiley Blackwell: Oxford, UK, 2017; pp. 45–60. [Google Scholar] [CrossRef]
- Lazarkevich, I.; Engibarov, S.; Mitova, S.; Popova, S.; Vacheva, E.; Stanchev, N.; Eneva, R.; Gocheva, Y.; Lalovska, I.; Paunova-Krasteva, T.; et al. Pathogenic Potential of Opportunistic Gram-Negative Bacteria Isolated from the Cloacal Microbiota of Free-Living Reptile Hosts Originating from Bulgaria. Life 2024, 14, 566. [Google Scholar] [CrossRef] [PubMed]
- Barazorda Romero, S.; Cizek, A.; Masarikova, M.; Knotek, Z. Choanal and cloacal aerobic bacterial flora in captive green iguanas: A comparative analysis. Acta. Vet. Brno. 2015, 84, 19–24. [Google Scholar] [CrossRef]
- Stahl, S.J. How I approach snake respiratory disease: The five-minute consult. In Proceedings of the NAVC Conference, Orlando, FL, USA, 16–20 January 2010. [Google Scholar]
- Knotek, Z.; Divers, S.J. 76-Pulmonology. In Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed.; Divers, S.J., Stahl, S.J., Eds.; W.B. Saunders: St. Louis, MO, USA, 2019; pp. 786–804.e1. [Google Scholar]
- Isaza, R.; Jacobson, E.R. Antimicrobial Drug Use in Reptiles. In Antimicrobial Therapy in Veterinary Medicine; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 623–636. [Google Scholar]
- Lawrence, K.; Muggleton, P.W.; Needham, J.R. Preliminary study on the use of ceftazidime, a broad spectrum cephalosporin antibiotic, in snakes. Res. Vet. Sci. 1984, 36, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.D.; Rüschoff, B.; Troll, C.; Heckers, K.O.; Marschang, R.E. Bacteria Associated with Clinically Suspected Respiratory Disease in Snakes and Effective Antimicrobial Treatment Options. J. Herpetol. Med. Surg. 2021, 30, 254–260. [Google Scholar] [CrossRef]
- Damerum, A.; Malka, S.; Lofgren, N.; Vecere, G.; Krumbeck, J.A. Next-generation DNA sequencing offers diagnostic advantages over traditional culture testing. Am. J. Vet. Res. 2023, 84, ajvr.23.03.0054. [Google Scholar] [CrossRef] [PubMed]
- Dipineto, L.; Russo, T.P.; Calabria, M.; De Rosa, L.; Capasso, M.; Menna, L.F.; Borrelli, L.; Fioretti, A. Oral flora of Python regius kept as pets. Lett. Appl. Microbiol. 2014, 58, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Starck, J.M.; Weimer, I.; Aupperle, H.; Müller, K.; Marschang, R.E.; Kiefer, I.; Pees, M. Morphological Pulmonary Diffusion Capacity for Oxygen of Burmese Pythons (Python molurus): A Comparison of Animals in Healthy Condition and with Different Pulmonary Infections. J. Comp. Pathol. 2015, 153, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Kolesnik, E. Detection of nidoviruses in live pythons and boas. Tierarztl Prax. Ausg. Kleintiere Heimtiere 2017, 45, 22–26. [Google Scholar] [CrossRef]
- Brown, D.R.; Crenshaw, B.C.; McLaughlin, G.S.; Schumacher, I.M.; McKenna, C.E.; Klein, P.A.; Jacobson, E.R.; Brown, M.B. Taxonomic analysis of the tortoise mycoplasmas Mycoplasma agassizii and Mycoplasma testudinis by 16S rRNA gene sequence comparison. Int. J. Syst. Bacteriol. 1995, 45, 348–350. [Google Scholar] [CrossRef] [PubMed]
- van Kuppeveld, F.J.; van der Logt, J.T.; Angulo, A.F.; van Zoest, M.J.; Quint, W.G.; Niesters, H.G.; Galama, J.M.; Melchers, W.J. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 1992, 58, 2606–2615. [Google Scholar] [CrossRef] [PubMed]

| Pathogens | Detection Method | Python regius (Ball Python) K09608 | Python regius (Ball Python) K09602 | Python regius (Ball Python) K09609 | Python regius (Ball Python) K02321 | Python regius (Ball Python) K02325 | Python curtus (Sumatra Python) K02330 | Malayo- python reticulatus (Reticulated Python) K02324 | Acrantophis dumerili (Dumeril’s Boa) K09605 | Acrantophis dumerili (Dumeril’s Boa) K09603 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bellinger River-related virus | TGS | − | − | − | − | − | − | − | + | + |
| Carpet python nidovirus 1 | TGS | + | − | + | − | − | − | − | − | − |
| Serpentovirus | TGS | − | − | − | − | − | + | − | − | − |
| Serpentovirus (PCR1, PCR2) | PCR | + | − | + | − | − | − | − | + | + |
| Mycoplasma agassizii like (PCR1, PCR2) | PCR | + | + | + | + | + | + | + | + | + |
| M. [Mycoplasmopsis] iguanae | TGS | − | − | − | − | − | − | − | − | − |
| M. [Mycoplasmoides] fastidiosum | TGS | − | + | − | − | − | + | − | + | + |
| M. [Mycoplasmopsis] agassizii | TGS | − | − | + | − | + | − | + | − | − |
| M. [Mycoplasmopsis] pulmonis | TGS | − | − | + | − | + | + | − | + | + |
| Mycoplasmopsis sp. | TGS | + | − | − | − | − | − | − | − | − |
| M. testudineum [Mycoplasmopsis testudinea] | TGS | + | − | − | − | − | − | + | − | − |
| Mesomycoplasma sp. | TGS | − | − | − | + | − | − | − | − | − |
| Chlamydia sp. | PCR | − | − | − | − | − | − | − | + | + |
| Chlamydia sp. | TGS | − | − | − | − | − | − | − | + | − |
| Bacteroides fragilis | TGS | − | − | − | − | − | − | − | − | − |
| Brucella intermedia comb. nov | MALDI- TOF | + | − | + | nd | nd | nd | nd | − | − |
| Chryseobacterium sp. | TGS | + | − | − | − | − | − | − | + | − |
| Citrobacter sp. | TGS | + | − | − | − | − | − | − | − | − |
| Elizabethkingia sp. | TGS | − | − | − | + | + | + | + | − | − |
| Escherichia sp. | TGS | − | − | − | + | + | + | + | − | − |
| Flavobacterium sp. | TGS | + | − | − | − | − | − | − | − | − |
| Lysobacter pythonis | TGS | − | − | − | − | − | − | − | + | − |
| Paracoccus sp. | TGS | − | − | − | − | − | − | − | + | − |
| Providencia rettgeri | TGS | − | − | − | − | − | − | − | − | − |
| Pseudomonas sp. | TGS | − | − | − | + | + | − | + | − | − |
| Clinical signs | None Deceased: 41 days after sampling (23 October 2023) | None | None | None | None | None | None | None Deceased 1 year, 6 months, and 19 days after sampling (31 May 2025) | None Deceased 1 year, 3 months after sampling (December 2024) |
| Pathogens | Detection Method | Acrantophis dumerili (Dumeril’s Boa) K09604 | Python anchietae (Angolan Python) K09606 | Python regius (Ball Python) K09601 | Python regius (Ball Python) K09607 | Python regius (Ball Python) K09610 | Python regius (Ball Python) K09611 |
|---|---|---|---|---|---|---|---|
| Bellinger River-related virus | TGS | + | − | − | − | − | − |
| Carpet python nidovirus 1 | TGS | − | + | − | + | + | + |
| Serpentovirus | TGS | − | − | − | − | − | − |
| Serpentovirus (PCR1, PCR2) | PCR | + | + | − | + | + | + |
| Mycoplasma agassizii like (PCR1, PCR2) | PCR | + | + | + | + | + | + |
| M. [Mycoplasmopsis] iguanae | TGS | − | + | − | − | − | − |
| M. [Mycoplasmoides] fastidiosum | TGS | + | − | + | − | − | + |
| M. [Mycoplasmopsis] agassizii | TGS | − | + | − | − | + | + |
| M. [Mycoplasmopsis] pulmonis | TGS | + | − | − | − | − | − |
| Mycoplasmopsis sp. | TGS | − | − | + | − | − | − |
| M. testudineum [Mycoplasmopsis testudinea] | TGS | − | − | + | − | + | + |
| Mesomycoplasma sp. | TGS | − | − | − | − | − | − |
| Chlamydia sp. | PCR | − | − | − | − | − | − |
| Chlamydia sp. | TGS | − | − | − | − | − | − |
| Bacteroides fragilis | TGS | + | − | − | − | − | − |
| Brucella intermedia comb. nov | MALDI- TOF | + | + | + | + | − | + |
| Chryseobacterium sp. | TGS | + | + | + | − | − | − |
| Citrobacter sp. | TGS | − | − | − | − | − | − |
| Elizabethkingia sp. | TGS | − | + | − | + | − | + |
| Escherichia sp. | TGS | − | − | − | − | − | + |
| Flavobacterium sp. | TGS | − | − | − | − | − | + |
| Lysobacter pythonis | TGS | − | − | − | − | − | + |
| Paracoccus sp. | TGS | + | − | − | − | − | − |
| Providencia rettgeri | TGS | + | − | − | − | − | − |
| Pseudomonas sp. | TGS | − | − | − | − | − | − |
| Clinical signs | Nares: nasal discharge. Oral cavity: mucous fluid, hyperemia of the mucous membranes. Deceased 1 year, 6 months, and 12 days after sampling (24 May 2025). | Oral cavity: mucous fluid. Deceased: 13 days after sampling (25 September 2023). | Oral cavity: mucous fluid, hyperemia of the mucous membranes, wheezing. | Oral cavity: mucous fluid, cream-colored coating. | Oral cavity: mucous fluid wheezing. | Oral cavity: mucous fluid. |
| Pathogens | Detection Method | No. of Positive Snakes/ All Examined Snakes (%) | Diseased Pythons: No. Positive/All Diseased Pythons (%) | Healthy Pythons: No. Positive/ All Healthy Pythons (%) | Boas: No. Positive/ All Boas * (%) |
|---|---|---|---|---|---|
| Viruses | |||||
| Bellinger River-related virus | TGS | 3/15 (20%) | 0/5 (0%) | 0/7 (0%) | 3/3 (100%) |
| Carpet python nidovirus 1 | TGS | 6/15 (40%) | 4/5 (80%) | 2/7 (29%) | 0/3 (0%) |
| Serpentovirus | TGS | 1/15 (7%) | 0/5 (0%) | 1/7 (14%) | 0/3 (0%) |
| Serpentovirus (PCR1, PCR2) | PCR | 9/15 (60%) | 4/5 (80%) | 2/7 (29%) | 3/3 (100%) |
| Bacteria | |||||
| Mycoplasma agassizii like (PCR1, PCR2) | PCR | 15/15 (100%) | 5/5 (100%) | 7/7 (100%) | 3/3 (100%) |
| M. [Mycoplasmopsis] iguanae | TGS | 1/15 (7%) | 1/5 (20%) | 0/7 (0%) | 0/3 (0%) |
| M. [Mycoplasmoides] fastidiosum | TGS | 7/15 (47%) | 2/5 (40%) | 2/7 (29%) | 3/3 (100%) |
| M. [Mycoplasmopsis] agassizii | TGS | 6/15 (40%) | 3/5 (60%) | 3/7 (43%) | 0/3 (0%) |
| M. [Mycoplasmopsis] pulmonis | TGS | 6/15 (40%) | 0/5 (0%) | 3/7 (43%) | 3/3 (100%) |
| Mycoplasmopsis sp. | TGS | 2/15 (13%) | 1/5 (20%) | 1/7 (14%) | 0/3 (0%) |
| M. testudineum [Mycoplasmopsis testudinea] | TGS | 5/15 (33%) | 3/5 (60%) | 2/7 (29%) | 0/3 (0%) |
| Mesomycoplasma sp. | TGS | 1/15 (7%) | 0/5 (0%) | 1/7 (14%) | 0/3 (0%) |
| Chlamydia sp. | PCR | 2/15 (13%) | 0/5 (0%) | 0/7 (0%) | 2/3 (67%) |
| Chlamydia sp. | TGS | 1/15 (7%) | 0/5 (0%) | 0/7 (0%) | 1/3 (33%) |
| Bacteroides fragilis | TGS | 1/15 (7%) | 0/5 (0%) | 0/7 (0%) | 1/3 (33%) |
| Brucella intermedia comb. nov basionym: Ochrobactrum intermedium | MALDI-TOF ** | 7/11 (64%) | 4/5 (80%) | 2/7 (29%) | 1/3 (33%) |
| Chryseobacterium sp. | TGS | 5/15 (33%) | 2/5 (40%) | 1/7 (14%) | 2/3 (67%) |
| Citrobacter sp. | TGS | 1/15 (7%) | 0/5 (0%) | 1/7 (14%) | 0/3 (0%) |
| Elizabethkingia sp. | TGS | 7/15 (47%) | 3/5 (60%) | 4/7 (57%) | 0/3 (0%) |
| Escherichia sp. | TGS | 5/15 (33%) | 1/5 (20%) | 4/7 (57%) | 0/3 (0%) |
| Flavobacterium sp. | TGS | 2/15 (13%) | 1/5 (20%) | 1/7 (14%) | 0/3 (0%) |
| Lysobacter pythonis | TGS | 2/15 (13%) | 1/5 (20%) | 0/7 (0%) | 1/3 (33%) |
| Paracoccus sp. | TGS | 2/15 (13%) | 0/5 (0%) | 0/7 (0%) | 2/3 (67%) |
| Providencia rettgeri | TGS | 1/15 (7%) | 0/5 (0%) | 0/7 (0%) | 1/3 (33%) |
| Pseudomonas sp. | TGS | 3/15 (20%) | 0/5 (0%) | 3/7 (43%) | 0/3 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faulhaber, M.M.; Tardy, F.; Gautier-Bouchardon, A.V.; Öfner, S.; Theuns, S.; Coppens, S.; Müller, E.; Pees, M.; Marschang, R.E. Identifying Infectious Agents in Snakes (Boidae and Pythonidae) with and Without Respiratory Disease. Animals 2025, 15, 2187. https://doi.org/10.3390/ani15152187
Faulhaber MM, Tardy F, Gautier-Bouchardon AV, Öfner S, Theuns S, Coppens S, Müller E, Pees M, Marschang RE. Identifying Infectious Agents in Snakes (Boidae and Pythonidae) with and Without Respiratory Disease. Animals. 2025; 15(15):2187. https://doi.org/10.3390/ani15152187
Chicago/Turabian StyleFaulhaber, Marline M., Florence Tardy, Anne V. Gautier-Bouchardon, Sabine Öfner, Sebastiaan Theuns, Sieglinde Coppens, Elisabeth Müller, Michael Pees, and Rachel E. Marschang. 2025. "Identifying Infectious Agents in Snakes (Boidae and Pythonidae) with and Without Respiratory Disease" Animals 15, no. 15: 2187. https://doi.org/10.3390/ani15152187
APA StyleFaulhaber, M. M., Tardy, F., Gautier-Bouchardon, A. V., Öfner, S., Theuns, S., Coppens, S., Müller, E., Pees, M., & Marschang, R. E. (2025). Identifying Infectious Agents in Snakes (Boidae and Pythonidae) with and Without Respiratory Disease. Animals, 15(15), 2187. https://doi.org/10.3390/ani15152187





