1. Introduction
Antibiotics are used in poultry production to promote rapid growth and disease prevention [
1]. For more than 60 years, antibiotics have been supplemented in animal feed at subtherapeutic doses to improve growth and feed conversion efficiency and prevent infections [
2]. However, the antibiotics used in poultry feed have increased the health concerns in humans due to the production of resistant strains of pathogenic bacteria [
3] and the bioaccumulation of antibiotic residues in poultry meat and eggs [
4,
5]. The human intake of poultry products containing residues above safe maximum residual levels is associated with numerous health hazards such as allergic reactions, dermatitis, alteration of intestinal microflora, development of antibiotic resistance, and sometimes drug toxicity [
6,
7]. In response to health concerns, food regulatory and health authorities have restricted antibiotic growth promoters (AGPs) in poultry feed, which has intensified the search for a non-antibiotic alternative in poultry feed to maintain or improve poultry health and performance [
8]. Prebiotics, probiotics, synbiotics, and phytogenic feed additives (PFA) have been presented as potential alternatives to AGPs [
9]. The demand for phytogenic feed additives has increased due to the growing preference for natural and safe alternatives, enabling them to be utilized in poultry feed to improve productivity and replace AGPs.
The PFA used in the present study is the fenugreek (
Trigonella foenum-graecum L.) seeds powder, which has been reported to possess hypoglycemic [
10], hypocholesterolemic [
11], antioxidant [
12], immunomodulatory [
13], digestion enhancing, anthelmintic, antibacterial, anti-inflammatory, antipyretic, and antimicrobial effects [
14]. Fenugreek seed contains bioactive constituents such as alkaloids, steroids, flavonoids, and saponins [
15]. Our previous study with the dietary inclusion of 15 g/kg of FS and a blend of probiotics also improved the growth performance and nutrient digestibility of New Zealand White rabbits [
16]. Similarly, studies with the dietary inclusion of 5, 10, and 15 g/kg FS [
17], 10 g/kg normal, and enzyme-treated FS [
18] reported improved growth performance in broilers. In contrast, other studies have reported a reduction in growth performance of broiler with the inclusion of 5 g/kg FS at 42 days of age [
19], 5 and 10 g/kg FS at 21 days of age [
20], 30 g/kg FS at 21 and 28 days of age [
21], and 40 and 50 g/kg FS at 38 days of age [
22]. However, the limited studies on broilers do not provide enough evidence to support the hypothesis that the inclusion of FS may improve the growth performance and overall health of broiler chickens.
This study aims to determine the effect of different levels of fenugreek seeds on the growth performance, hematological parameters, and intestinal histomorphology of straight-run and male broiler chickens. We hypothesized that the overall performance and intestinal histomorphology of the broilers would be positively affected by fenugreek seeds consumption.
2. Materials and Methods
2.1. Preparation of Fenugreek Seeds
Dried fenugreek seeds (Deep Foods Inc., Union City, NJ, USA) were obtained from the local vendor in Little Rock, AR. Seeds were ground in a grinding grain mill (Thomas Scientific, Swedesboro, NJ, USA) using a 1 mm sieve to bring them to medium texture. Ground seeds were evenly mixed with the starter and finisher diets as per the requirements in the experimental designs of the two experiments. The analysis of fenugreek seeds was conducted in our previous study by Abdel-Wareth et al., 2021, using gas chromatography–mass spectrometry [
16].
2.2. Animals and Experimental Design
In the first experiment, one-day-old straight-run broiler chicks (Ross, n = 160) were weighed on arrival from the hatchery (Keith Smith, Hot Springs, AR, USA) and randomly allocated to 16 floor pens (10 birds/pen). Each floor pen (71 × 35 × 19 inches) was covered with pine wood shavings and equipped with a separate feeder (Harris Farms Hanging Feeders, Tractor Supply Co., Pine Bluff, AR, USA) and drinker (Harris Farms Drinkers, Tractor Supply Co., Pine Bluff, AR, USA). Four pens were randomly assigned to 1 of 4 treatments in a completely randomized design. The dietary treatments included a corn-soybean-based basal diet with the inclusion of FS powder at 0 g, 2.5 g, 5 g, and 10 g per kilogram of the diet. The basal diets (
Table 1) were formulated for starter (from 1 to 21 days) and finisher period (from 22 to 35 days) to meet or exceed the nutrient requirement of NRC, 1994.
In the second experiment, one-day-old male broiler chicks (Ross, n = 144) were weighed on arrival from the hatchery (Keith Smith, Hot Springs, AR, USA) and randomly allocated to 18 floor pens (8 birds/pen). Each floor pen was covered with pine wood shavings and equipped with a separate feeder (Harris Farms Hanging Feeders, Tractor Supply Co., Pine Bluff, AR, USA) and drinker (Harris Farms Drinkers, Tractor Supply Co., Pine Bluff, AR, USA). Six pens were randomly assigned to 1 of 3 treatments in a completely randomized design. The dietary treatments included a corn-soybean-based basal diet with the inclusion of 0 g, 5 g, and 10 g FS powder per kilogram of diet. The basal diets (
Table 1) were formulated for starter (from 0 to 21 d) and finisher period (from 21 to 42 d) to meet or exceed the nutrient requirement of NRC, 1994.
In both experiments, the feed was provided in mash form. All the birds were offered ad libitum access to feed and water during the experiments. The ambient temperature was gradually decreased from 34 °C (40 RH%) for days 1–3, 31 °C (40 RH%) for days 4–6, 29 °C (35 RH%) for days 7–10, 27 °C (45 RH%) for days 11–14, and 25 °C (35 RH%) thereafter.
2.3. Sample and Data Collection
Birds were weighed on a pen basis after arrival from the hatchery (d 1), at the end of the starter period (d 21), and at the end of the finisher period (d 35 in the first experiment and d 42 in the second experiment) to calculate mean BW and BWG. Feed consumption was recorded on a pen basis for the starter and finisher period to calculate FI and FCR. For the second experiment, two birds were selected from each pen on d 42 that represented the pen, individually weighed, and euthanized by decapitation technique. Blood was collected from the jugular vein in two different tubes for whole blood and serum collections.
2.4. Hematological Parameters
On day 42, blood samples were collected from a jugular vein of two birds per pen into 5 mL BD Vacutainer EDTA blood collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) to collect whole blood. Whole blood samples were analyzed for white blood cell (WBC) count, hemoglobin, hematocrit, total protein, heterophils, lymphocyte, monocyte, and basophil counts. Another 5 mL of blood was collected in a BD Vacutainer serum tube (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) to obtain blood serum. The blood was allowed to clot for approximately one hour at room temperature and then centrifuged at 2000× g for 15 min in a Centrifuge 5430R (Eppendorf SE, Enfield, CT, USA) to collect the blood serum. Serum samples were analyzed for glucose (GL), blood urea nitrogen (BUN), albumin (A), globulin (G), albumin/globulin ratio (A: G), total bilirubin (TB), and gamma-glutamyl transferase (GGT). Whole blood and serum samples were analyzed by an external laboratory (Arkansas State Veterinary Laboratory, Little Rock, AR, USA).
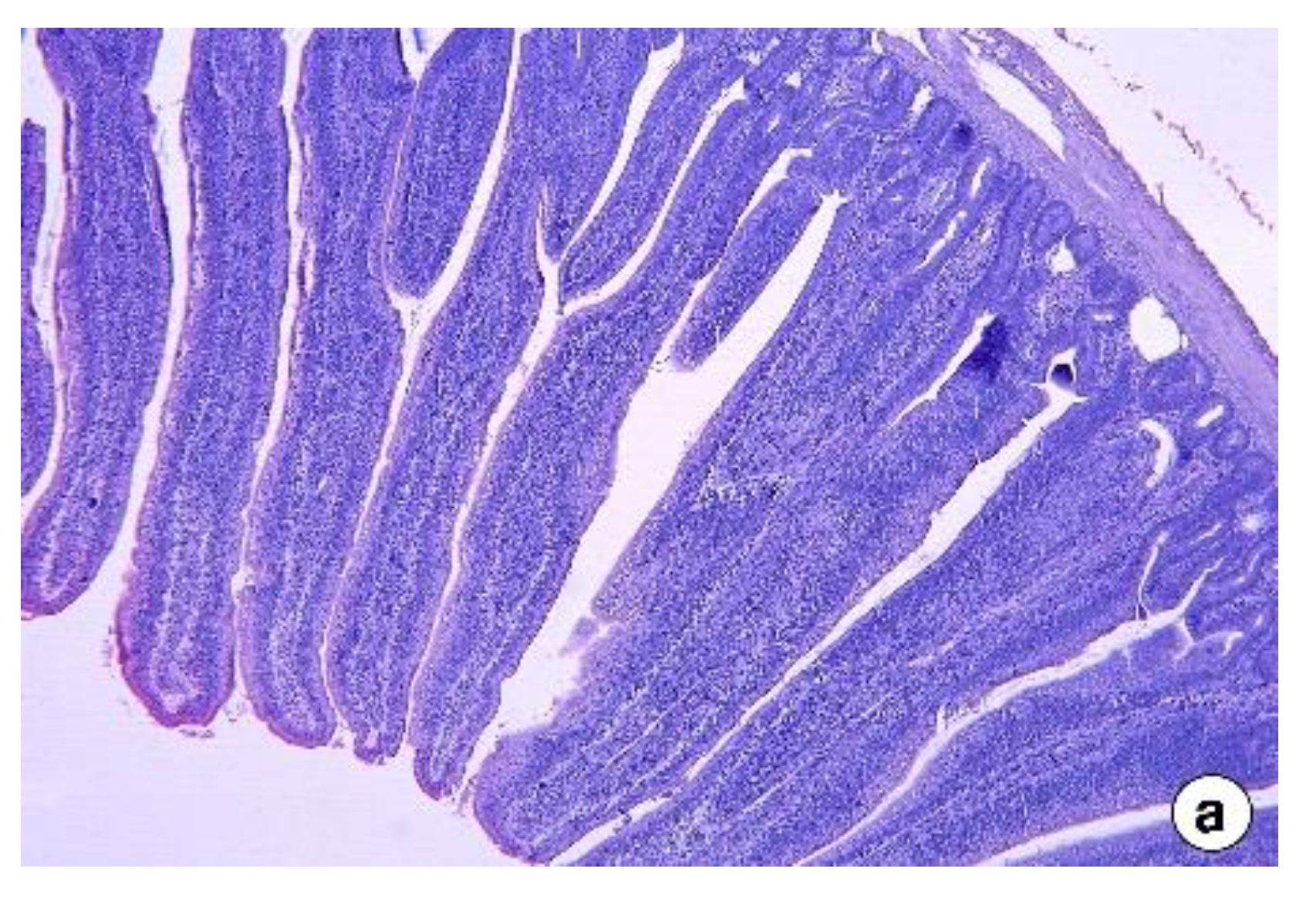
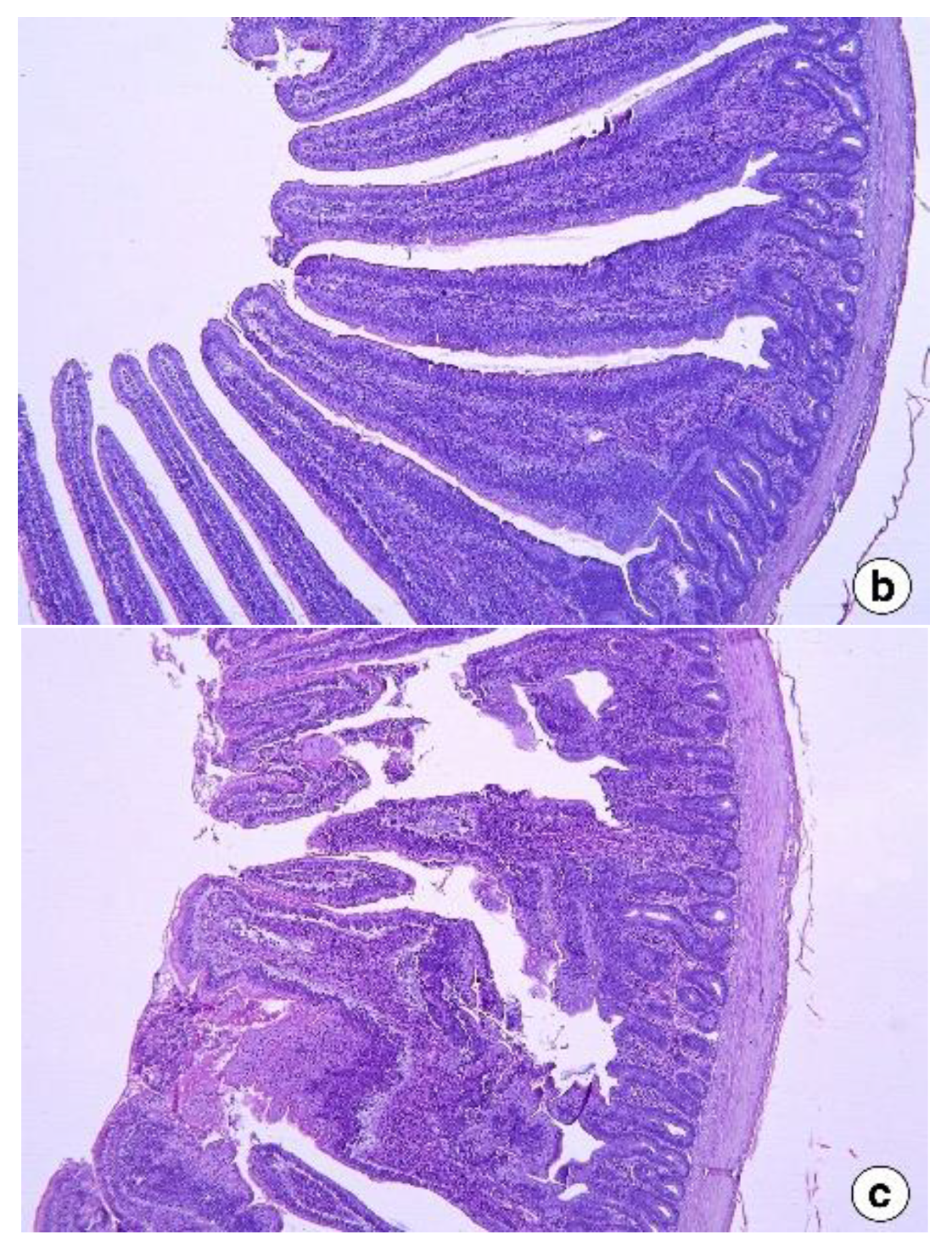
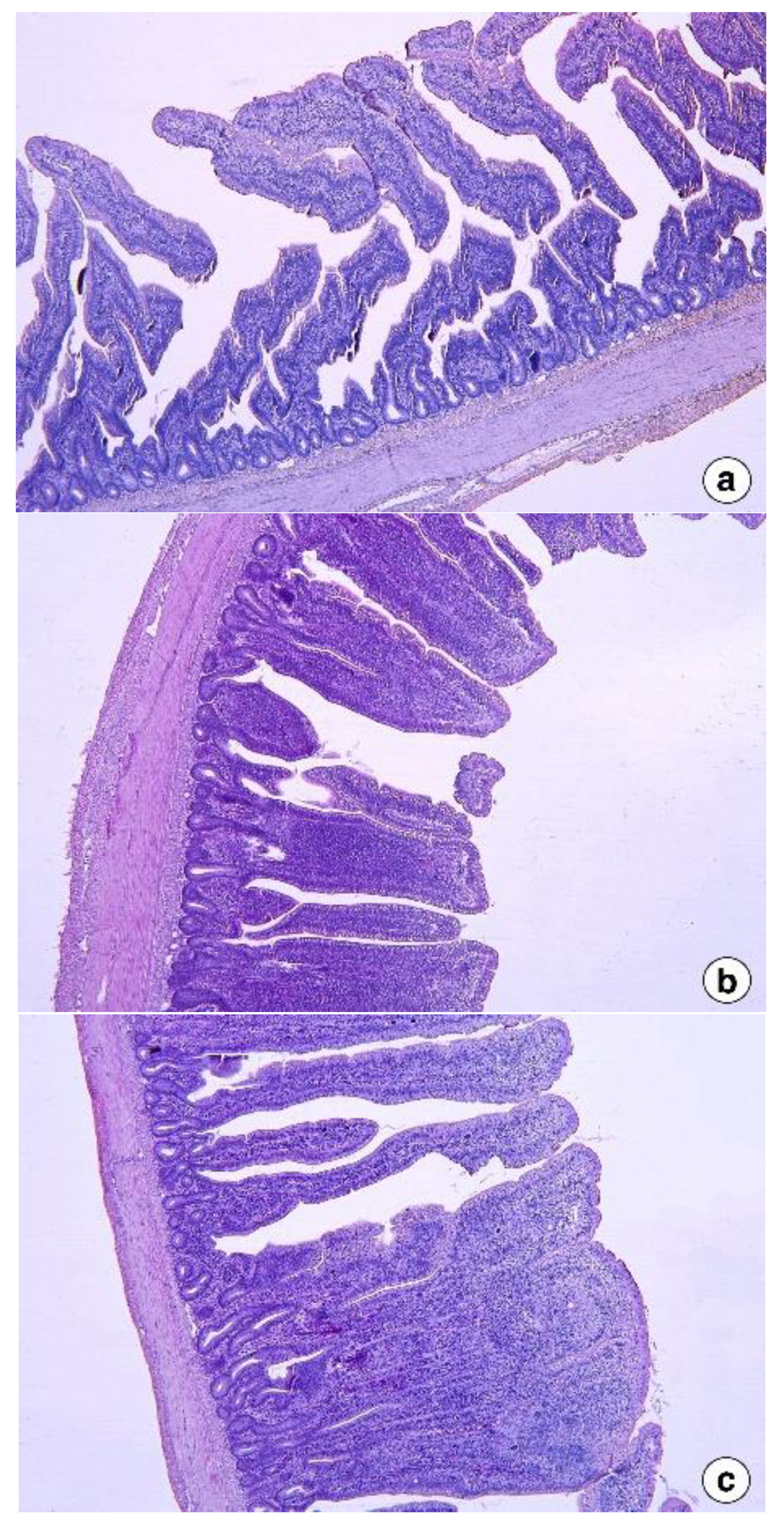
2.5. Intestinal Histomorphology
Intestine samples (about 2 cm segments) were collected from the mid-region of the intestinal segments: jejunum (from the pancreatic loop to Meckel’s diverticulum) and ileum (from Meckel’s diverticulum to the ileocecal junction) of two birds per pen. The samples were washed with 0.1 M phosphate-buffered saline (pH 7.4) and then fixed in Bouin’s solution for 24 h, followed by preservation in 70% (v/v) ethanol for a day. The samples were then progressively dehydrated at increasing concentrations of ethanol, which was then cleared in xylene. The samples were then embedded with paraffin wax and sectioned at 5 µm thickness using Manual Rotary Microtome (Leica Biosystems, Buffalo Grove, IL, USA). Two sections, at different depths, were made for each sample, which was then stained with hematoxylin and eosin. Pictures at ×50 magnification were taken, and the villus height, width, and crypt depth were measured from five random villi from each replicate using Leica software (Leica DM3000, Leica Biosystems, Buffalo Grove, IL, USA).
2.6. Statistical Analysis
Replicate pens were considered as the experimental unit for all analyses. Data collected for growth performance, hematological parameters, serum biochemistry, and intestinal histomorphology were analyzed by one-way analysis of variance (ANOVA) using IBM SPSS Statistics V28.0 software (IBM Corp., Armonk, NY, USA), evaluating the effect of dietary FS inclusion levels by polynomial contrasts. Duncan’s multiple range test was used to compare the difference among the group means. P-values ≤ 0.05 were considered statistically significant, and 0.05 ≤ p-values ≤ 0.10 were considered a tendency. Results were presented as means and their pooled standard error.
4. Discussion
This study examined the effect of fenugreek seeds on the growth performance, hematological parameters, and intestinal histomorphology of broiler chickens. Two experiments were conducted independently. The first experiment evaluated the effect of four different levels of fenugreek seed powder (0 g, 2.5 g, 5 g, and 10 g/kg of diet) on the growth performance of straight-run broilers. The second experiment examined the effect of three different levels of fenugreek seed powder (0 g, 5 g, and 10 g/kg of diet) on the growth performance, hematological parameter, and intestinal histomorphology of male broilers.
The first experiment showed a linear decrease (
p < 0.05) in BWG and FI, whereas a linear increase (
p < 0.05) in FCR with increasing levels of fenugreek seeds during the starter phase in straight-run broilers. However, no effects were observed on the BWG, FI, and FCR during the finisher phase. BWG was also linearly decreased (
p < 0.05) in the overall phase with the increasing levels of FS. In line with the first experiment, Laudadio et al. (2020) [
20] reported the reduction in overall growth performance of broilers with the inclusion of 5 g/kg and 10 g/kg of fenugreek seeds in the broiler diet. Al-Homidan et al. (2020) [
23] also reported the dose-dependent reduction of body weight in broilers with the inclusion of 5 g, 15 g, and 25 g/kg FS of fenugreek seeds. In contrast, other studies reported improvement in the growth performance of broilers with the inclusion of 3 g/kg or lower levels of fenugreek seed powder in the diet [
24,
25]. The decrease in the BWG might be driven by the reduced feed intake during the starter period. The reduced overall performance might be associated with the reduced palatability and lower feed utilization of the diets during the adaptation period of broiler chicken. Fenugreek seeds contain two main constituents that cause a strong odor and bitter taste: volatile oils and alkaloids [
15], which might have reduced the palatability of feed. Future research should investigate the organoleptic properties of FS and the intestinal morphometric changes during the starter phases to unfold the taste preferences and histological changes that might be associated with the reduction in feed intake and feed utilization. However, the body weight gain and feed intake were not affected during the finisher phase. Duru et al. (2013) [
26] also reported the unchanged body weight gain and feed intake from 22 to 42 days of age, including 5 g, 10 g, 20 g, and 40 g/kg fenugreek [
26]. Therefore, it appears that younger broilers are more sensitive to fenugreek, possibly due to being more responsive to factors affecting palatability and/or more susceptible to factors affecting their gastrointestinal system. Birds fed the control diet exhibited the best feed conversion ratio in this experiment. The feed conversion ratio was significantly increased (
p < 0.05) with the increasing levels of fenugreek seeds and recorded maximum value in 10 g/kg fenugreek seeds inclusion. The increase in feed conversion ratio along with the reduction of body weight gain might be due to poor digestion and absorption of nutrients in the diet. It is also possible that higher fenugreek seed levels may amplify adverse effects on growth performance due to higher doses of bioactive compounds such as alkaloids and steroidal sapogenins delivered by fenugreek seeds.
The second experiment also showed a linear decrease (p < 0.05) in BWG and FI, whereas a linear increase (p < 0.05) in FCR with increasing levels of fenugreek seeds (0 g, 5 g, and 10 g/kg FS) was noted during the starter phase in male broilers. However, no effects (p > 0.05) were observed on the BWG, FI, and FCR during the finisher phase. BWG and FI were linearly decreased (p < 0.05) in the overall phase with the increasing levels of FS. These results were similar to the results obtained in the first experiment with straight-run broilers.
WBC count, heterophil count, and lymphocyte count were linearly increased (
p < 0.05) with increasing levels of FS in the diet. It might be possible that inflammation and damage to the jejunum that was characterized by infiltrations of WBC may have led to an increase in the WBC count, heterophil count, and lymphocyte counts. In addition, there was a decrease in the hematocrit percentage compared to the control and 5 g FS inclusion. However, the values lie within the normal hematological range of broiler chickens. Alterations in the hematological count might result from inhibition of hematopoietic regulatory elements in the bone marrow with the high inclusion of FS in the diet [
27].
Dietary fenugreek seeds inclusion did not influence (
p > 0.05) the serum biochemical parameters of the broilers except for total bilirubin in experiment 2. The total bilirubin levels showed a linear decrease (
p < 0.05) with increasing levels of fenugreek seeds in the diet. Qureshi et al. (2017) [
28] also reported a decrease in total bilirubin with the inclusion of fenugreek in the broiler diet. Lower bilirubin levels in the blood serum indicate no negative effects on liver functions [
29].
The intestinal histomorphology studies in the second experiment revealed that the jejunum and ileum showed adverse responses to dietary fenugreek seeds. This included shorter villi and deeper crypts that could have reduced the nutrient digestion and absorption, culminating in reduced body weight gain in FS added groups in the present study compared to control. The small intestine morphology, especially the villi and crypt, plays a vital role in nutrient digestion and assimilation in animals [
30]. Long villi and more shallow crypts are important features of gut morphological development in any animal. Longer villi are associated with a larger total luminal absorptive area, leading to better digestive enzyme function and better nutrient transport [
31]. Simultaneously, shallower crypts represent a longer turnover time for the renewal of the villus [
32], resulting in reduced energy expenditure, and as a result, energy can be channeled to other tissues [
33]. The presence of toxins might be associated with alterations in intestinal architecture, such as shorter villi and deeper crypts, resulting in inadequate nutrient absorption, increased gastrointestinal secretion, and reduced growth performance [
27,
34]. Therefore, the altered intestinal morphology observed in the broiler chickens fed with 5 g and 10 g fenugreek seeds could be related to the toxic effects of high levels of saponins present in the higher inclusion levels of fenugreek seeds. In fact, in the 10 g/kg inclusion of fenugreek, there were instances of damage to the jejunum. Further studies are warranted to analyze the components of fenugreek seeds, such as volatile oils and alkaloids, that affect the intake of the diets in broiler chickens and affect intestinal morphology, especially during the starter phase.