Antibiotic Toxicity Isolated and as Binary Mixture to Freshwater Algae Raphidocelis subcapitata: Growth Inhibition, Prediction Model, and Environmental Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algae Culture Procedures
2.2. Chemicals
2.3. Algal Growth Inhibition Test
2.4. Mixture Toxicity Predictions
2.5. Ecotoxicological Risk Assessment
2.6. Antibiotics Analysis Procedures
2.7. Statistical Analysis
3. Results
3.1. Single Toxicity Assessments
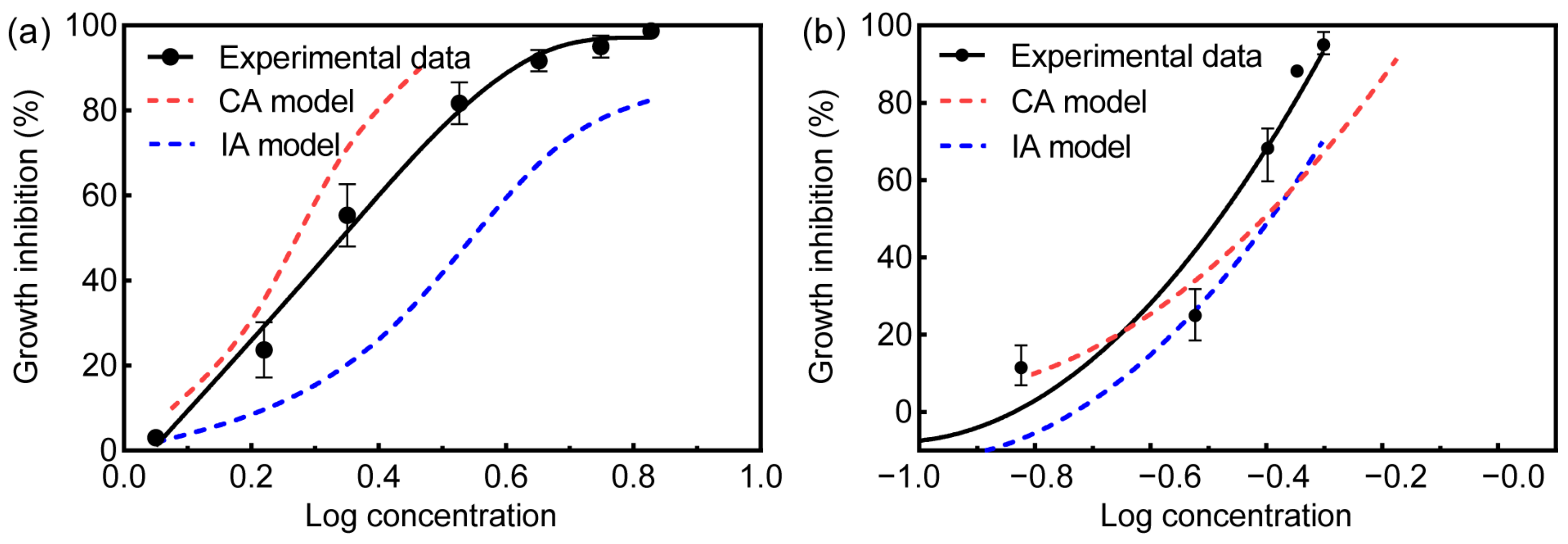
3.2. Toxicity Assessments of Binary Mixtures
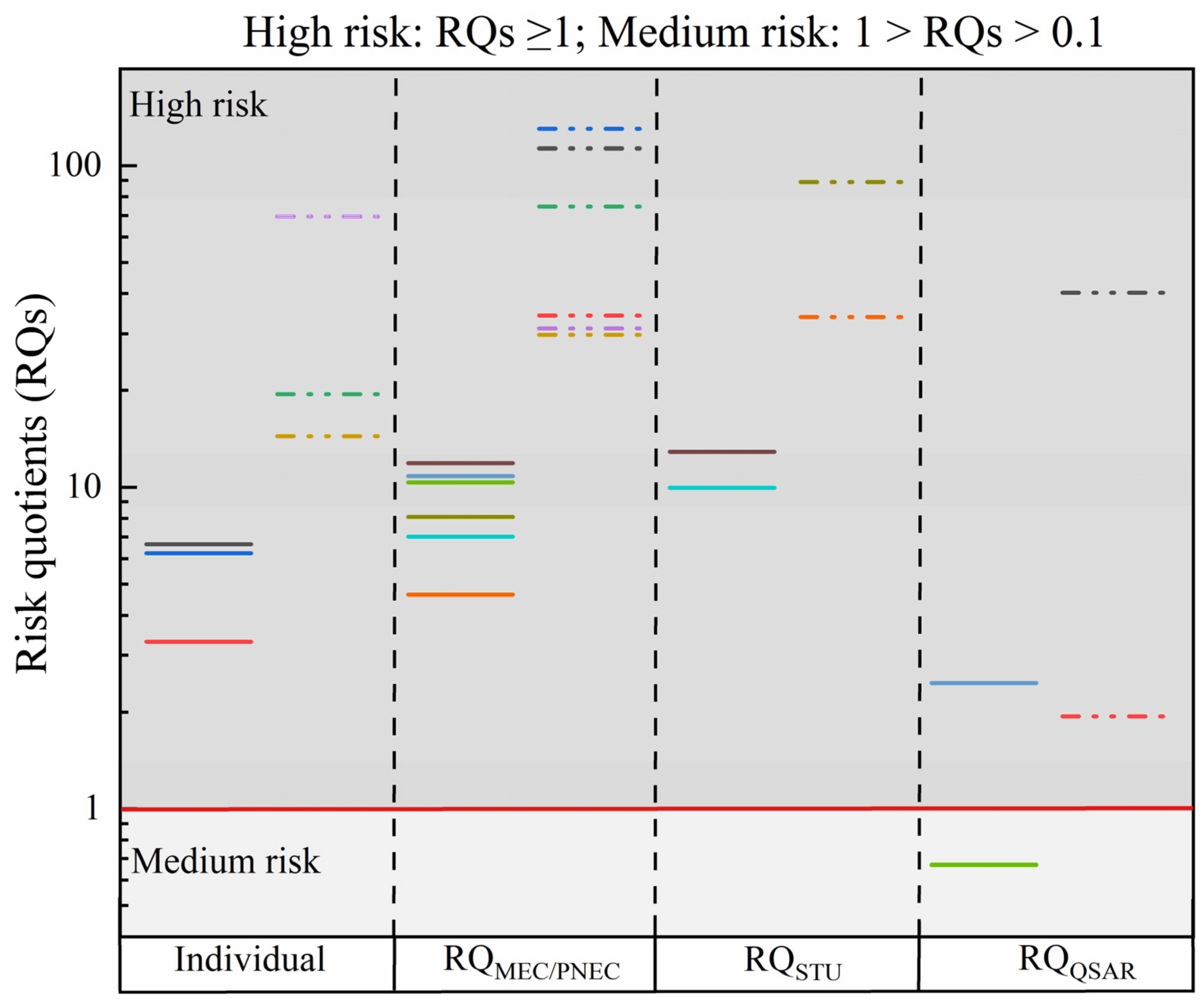
3.3. Environmental Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bu, Z.; Hou, M.; Li, Z.; Dong, Z.; Zeng, L.; Zhang, P.; Wu, G.; Li, X.; Zhang, Y.; Pan, Y. Fe3+/Fe2+ cycle promoted peroxymonosulfate activation with addition of boron for sulfamethazine degradation: Efficiency and the role of boron. Sep. Purif. Technol. 2022, 298, 121596. [Google Scholar] [CrossRef]
- Dang, C.; Xia, Y.; Zheng, M.; Liu, T.; Liu, W.; Chen, Q.; Ni, J. Metagenomic insights into the profile of antibiotic resistomes in a large drinking water reservoir. Environ. Int. 2020, 136, 105449. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gong, Y.; Cai, Z.; O’Reilly, S.; Zhao, D. Mechanistic investigation into sunlight-facilitated photodegradation of pyrene in seawater with oil dispersants. Mar. Pollut. Bull. 2017, 114, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Chang, F.; Yi, M.; Ge, H.; Fu, J.; Dang, C. The distinct resistance mechanisms of cyanobacteria and green algae to sulfamethoxazole and its implications for environmental risk assessment. Sci. Total Environ. 2023, 854, 158723. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.-K.; Choi, J.; Kim, J.O.; Jeon, B.-H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Magdaleno, A.; Saenz, M.; Juárez, A.; Moretton, J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol. Environ. Saf. 2015, 113, 72–78. [Google Scholar] [CrossRef]
- Fu, J.; Lee, W.-N.; Coleman, C.; Nowack, K.; Carter, J.; Huang, C.-H. Removal of pharmaceuticals and personal care products by two-stage biofiltration for drinking water treatment. Sci. Total. Environ. 2019, 664, 240–248. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Chang, F.; Dang, C.; Fu, J. Combined Effects of Sulfamethoxazole and Erythromycin on a Freshwater Microalga, Raphidocelis subcapitata: Toxicity and Oxidative Stress. Antibiotics 2021, 10, 576. [Google Scholar] [CrossRef]
- He, K.; Borthwick, A.G.; Lin, Y.; Li, Y.; Fu, J.; Wong, Y.; Liu, W. Sale-based estimation of pharmaceutical concentrations and associated environmental risk in the Japanese wastewater system. Environ. Int. 2020, 139, 105690. [Google Scholar] [CrossRef]
- Cen, C.; Zhang, K.; Fu, J.; Wu, X.; Wu, J.; Zheng, Y.; Zhang, Y. Odor-producing response pattern by four typical freshwater algae under stress: Acute microplastic exposure as an example. Sci. Total Environ. 2022, 821, 153350. [Google Scholar] [CrossRef]
- Baruah, P.; Chaurasia, N. Ecotoxicological effects of alpha-cypermethrin on freshwater alga Chlorella sp.: Growth inhibition and oxidative stress studies. Environ. Toxicol. Pharmacol. 2020, 76, 103347. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamaguchi, H.; Nakajima, N.; Kawachi, M. Raphidocelis subcapitata (=Pseudokirchneriella subcapitata) provides an insight into genome evolution and environmental adaptations in the Sphaeropleales. Sci. Rep. 2018, 8, 8058. [Google Scholar] [CrossRef]
- Reynolds, A.; Giltrap, M.; Chambers, G. Acute growth inhibition & toxicity analysis of nano-polystyrene spheres on Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2020, 207, 111153. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, L.; Chen, Q. Combined effects of binary antibiotic mixture on growth, microcystin production, and extracellular release of Microcystis aeruginosa: Application of response surface methodology. Environ. Sci. Pollut. Res. 2017, 25, 736–748. [Google Scholar] [CrossRef]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbons in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2020, 207, 111554. [Google Scholar] [CrossRef]
- Mohtar, W.H.M.W.; Maulud, K.N.A.; Muhammad, N.S.; Sharil, S.; Yaseen, Z.M. Spatial and temporal risk quotient based river assessment for water resources management. Environ. Pollut. 2019, 248, 133–144. [Google Scholar] [CrossRef]
- Witthayawirasak, B.; Kingsley, O. Occurrence, Ecological and Health Risk Assessment of Phthalate Esters in Surface Water of U-Tapao Canal, Southern, Thailand. Toxics 2020, 8, 58. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, Z.; Hollert, H.; Zhou, S.; Deutschmann, B.; Seiler, T.-B. Toxicity of 10 organic micropollutants and their mixture: Implications for aquatic risk assessment. Sci. Total. Environ. 2019, 666, 1273–1282. [Google Scholar] [CrossRef]
- Neale, P.A.; Ait-Aissa, S.; Brack, W.; Creusot, N.; Denison, M.S.; Deutschmann, B.; Hilscherová, K.; Hollert, H.; Krauss, M.; Novák, J.; et al. Linking in Vitro Effects and Detected Organic Micropollutants in Surface Water Using Mixture-Toxicity Modeling. Environ. Sci. Technol. 2015, 49, 14614–14624. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Leszczynski, J. Exploration of Computational Approaches to Predict the Toxicity of Chemical Mixtures. Toxics 2019, 7, 15. [Google Scholar] [CrossRef]
- Escher, B.; Braun, G.; Zarfl, C. Exploring the Concepts of Concentration Addition and Independent Action Using a Linear Low-Effect Mixture Model. Environ. Toxicol. Chem. 2020, 39, 2552–2559. [Google Scholar] [CrossRef]
- Backhaus, T. Environmental Risk Assessment of Pharmaceutical Mixtures: Demands, Gaps, and Possible Bridges. AAPS J. 2016, 18, 804–813. [Google Scholar] [CrossRef]
- OECD TG 201. Organization for Economic Co-operation and Development (OECD) Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems Test No. 201: Freshwater Alga and Cyanobacteria; Growth Inhibition Test OECD: Paris, France, 2011. [Google Scholar]
- Cheng, L.; He, Y.; Tian, Y.; Liu, B.; Zhang, Y.; Zhou, Q.; Wu, Z. Comparative biotoxicity of N-Phenyl-1-naphthylamine and N-Phenyl-2-naphthylamine on cyanobacteria Microcystis aeruginosa. Chemosphere 2017, 176, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kim, S.-J.; Kurade, M.B.; Govindwar, S.; Abou-Shanab, R.A.I.; Kim, J.-R.; Roh, H.-S.; Khan, M.A.; Jeon, B.-H. Combined effects of sulfamethazine and sulfamethoxazole on a freshwater microalga, Scenedesmus obliquus: Toxicity, biodegradation, and metabolic fate. J. Hazard. Mater. 2019, 370, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, Y.; Ge, X.; Nie, D.; Wang, M.; Zhou, H.; Chen, M. In vitro toxicity evaluation of heavy metals in urban air particulate matter on human lung epithelial cells. Sci. Total Environ. 2019, 678, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Zuccato, E.; Davoli, E.; Fattore, E.; Castiglioni, S. Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in Italy. J. Hazard. Mater. 2018, 361, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, Y.-S.; Hu, L.-X.; Shi, Z.-Q.; Ying, G.-G. Levofloxacin and sulfamethoxazole induced alterations of biomolecules in Pseudokirchneriella subcapitata. Chemosphere 2020, 253, 126722. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total. Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Borecka, M.; Białk-Bielińska, A.; Haliński, Ł.P.; Pazdro, K.; Stepnowski, P.; Stolte, S. The influence of salinity on the toxicity of selected sulfonamides and trimethoprim towards the green algae Chlorella vulgaris. J. Hazard. Mater. 2016, 308, 179–186. [Google Scholar] [CrossRef]
- Sharma, L.; Siedlewicz, G.; Pazdro, K. The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art. Plants 2021, 10, 591. [Google Scholar] [CrossRef]
- Yang, L.-H.; Ying, G.-G.; Su, H.-C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, L.; Feng, W.; Yuan, M.; Li, J.; Xu, H.; Zheng, X.; Zhang, W. Sulfonamides-induced oxidative stress in freshwater microalga Chlorella vulgaris: Evaluation of growth, photosynthesis, antioxidants, ultrastructure, and nucleic acids. Sci. Rep. 2020, 10, 8243. [Google Scholar] [CrossRef]
- Aderemi, A.O.; Roberts, J.; Hunter, C.; Pahl, O. Microalgal Exposure to Human Antibiotics Triggers Similarities in Growth and Photosynthetic Responses. J. Environ. Prot. 2021, 12, 509–525. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Sensitivity of freshwater and marine green algae to three compounds of emerging concern. J. Appl. Phycol. 2018, 31, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Geiger, E.; Hornek-Gausterer, R.; Saçan, M.T. Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2016, 129, 189–198. [Google Scholar] [CrossRef]
- U.S. EPA-OPP-EFED. Appendix F. The Risk Quotient Method and Levels of Concern; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- Duan, L.; Yang, S.; Sun, Y.; Ye, F.; Jiang, J.; Kou, X.; Yang, F. Spatial and temporal distribution characteristics and potential risks of sulfonamides in theshaanxi section of the weihe river. Int. J. Environ. Res. Public Health 2022, 19, 8607. [Google Scholar] [CrossRef]
- Qin, L.-T.; Chen, Y.-H.; Zhang, X.; Mo, L.-Y.; Zeng, H.-H.; Liang, Y.-P. QSAR prediction of additive and non-additive mixture toxicities of antibiotics and pesticide. Chemosphere 2018, 198, 122–129. [Google Scholar] [CrossRef]
- Lunghini, F.; Marcou, G.; Azam, P.; Enrici, M.; Van Miert, E.; Varnek, A. Consensus QSAR models estimating acute toxicity to aquatic organisms from different trophic levels: Algae, Daphnia and fish. SAR QSAR Environ. Res. 2020, 31, 655–675. [Google Scholar] [CrossRef]
- Almeida, L.C.; Mattos, A.C.; Dinamarco, C.P.G.; Figueiredo, N.G.; Bila, D.M. Chronic toxicity and environmental risk assessment of antivirals in Ceriodaphnia dubia and Raphidocelis subcapitata. Water Sci. Technol. 2021, 84, 1623–1634. [Google Scholar] [CrossRef]
- Alho, L.D.O.G.; Gebara, R.C.; Paina, K.D.A.; Sarmento, H.; Melão, M.D.G.G. Responses of Raphidocelis subcapitata exposed to Cd and Pb: Mechanisms of toxicity assessed by multiple endpoints. Ecotoxicol. Environ. Saf. 2018, 169, 950–959. [Google Scholar] [CrossRef]
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Comput. Mater. Sci. 2016, 6, 147–172. [Google Scholar]
- Guo, J.; Zhang, Y.; Mo, J.; Sun, H.; Li, Q. Sulfamethoxazole-Altered Transcriptomein Green Alga Raphidocelis subcapitata Suggests Inhibition of Translation and DNA Damage Repair. Front. Microbiol. 2021, 12, 541451. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Soares, E.V. Impact of erythromycin on a non-target organism: Cellular effects on the freshwater microalga Pseudokirchneriella subcapitata. Aquat. Toxicol. 2019, 208, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.D.; Soares, E.V. Exposure of the alga Pseudokirchneriella subcapitata to environmentally relevant concentrations of the herbicide metolachlor: Impact on the redox homeostasis. Ecotoxicol. Environ. Saf. 2020, 207, 111264. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Soares, E.V. Toxicological effects induced by the biocide triclosan on Pseudokirchneriella subcapitata. Aquat. Toxicol. 2020, 230, 105706. [Google Scholar] [CrossRef]
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official Journal of the European Union, 24 August 2013; 1–17.
- Liu, L.; Wu, W.; Zhang, J.; Lv, P.; Xu, L.; Yan, Y. Progress of research on the toxicology of antibiotic pollution in aquatic organisms. Acta Ecol. Sin. 2018, 38, 36–41. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhang, J.; Li, X.; Gao, B. Stimulation effects of ciprofloxacin and sulphamethoxazole in Microcystis aeruginosa and isobaric tag for relative and absolute quantitation-based screening of antibiotic targets. Mol. Ecol. 2017, 26, 689–701. [Google Scholar] [CrossRef]
- Commission Directive 93/67/EEC of 20 July 1993 Laying Down the Principles for Assessment of Risks to Man and the Environment of Subtances Notified in Accordance with Council Directive 67/548/EEC. Official Journal of the European Union, 8 September 1993; 9–18.
- Di Poi, C.; Costil, K.; Bouchart, V.; Halm-Lemeille, M.-P. Toxicity assessment of five emerging pollutants, alone and in binary or ternary mixtures, towards three aquatic organisms. Environ. Sci. Pollut. Res. 2017, 25, 6122–6134. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Castiglioni, S.; Fent, K. Synthetic Progestins Medroxyprogesterone Acetate and Dydrogesterone and Their Binary Mixtures Adversely Affect Reproduction and Lead to Histological and Transcriptional Alterations in Zebrafish (Danio rerio). Environ. Sci. Technol. 2015, 49, 4636–4645. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Li, J.; Chen, X.; Lang, Q.; Kuang, S. Combined effects of erythromycin and enrofloxacin on antioxidant enzymes and photosynthesis-related gene transcription in Chlorella vulgaris. Aquat. Toxicol. 2019, 212, 138–145. [Google Scholar] [CrossRef]
- Drzymała, J.; Kalka, J. Ecotoxic interactions between pharmaceuticals in mixtures: Diclofenac and sulfamethoxazole. Chemosphere 2020, 259, 127407. [Google Scholar] [CrossRef]
- Jakobs, G.; Krüger, J.; Schüttler, A.; Altenburger, R.; Busch, W. Mixture toxicity analysis in zebrafish embryo: A time and concentration resolved study on mixture effect predictivity. Environ. Sci. Eur. 2020, 32, 143. [Google Scholar] [CrossRef]
- Carusso, S.; Juárez, A.; Moretton, J.; Magdaleno, A. Effects of three veterinary antibiotics and their binary mixtures on two green alga species. Chemosphere 2017, 194, 821–827. [Google Scholar] [CrossRef]
- Pinheiro, C.; Azevedo, J.; Campos, A.; Vasconcelos, V.; Loureiro, S. The interactive effects of microcystin-LR and cylindrospermopsin on the growth rate of the freshwater algae Chlorella vulgaris. Ecotoxicology 2016, 25, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.-L.; He, W.; Li, Y.-L.; Li, Y.-Y.; Qin, Y.-F.; Meng, F.-Q.; Wang, L.-G.; Xu, F.-L. Residual concentrations and ecological risks of neonicotinoid insecticides in the soils of tomato and cucumber greenhouses in Shouguang, Shandong Province, East China. Sci. Total Environ. 2020, 738, 140248. [Google Scholar] [CrossRef]
- Ågerstrand, M.; Breitholtz, M.; Rudén, C. Comparison of four different methods for reliability evaluation of ecotoxicity data: A case study of non-standard test data used in environmental risk assessments of pharmaceutical substances. Environ. Sci. Eur. 2011, 23, 17. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Wu, Y.; Zhang, W.; Zhong, J.; Lou, Q.; Yang, P.; Fang, Y. Occurrence, distribution, and risk assessment of antibiotics in the surface water of Poyang Lake, the largest freshwater lake in China. Chemosphere 2017, 184, 137–147. [Google Scholar] [CrossRef]
- Magdaleno, A.; Juárez, Á.B.; Dragani, V.; Saenz, M.E.; Paz, M.; Moretton, J. Ecotoxicological and Genotoxic Evaluation of Buenos Aires City (Argentina) Hospital Wastewater. J. Toxicol. 2014, 2014, 248461. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Bu, Z.; Li, Y.; Guo, J.; Li, Q. The transcriptomic analysis revealed sulfamethoxazole stress at environmentally relevant concentration on the mechanisms of toxicity of cyanobacteria Synechococcus sp. J. Environ. Chem. Eng. 2022, 10, 107637. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J.; Li, P. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment. J. Hazard. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, M.E.; Ulvi, A.; Kilic, H. Antibiotics in hospital effluents: Occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ. Sci. Pollut. Res. 2018, 26, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics | Algal Species | EC50 (mg/L) | Endpoint and Duration of Test | References |
|---|---|---|---|---|
| SMX | R. subcapitata | 4.74 | 96 h | [28] |
| SMX | R. subcapitata | 0.52 | 72 h | [29] |
| SMX | C. vulgaris | 1.51 | 72 h | [30] |
| SMX | R. subcapitata | 0.612 | 96 h | This study |
| SMZ | R. subcapitata | 7.8 | 72 h | [31] |
| SMZ | R. subcapitata | 8.7 | 72 h | [32] |
| SMZ | C. vulgaris | 31.35 | 96 h | [33] |
| SMZ | R. subcapitata | 3.235 | 96 h | This study |
| ERY | R. subcapitata | 0.0246 | 96 h | [34] |
| ERY | R. subcapitata | 0.038 | 72 h | [35] |
| ERY | R. subcapitata | 0.044 | 96 h | [8] |
| ERY | C. vulgaris | 0.36 | 96 h | [31] |
| ERY | R. subcapitata | 0.056 | 96 h | This study |
| Growth Inhibition | SMX + SMZ | SMX + ERY | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | ED a | CA | CA-MDR b | IA | IA-MDR c | ED a | CA | CA-MDR b | IA | IA-MDR c |
| 10 | 1.283 | 1.185 | 1.082 | 1.711 | 0.75 | 0.2534 | 0.1561 | 1.623 | 0.3260 | 0.7773 |
| 30 | 1.760 | 1.572 | 1.120 | 2.539 | 0.6932 | 0.2907 | 0.2807 | 1.036 | 0.3206 | 0.9067 |
| 50 | 2.146 | 1.864 | 1.151 | 3.253 | 0.6597 | 0.3716 | 0.4077 | 0.9115 | 0.4267 | 0.8709 |
| 70 | 2.617 | 2.226 | 1.176 | 4.168 | 0.6278 | 0.4308 | 0.4921 | 0.8754 | 0.4734 | 0.91 |
| 90 | 3.589 | 2.932 | 1.224 | 6.186 | 0.58 | 0.5451 | 0.669 | 0.8148 | 0.5585 | 0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, F.; Yi, M.; Li, H.; Wang, J.; Zhao, X.; Hu, X.; Qi, Q. Antibiotic Toxicity Isolated and as Binary Mixture to Freshwater Algae Raphidocelis subcapitata: Growth Inhibition, Prediction Model, and Environmental Risk Assessment. Toxics 2022, 10, 739. https://doi.org/10.3390/toxics10120739
Chang F, Yi M, Li H, Wang J, Zhao X, Hu X, Qi Q. Antibiotic Toxicity Isolated and as Binary Mixture to Freshwater Algae Raphidocelis subcapitata: Growth Inhibition, Prediction Model, and Environmental Risk Assessment. Toxics. 2022; 10(12):739. https://doi.org/10.3390/toxics10120739
Chicago/Turabian StyleChang, Fang, Malan Yi, Huiting Li, Jiangnan Wang, Xuefeng Zhao, Xiaoyue Hu, and Qianju Qi. 2022. "Antibiotic Toxicity Isolated and as Binary Mixture to Freshwater Algae Raphidocelis subcapitata: Growth Inhibition, Prediction Model, and Environmental Risk Assessment" Toxics 10, no. 12: 739. https://doi.org/10.3390/toxics10120739
APA StyleChang, F., Yi, M., Li, H., Wang, J., Zhao, X., Hu, X., & Qi, Q. (2022). Antibiotic Toxicity Isolated and as Binary Mixture to Freshwater Algae Raphidocelis subcapitata: Growth Inhibition, Prediction Model, and Environmental Risk Assessment. Toxics, 10(12), 739. https://doi.org/10.3390/toxics10120739







