Environmental Contamination and Human Exposure to Select Endocrine-Disrupting Chemicals: A Review
Abstract
1. Introduction
2. Mechanism of Action of EDCs
3. Applications/Use of Compounds That Elicit Endocrine-Disrupting Properties
- Short-chain parabens: methyl paraben (MePB) and ethyl paraben (EtPB);
- Long-chain parabens: propyl paraben (PrPB), isopropyl paraben (iPrPB), butyl paraben (BuPB), isobutyl paraben (iBuPB), and benzyl paraben (BePB) [26].
- Short-chain phthalates: dimethyl phthalate (DMP) and dibutyl phthalate (DBP);
- Long-chain phthalates: butyl-benzyl phthalate (BBP), di-n-hexyl phthalate (DNHP), di-2-ethylhexyl phthalate (DEHP), di-n-octyl phthalate (DNOP), di-iso-nonyl phthalate (DINP), and di-iso-decyl phthalate (DIDP).
4. Endocrine-Disrupting Compounds in Environmental Matrices
4.1. Parabens in Water
4.2. Parabens in Soil and Sediment
4.3. Parabens in Biota/Fish
| Sampling Area | MePB | EtPB | PrPB | BuPB | BePB | n-PrPB | Unit | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Manzanares River, Spain | 13.5 | 32 | ng/L | [103] | |||||
| Jarama River, Spain | 4.2 (Sunday) 30 (Thursday) | ng/L | |||||||
| Northern Antarctic Peninsula Region | 16.05 | ng/L | |||||||
| Rivers in the Iberian Peninsula | ND–142 | ND–49 | ND–26 | ND–7.3 | ng/L | [104] | |||
| Ria de Aveiro (Rivers of Agueda & Vouga) | ND–45 | ND–2.2 | ND–6.2 | ng/L | [24] | ||||
| Rivers Caster & Antua | 3.3–16 | <0.3–6.4 | <0.5–64 | <0.2–42 | <0.2–0.3 | ng/L | |||
| Lagoon in Ria de Aveiro | 2.1–51 | <0.3–6.7 | <0.5–7.9 | <0.2–0.2 | <0.2–0.3 | ng/L | |||
| Sea | 5.1–21 | <0.3–1.6 | <0.5–1.6 | <0.2–0.7 | <0.2 | ng/L | |||
| Sea near outfall | 5.7–62 | <0.3–15 | <0.5–6.1 | <0.2–7.1 | <0.2 | ng/L | |||
| Japanese rivers | ND–525 | ND-181 | ng/L | [78] | |||||
| Antarctic seawater | <0.8–37.4 | ng/L | [84] | ||||||
| Southern India (29 sites) | ND–22.8 | 2.47–147 | ng/L | [77] | |||||
| Urban, streams in Tokushima and Osaka, Japan (12 sites) | 25–676 | <1.3–64 | <0.8–207 | <0.6–163 | <0.2–2.3 | ng/L | [105] | ||
| CentralPacific region, Japan (4 sites) | LOQ––5.4 | LOQ––25 | LOQ–12 | ng/L | [106] | ||||
| Greater Pittsburgh area, USA (6 sites) | 2.2–17.3 | ND–12 | ng/L | [38] | |||||
| Drinking water from Turia River Basin, Spain | 12 | <0.3 | 9 | 28 | ng/L | [36] | |||
| Jiulong River Estuary, China Winter | 2.65–29.1 | 1.11–5.22 | [82] | ||||||
| 2.23–53.4 | 1.91–68.3 | ng/L | |||||||
| Spring | ng/L | ||||||||
| Autumn | 1.41–7.27 | 0.4–1.59 | ng/L | ||||||
| Summer | 2.98–68.8 | 1.06–10.1 | ng/L | ||||||
| Wet Season | 1.68–39.4 | 3.4–69.9 | ng/L | ||||||
| Pearl River Estuary, China (Seawater) | 2.21 | 0.94 | 1.12 | 0.21 | 0.01 | 0.04 | ng/L | [107] | |
| Florida coast, USA | 14.7 ± 10.9 | 6.12 ± 7.09 | <0.5–9.04 | [85] | |||||
| Concentrations of Parabens in Sediment | |||||||||
| Sampling Area | MePB | EtPB | PrPB | BuPB | BePB | n-PrPB | Unit | Reference | |
| Turia River Basin, Spain | 476 | 60 | ng/g | [36] | |||||
| Korea | 0.13–11.2 | <LOQ–0.08 | <LOQ–0.10 | <LOQ–0.07 | <LOQ–0.06 | ng/g dw | [89] | ||
| Ebro River, Spain | <LOQ–435 | <LOQ–2.7 | <LOQ–51 | ng/g dw | [104] | ||||
| Guadalquivir River, Spain | <LOQ–63.0 | <LOQ–1.8 | <LOQ–3.5 | ||||||
| Jucar River, Spain | <LOQ–22.6 | <LOQ–0.3 | <LOQ–5.3 | ||||||
| Llobregat River, Spain | <LOQ–95 | <LOQ–0.91 | <LOQ–3.9 | ||||||
| Florida coast, USA | 0.85–9.00 | 2.15–12.38 | ng/g | [91] | |||||
| Tokyo Bay, Japan | 2.59–17.8 | <LOQ–0.13 | <LOQ–2.84 | <LOQ–29.1 | <LOQ–0.64 | ng/g | [31] | ||
| Pearl River, china | 0.9–8.8 | ng/g | [92] | ||||||
| Yellow River, China | 7.07–27.6 | 0.61–2.43 | 2.52–6.91 | 0.96–3.90 | 0.13–2.09 | ng/g | [90] | ||
| Huai River, China | 6.97–18.8 | 1.02–2.14 | 2.72–9.17 | 1.84–7.6 | 0.17–0.4 | ng/g | |||
| Guangzhou River, China | 1.03–69.9 | <LOQ–1.97 | <LOQ–21.3 | ng/g | [93] | ||||
| Dongjiang River, China | 1.83–26.2 | 0.28–0.75 | 0.16–0.86 | ng/g | [108] | ||||
| Sha River, China | 1.95–42.8 | 0.26–3.19 | ng/g | [109] | |||||
| Yangtze River, China | 1.43–15.1 | <LOQ-0.63 | <LOQ-2.40 | ng/g | [110] | ||||
| Pearl River Estuary, China | 118 | 45.4 | 10.0 | 2.09 | 2.75 | 1.07 | ng/g | [107] | |
| Concentration of Parabens in Fish | |||||||||
| Sampling Area | MePB | EtPB | PrPB | BuPB | BePB | n-PrPB | Unit | Reference | |
| Northern coast of Spain | Mussel | 7 ± 2 | 0.3 ± 0.1 | 0.56 ±0.01 | ng/g dw | [99] | |||
| Manila Clam | 1.6 ± 0.3 | ||||||||
| Cockle | 2.0 ± 0.5 | 0.37 ± 0.08 | |||||||
| Manila Bay, Philippines | 46.6–195 | 46–1140 | 6.61–37.3 | ng/g lw | [34] | ||||
| Manila Bay (fish muscle) (20 species) | <0.05–3600 | <0.011–840 | <0.024–1100 | <0.003–70 | ng/g lw | [35] | |||
| Mediterranean Rivers, Spain (fish homogenate) | 84.69 ± 6.58 | 0.19 ± 0.04 | ng/g | [33] | |||||
| Pearl River Estuary, China (shellfish and fish) | 5.2 | 2.35 | 0.25 | 0.48 | 0.01 | ng/g | [107] | ||
| Llobregat River, Spain (Barbus graellsii) | Adult | 62.85 ± 6.52 | 3.48 ± 0.58 | ng/ g dw | [33] | ||||
| Juvenile | 33.65 ± 3.70 | 0.19 ± 0.04 | |||||||
| Cyprinus carpio | Llobregat River, Spain | 2.53 ± 0.38 | ng/ g dw | ||||||
| Ebro River, Spain | 3.41 ± 0.59 | ||||||||
| Llobregat River, Spain (Lepomis gibbosus) | 9.08 ± 1.06 | 0.64 ± 0.13 | 0.35 ± 0.02 | ng/ g dw | |||||
| Jucar River, Spain | Salmo truta | 4.45 ± 0.44 | 0.82 (Adult) 0.78 (Juvenile) | 1.43 ± 0.69 | ng/g dw | ||||
| Micropterus salmoides | 4.45 ± 0.44 | ||||||||
| Anguilla anguilla | 2.97 ± 0.13 | 0.50 ± 0.04 | |||||||
| Lepomis gibbosus | 0.54 | ||||||||
| Sampling Area | MePB | EtPB | PrPB | BuPB | BePB | n-PrPB | Unit | Reference | |
| Urinary concentration of parabens in U.S population (≥ 6 years) | 5.60–974 | ND-57.2 | 0.30–299 | ND-19.6 | µg/L | [111] | |||
| Spain | Pregnant Women | 100 | 98 | 88 | 90 | ng/L | [112] | ||
| Children | 100 | 100 | 80 | 83 | ng/L | ||||
| Newborn infants, Korea | 79.6 | 2.4 | 3.4 | µg/L | [30] | ||||
| Serum level in Danish women | ND-59.6 | ND-20.8 | ND-5.50 | ND-0.87 | ND-0.29 | ng/L | [113] | ||
| Breast milk (28–40-year-old women) (Valencian region, Spain) | 0.11–7.00 | 0.49–4.05 | 0.13–0.76 | 0.17–0.34 | ng/mL | [114] | |||
| Breast milk, North Carolina | 0.5–21 | 0.1–12 | ng/mL | [115,116,117] | |||||
| Breast milk, Spain | 0.6–22 | 0.81–1.10 | ng/mL | [118] | |||||
| The Belgian ENVIRONAGE cohort (placenta samples) | 0.5–7.1 | 0.5–4.5 | 0.5–9.1 | ng/g | [119] | ||||
| Hospital Sant Joan de Deu, Barcelona, Spain (mothers at first trimester) | 11.77 | ng/g fw (fresh weight) | [120] | ||||||
| Taiwan (urine) | Male | ND-56.8 | ND-52.0 | ND-1.8 | ND-19.5 | ng/mL | [121] | ||
| Female | ND-174 | ND-40.4 | ND-61.4 | ND-84.7 | |||||
4.4. Phenols in Water
4.5. Phenols in Soil/Sediment
4.6. Phenols in Biota/Fish
| Concentration of Phenolic Compounds in Water | ||||||
|---|---|---|---|---|---|---|
| Sampling Area | Compound | Concentration | Unit | Reference | ||
| Manzanares River, Spain | BPA | 36.5 (Sunday) 37 (Thursday) | ng/L | [103] | ||
| OP | 109.5 (Sunday) 125 (Thursday) | |||||
| NP | 850 (Sunday) 622.5 (Thursday) | |||||
| Nonylphenolmonocarboxylate | 1342.5 (Sunday) 938 (Thursday) | |||||
| Octylphenoldiethoxylate | 46.5 (Sunday) 15.5 (Thursday) | |||||
| Nonylphenoldiethoxylate | 279.5 (Sunday) 168 (Thursday) | |||||
| Jarama River, Spain | BPA | 106 (Sunday) 47.5 (Thursday) | ||||
| OP | 60 (Sunday) 96 (Thursday) | |||||
| NP | 123 (Sunday) 813 (Thursday) | |||||
| Nonylphenolmonocarboxylate | 734 (Sunday) 926 (Thursday) | |||||
| Octylphenoldiethoxylate | 68 (Sunday) 49 (Thursday) | |||||
| Nonylphenoldiethoxylate | 345 (Sunday) 637 (Thursday) | |||||
| Llobregat River and other rivers of Spain | BPA | 2970 | ng/L | [146,147,148] | ||
| Jialu River, China | BPA | 2990 | ng/L | [149] | ||
| Liao River and Yellow River, China | 755.6 | ng/L | [150] | |||
| Rio das Velhas River, Brazil | 168.3 | ng/L | [151] | |||
| Qiantang River and Tiesha River | NP | 8540 | ng/L | [152] | ||
| Rio das Velhas River, Brazil | 1582 | ng/L | [151] | |||
| Liao River and Yellow River, China | 2065.7 | ng/L | [150] | |||
| Liao River and Yellow River, China | OP | 577.9 | ng/L | [150] | ||
| Llobregat River and other rivers of Spain | 6200 | ng/L | [146,147,148] | |||
| Jialu River, China | 63.2 | ng/L | [153] | |||
| Liao River and Yellow River, China | 52.1 | ng/L | [150] | |||
| Rio das Velhas River, Brazil | 1435 | ng/L | [151] | |||
| Northern Antarctic Peninsula region | BPA | 18.74 | ng/L | [154] | ||
| NP | 138.32 | |||||
| Mississippi | BPA | 57.14 | ng/L | [155] | ||
| Rivers in Portugal | BPA | 5.4 | ng/L | [24] | ||
| PulauKukup, Johor (estuarine water) | BPA | 0.19–0.47 | ng/L | [125] | ||
| Seoul, South Korea | Surface river water | BPA | 6.90–59.00 | ng/L | [126] | |
| estuarine water | 5.00–1918 | ng/L | ||||
| Xiangjiang River | Alkylphenol | 0.79–3079.4 | ng/L | [156] | ||
| Pearl River, China | 8–15688 | ng/L | [157] | |||
| Han River, South Korea | 6.9–5.9 | ng/L | [126] | |||
| Rio de Janeiro, Brazil | 204–13016 | ng/L | [158] | |||
| Iberian River, Spain | BPA | ND-649 | ng/L | [104] | ||
| OP | ND-85 | ng/L | ||||
| NP | ND-391 | ng/L | ||||
| Lamone River, northeastern part of Italy | BPA | 16 | ng/L | [130] | ||
| NP | 39 | |||||
| Fiumi Uniti River, Italy | BPA | 19 | ng/L | |||
| NP | 94 | |||||
| Bevano River, Italy | BPA | 46 | ng/L | |||
| NP | 41 | |||||
| Savio River, Italy | BPA | 23 | ng/L | |||
| NP | 79 | |||||
| Marecchia River, Italy | BPA | 195 | ng/L | |||
| NP | 9.7 | |||||
| Guangzhou tap water | BPA | 317 | [159] | |||
| Langat River, Peninsular, Malaysia | BPA | 1.18–8.24 | ng/L | [127] | ||
| Malaysia (drinking water sources) | BPA | ND-215 | ng/L | [128] | ||
| Jiulong River Estuary, China | BPA | ND-364 | ng/L | [82] | ||
| Tokyo Bay, Japan | BPA | ND-431 | ng/L | [54] | ||
| BPF | ND-1470 | |||||
| Pearl River Estuary, China | BPA | 24.6 | ng/L | [107] | ||
| Ria de Aveiro, Portugal | BPA | <1.1 | ng/L | [24] | ||
| Ross Island, Antarctic | BPA | <1.3–7.7 | ng/L | [84] | ||
| Laizhou Bay, China | BPA | 11.1–101 | [122] | |||
| Pearl River, China | 4-NP | 61–2996 | ng/L | [160] | ||
| 4-t-OP | ND-198 | |||||
| BPA | 66–556 | |||||
| The Pearl River Delta region, China | BPA | 5.84–469 | ng/L | [143] | ||
| 4-NP | 52.0–8643 | |||||
| 4-t-OP | 1539 | |||||
| Gernika | 4-t-OP | 41 ± 2 | ng/L | [161] | ||
| 4nOP | 22 ± 2 | |||||
| Santurtzi | 4-t-OP | 17 ± 2 | ||||
| Cangzhou, Hebei, China (irrigation with ground water) | 4-t-OP | 6.8 ± 2.1 | ng/L | [162] | ||
| 4nOP | 350 ± 37.2 | |||||
| BPA | 61.2 ± 5.2 | |||||
| Shijazhuang, Heibei, China (irrigation with ground water) | 4-t-OP | 9.0 ± 1.4 | ||||
| 4nOP | 396 ± 51.2 | |||||
| BPA | 51.7 ± 2.9 | |||||
| Baoding, Heibei, China (irrigation with ground water) | 4-t-OP | 5.2 ± 0.66 | ||||
| 4nOP | 202 ± 69.6 | |||||
| BPA | 44.8 ± 2.8 | |||||
| Concentration of Phenolic Compounds in Sediments | ||||||
| Sampling Area | Compound | Concentration | Unit | Reference | ||
| Pearl River estuary, China | BPA | 69.4 | ng/g | [107] | ||
| BPS | 41.6 | |||||
| BPF | 183 | |||||
| BPAF | 167 | |||||
| BPB | 73.3 | |||||
| Pearl River, China | BPA | 7.3–627 | ng/g | [160] | ||
| 4-NP | 53–12042 | |||||
| 4-t-OP | 8.3–176 | |||||
| Concentration of Phenolic Compounds in Fish | ||||||
| Sampling Area | Compound | Concentration | Unit | Reference | ||
| Mariculture production, Malaysia (fish muscle) | BPA | 0.023–0.322 | ng/g | [129] | ||
| BPS | 10.3 | |||||
| BPF | 35.0 | |||||
| BPAF | 0.70 | |||||
| BPB | 1.51 | |||||
| Pearl River estuary, China (shellfish and fish) | BPP | 25.4 | ng/mL | [107] | ||
| BPA | 0.81 | |||||
| BPS | 1.27 | |||||
| BPF | 1.45 | |||||
| BPAF | 0.22 | |||||
| BPB | 12.3 | |||||
| Llobregat River, Spain (Barbusgraellsii) | BPA | 223.91 ± 11.51 | ng/g dw | [33] | ||
| Guadalquivir River, Spain (luciobarbuss clateri) | BPA | 59.09 ± 8.12 | ||||
| Sampling Area | Compound | Concentration | Unit | Reference | ||
| Pearl River delta region (the Dongjang River, Shima River, Danshui River, and Xizhijiang River) | Wet season | Dry season | ng/g ww | [143] | ||
| Bile | BPA | 2.45–1,3610 | 0–1,3070 | |||
| 4-t-OP | 38.6–1938 | 35.9–2625 | ||||
| 4-NP | 4695–21160 | 3216–27420 | ||||
| Liver | BPA | 2.17–40920 | 1.27–16070 | |||
| 4-t-OP | 0–261 | 0–50.8 | ||||
| 4-NP | 0–5978 | 0–3535 | ||||
| Plasma | BPA | 6.90–141 | 8.51–1571 | |||
| 4-t-OP | 26.7–135 | 31.2–56.0 | ||||
| 4-NP | 2743–5530 | 3136–5901 | ||||
| Muscle | BPA | 3.76–65.5 | 0.70–2053 | |||
| 4-t-OP | 0–4.53 | 0-6.98 | ||||
| 4-NP | 9.54–307 | 14.2–329 | ||||
| Xiangjang River, China (Parabramis pekinensis, Cyprinus carpio, Siniperca chuatsi) | Muscle | 4-n-NP | ND-2.07 | ng/g | [121] | |
| BPA | ND-3.51 | |||||
| Liver | 4-n-NP | ND-148 | ||||
| BPA | ND-61.9 | |||||
| Gill | 4-n-NP | ND-29.7 | ||||
| BPA | ND-48.2 | |||||
| Gonad | 4-n-NP | ND-20.8 | ||||
| BPA | ND-1379 | |||||
| Pearl River estuary, China (muscle tissue) | Mugilcephalus | BPA | 0.19–1.27 | ng/g dw | [163] | |
| Parabramispekinensis | BPA | 0.43–4.51 | ||||
| Loma Lake, China (Grass carp and Lateolabrax japonicas) | Muscle | BPA | 7.56 | ng/g dw | [164] | |
| Northern coast of Sicily, Italy (Red mullet) | Muscle | BPA | 46.7–58.9 | ng/g | [140] | |
| Liver | BPA | 35.0–77.6 | ||||
| Panlong River, Chin (Crucian carp and carp) | Muscle | BPA | 1.9–69 | ng/g | [144] | |
| Gill | BPA | 23 | ||||
| Basque coast, Spain (Grey mullet) | Muscle | BPA | 20–28 | ng/g | [141] | |
| Liver | BPA | 47–97 | ||||
| Brain | BPA | 31–46 | ||||
| Taihu Lake, China | Muscle | BPA | 37.3–475 | ng/g | [132] | |
| Pearl River delta, China (Carp) | Bile | BPA | 70–1020 | ng/g | [165] | |
| Rhone River, France | Barbel | BPA | 3.2 | ng/g | [166] | |
| Common bream | 19.8 | |||||
| White bream | 9.6 | |||||
| Chub | 18.6 | |||||
| (Cyprinus carpio) | Muscle | BPA | 1.58 ± 0.26 | mg/g | [167] | |
| Liver | 2.15 ± 0.19 | |||||
| Dianchi Lake, China (Crucian carp and carp) | Muscle | BPA | 38.7 | ng/g | [168] | |
| Liver | 107 | |||||
| Gill | 37.5 | |||||
| Concentration of Phenolic Compounds in Human Samples | ||||||
| Sampling Area | Compound | Concentration | Unit | Reference | ||
| Valencian region, Spain (28–40-year-old women’s breast milk) | BPF | 0.13–0.32 | ng/mL | [114] | ||
| BPS | <LOQ-0.37 | |||||
| BPA | <LOQ-1.62 | |||||
| The Belgian ENVIRONAGE cohort (placenta samples) | BPA | 0.5–3.9 | ng/g | [119] | ||
| BPF | 0.6–2.1 | |||||
| BPS | 0.8–1.3 | |||||
| OP | 0.5–3.7 | |||||
4.7. Phthalates in Water
4.8. Phthalates in Soil/Sediment
4.9. Phthalates in Fish
| Concentration of Phthalate Compounds in Water | |||||
|---|---|---|---|---|---|
| Sampling Area | Compound | Concentration | Unit | Reference | |
| Chaohu Lake, China | DMP | 0.015–3.670 | µg/L | [173] | |
| DEP | 0.006–0.283 | ||||
| BBP | ND-0.107 | ||||
| DnBP | 0.070–17.529 | ||||
| DEHP | ND-0.576 | ||||
| DnOP | ND-0.045 | ||||
| Songhua River, China | DMP | 0.98–4.12 | ng/mL | [191] | |
| DEP | 1.33–6.67 | ||||
| BBP | ND-4.39 | ||||
| DBP | 1.69–11.8 | ||||
| DEHP | 2.26–11.6 | ||||
| DOP | 0.69–6.14 | ||||
| Jiulong River, China | DMP | 0.03–0.24 | µg/L | [169] | |
| DEP | 0.03–0.22 | ||||
| DBP | 0.3–2.4 | ||||
| DEHP | 0.9–3.6 | ||||
| Rhone River, France | DMP | 0.003–0.005 | µg/L | [192] | |
| DEP | 0.016–0.031 | ||||
| DBP | 0.022–0.041 | ||||
| DEHP | 0.039–0.407 | ||||
| Al-Khobar, Saudi Arabia | DEP | 6.98 | µg/L | [193] | |
| DBP | 7.9 | ||||
| Taihu Lake | DMP | ND-1.32 | µg/L | [171] | |
| DEP | 0.08–4.79 | ||||
| BBP | 0.08–4.72 | ||||
| DBP | ND-2.54 | ||||
| DEHP | ND-1.41 | ||||
| DnOP | 0.07–0.590 | ||||
| Chaohu Lake, China | Summer | DMP | 0.021–0.193 | µg/L | [179] |
| DEP | 0.078–0.174 | ||||
| BBP | 0.001–0.003 | ||||
| DBP | 0.463–11.2 | ||||
| DEHP | ND-0.067 | ||||
| DiBP | 0.918–11.1 | ||||
| Autumn | DMP | ND-0.111 | |||
| DEP | 0.024–0.160 | ||||
| BBP | 0.001–0.011 | ||||
| DBP | 0.426–3.65 | ||||
| DEHP | ND-0.086 | ||||
| DiBP | 0.832–2.64 | ||||
| Winter | DMP | 0.006–0.099 | |||
| DEP | 0.010–0.102 | ||||
| BBP | 0.001–0.004 | ||||
| DBP | 0.098–0.465 | ||||
| DEHP | 0.002–0.217 | ||||
| DiBP | 0.210–1.08 | ||||
| Asan Lake, Korea | DMP | ND-0.18 | µg/L | [89] | |
| DEP | ND-0.05 | ||||
| DBP | ND-0.34 | ||||
| DEHP | ND-1.34 | ||||
| DnOP | ND-0.02 | ||||
| DiBP | ND-0.07 | ||||
| Gernika | BBP | 19 ± 1 | ng/L | [161] | |
| DEHP | 641 ± 195 | ||||
| Ondarroa | BBP | 16 ± 3 | |||
| DEHP | 350 ± 26 | ||||
| Deba | BBP | 20 ± 1 | |||
| DEHP | 1595 ± 416 | ||||
| Pasaia | BBP | 20 ± 3 | |||
| DEHP | 806 ± 380 | ||||
| Concentration of Phthalates in Sediment | |||||
| Sampling Area | Compound | Concentration | Unit | Reference | |
| Pearl River, China | DMP | 0.001–0.019 | mg/kg | [194] | |
| DEP | 0.001–0.091 | ||||
| BBP | ND-0.113 | ||||
| DBP | 0.042–5.03 | ||||
| DEHP | 0.415–29.5 | ||||
| DnOP | ND-0.181 | ||||
| Qiantang River, China | DMP | ND-0.179 | mg/kg | [170] | |
| DEP | ND-0.218 | ||||
| BBP | ND-0.021 | ||||
| DnBP | 0.034–0.241 | ||||
| DEHP | 0.365–6.24 | ||||
| DnOP | ND-0.019 | ||||
| Jiulong River, China | DMP | ND-0.004 | mg/kg | [169] | |
| DEP | ND-0.002 | ||||
| DBP | 0.004–0.23 | ||||
| DEHP | 0.053–1.28 | ||||
| Songhua River, China | DMP | 0.03–0.09 | mg/kg | [191] | |
| DEP | 0.03–0.04 | ||||
| BBP | ND-0.10 | ||||
| DBP | 0.06–0.88 | ||||
| DEHP | 0.23–0.57 | ||||
| DnOP | ND-0.38 | ||||
| Ogun River, Nigeria | DMP | ND-0.85 | mg/kg | [189] | |
| DEP | 0.08–0.35 | ||||
| DBP | 0.19–1.42 | ||||
| DEHP | 0.02–0.82 | ||||
| Gomti River, India | DMP | ND-0.05 | mg/kg | [195] | |
| DEP | ND-0.035 | ||||
| DBP | ND-0.034 | ||||
| DEHP | ND-0.324 | ||||
| DnOP | ND-0.053 | ||||
| Taihu Lake | DMP | 0.950–3.50 | mg/kg | [171] | |
| DEP | 0.590–2.290 | ||||
| BBP | 0.420–1.30 | ||||
| DBP | 0.5–1.75 | ||||
| DEHP | 0.550–4.77 | ||||
| DnOP | 0.480–16.2 | ||||
| Lake Chaohu, China | Summer | DMP | 0.627–13.4 | µg/g | [179] |
| DEP | 0.599–12.08 | ||||
| BBP | ND-0.688 | ||||
| DBP | 3.26–108 | ||||
| DEHP | 1.99–48.6 | ||||
| DiBP | 7.94–225 | ||||
| Autumn | DMP | 0.430–226 | |||
| DEP | 0.475–149 | ||||
| BBP | ND-4.69 | ||||
| DBP | 4.86–1307 | ||||
| DEHP | 1.69–1059 | ||||
| DiBP | 9.58–4383 | ||||
| Winter | DMP | 12.8–434 | |||
| DEP | 3.22–55.9 | ||||
| BBP | ND-28.4 | ||||
| DBP | 10.6–285 | ||||
| DEHP | 6.08–372 | ||||
| DiBP | 25.5–548 | ||||
| Asan lake, Korea | DMP | ND-6.4 | µg/kg dw | [89] | |
| DEP | ND-4.1 | ||||
| DBP | ND-535 | ||||
| DEHP | 3.6–8326 | ||||
| DiBP | ND-43 | ||||
| Concentration of Phthalates in Fish | |||||
| Sampling Area | Compound | Concentration | Unit | Reference | |
| Asan Lake, Korea (crucian carp, skygager, bass, bluegill) | DMP | ND-10.9 | µg/kg dw | [89] | |
| DEP | ND-13.6 | ||||
| DBP | ND-107 | ||||
| DEHP | ND-568 | ||||
| DnOP | ND-34.2 | ||||
| DiBP | ND-29.4 | ||||
| BBP | ND-65.0 | ||||
5. Human Exposure
5.1. Parabens
5.2. Phenols
- Age;
- Concentration (exposure magnitude);
- Duration of exposure both internal and external; and
5.3. Phthalates
- 5.
- Increased thyroid transcription factor I (TTF1) and paired-box gene 8 (Pax8) influence on the thyroid system growth and development;
- 6.
- Increased thyroid stimulating hormone beta-subunit (TSHβ) and sodium/iodide symporter (NIS) and thyroglobulin (TG) results in thyroid hormone synthesis; and
- 7.
- Decreased transthyretin (TTR) via thyroid transport [247].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and agriculture: The case of pesticides. Comptes Rendus Biol. 2017, 340, 406–409. [Google Scholar] [CrossRef]
- Quan, C.; Liu, Q.; Tian, W.; Kikuchi, J.; Fan, S. Biodegradation of an endocrine-disrupting chemical, di-2-ethylhexyl phthalate, by Bacillus subtilis No. 66. Appl. Microbiol. Biotechnol. 2005, 66, 702–710. [Google Scholar] [CrossRef]
- Anwer, F.; Chaurasia, S.; Khan, A.A. Hormonally active agents in the environment: A state-of-the-art review. Rev. Environ. Health 2016, 31, 415–433. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Bergman, Å.; Becher, G.; Bjerregaard, P.; Bornman, R.; Brandt, I.; Iguchi, T.; Jobling, S.; Kidd, K.A.; Kortenkamp, A. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ. Health A Glob. Access Sci. Source 2014, 13, 118. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Golub, M.S.; Doherty, J.D. Triphenyltin as a potential human endocrine disruptor. J. Toxicol. Environ. Healthpart B 2004, 7, 281–295. [Google Scholar] [CrossRef]
- Mazur, C.S.; Marchitti, S.A.; Zastre, J. P-glycoprotein inhibition by the agricultural pesticide propiconazole and its hydroxylated metabolites: Implications for pesticide–drug interactions. Toxicol. Lett. 2015, 232, 37–45. [Google Scholar] [CrossRef]
- Wadzinski, T.; Altowaireb, Y.; Gupta, R.; Conroy, R.; Shoukri, K. Luteoma of pregnancy associated with nearly complete virilization of genetically female twins. Endocr. Pract. 2014, 20, e18–e23. [Google Scholar] [CrossRef]
- Lee, H.R.; Jeung, E.B.; Cho, M.H.; Kim, T.H.; Leung, P.C.; Choi, K.C. Molecular mechanism (s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J. Cell. Mol. Med. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Legler, J.; Zeinstra, L.M.; Schuitemaker, F.; Lanser, P.H.; Bogerd, J.; Brouwer, A.; Vethaak, A.D.; de Voogt, P.; Murk, A.J.; van der Burg, B. Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenic activity. Environ. Sci. Technol. 2002, 36, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Munier, M.; Grouleff, J.; Gourdin, L.; Fauchard, M.; Chantreau, V.; Henrion, D.; Coutant, R.; Schiøtt, B.; Chabbert, M.; Rodien, P. In vitro effects of the endocrine disruptor p, p’-DDT on human follitropin receptor. Environ. Health Perspect. 2016, 124, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [PubMed]
- Schrader, T.J.; Cooke, G.M. Examination of selected food additives and organochlorine food contaminants for androgenic activity in vitro. Toxicol. Sci. 2000, 53, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L. Minireview: Epigenomic plasticity and vulnerability to EDC exposures. Mol. Endocrinol. 2016, 30, 848–855. [Google Scholar] [CrossRef]
- Walker, D.M.; Gore, A.C. Epigenetic impacts of endocrine disruptors in the brain. Front. Neuroendocr. 2017, 44, 1–26. [Google Scholar] [CrossRef]
- Zama, A.M.; Uzumcu, M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology 2009, 150, 4681–4691. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Zhang, G.; Guan, Y.; Wang, Z. Effect of low-dose malathion on the gonadal development of adult rare minnow Gobiocypris rarus. Ecotoxicol. Environ. Saf. 2016, 125, 135–140. [Google Scholar] [CrossRef]
- Zhou, Q.; Miao, M.; Ran, M.; Ding, L.; Bai, L.; Wu, T.; Yuan, W.; Gao, E.; Wang, J.; Li, G. Serum bisphenol-A concentration and sex hormone levels in men. Fertil. Steril. 2013, 100, 478–482. [Google Scholar] [CrossRef]
- Brucker-Davis, F. Effects of environmental synthetic chemicals on thyroid function. Thyroid 1998, 8, 827–856. [Google Scholar] [CrossRef]
- Bansal, R.; Zoeller, R.T. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology 2008, 149, 4001–4008. [Google Scholar] [CrossRef]
- Komesli, O.; Muz, M.; Ak, M.; Bakırdere, S.; Gokcay, C. Occurrence, fate and removal of endocrine disrupting compounds (EDCs) in Turkish wastewater treatment plants. Chem. Eng. J. 2015, 277, 202–208. [Google Scholar] [CrossRef]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- Preau, L.; Fini, J.B.; Morvan-Dubois, G.; Demeneix, B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim. Et Biophys. Acta Gene Regul. Mech. 2015, 1849, 112–121. [Google Scholar] [CrossRef]
- Jonkers, N.; Sousa, A.; Galante-Oliveira, S.; Barroso, C.M.; Kohler, H.-P.E.; Giger, W. Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ. Sci. Pollut. Res. 2010, 17, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Danish, E. Survey of parabens. Part Lous Rev. 2013, 1474, 2013. [Google Scholar]
- Soni, M.; Carabin, I.; Burdock, G. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; McGinity, J.W. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties of Eudragit® RS PO hot-melt extrudates. Eur. J. Pharm. Biopharm. 2003, 56, 95–100. [Google Scholar] [CrossRef]
- Núñez, L.; Tadeo, J.; García-Valcárcel, A.; Turiel, E. Determination of parabens in environmental solid samples by ultrasonic-assisted extraction and liquid chromatography with triple quadrupole mass spectrometry. J. Chromatogr. A 2008, 1214, 178–182. [Google Scholar] [CrossRef]
- Jewell, C.; Prusakiewicz, J.J.; Ackermann, C.; Payne, N.A.; Fate, G.; Voorman, R.; Williams, F.M. Hydrolysis of a series of parabens by skin microsomes and cytosol from human and minipigs and in whole skin in short-term culture. Toxicol. Appl. Pharmacol. 2007, 225, 221–228. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.; Park, J.; Kim, H.-J.; Lee, J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.-B. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 2013, 461, 214–221. [Google Scholar] [CrossRef]
- Liao, C.; Chen, L.; Kannan, K. Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ. Int. 2013, 57, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Xue, J.; Park, K.J.; Kannan, K.; Moon, H.-B. Tissue-specific accumulation and body burden of parabens and their metabolites in small cetaceans. Environ. Sci. Technol. 2018, 53, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Jakimska, A.; Huerta, B.; Bargańska, Ż.; Kot-Wasik, A.; Rodríguez-Mozaz, S.; Barceló, D. Development of a liquid chromatography–tandem mass spectrometry procedure for determination of endocrine disrupting compounds in fish from Mediterranean rivers. J. Chromatogr. A 2013, 1306, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Ramaswamy, B.R.; Chang, K.-H.; Isobe, T.; Tanabe, S. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3511–3520. [Google Scholar] [CrossRef]
- Ramaswamy, B.R.; Kim, J.-W.; Isobe, T.; Chang, K.-H.; Amano, A.; Miller, T.W.; Siringan, F.P.; Tanabe, S. Determination of preservative and antimicrobial compounds in fish from Manila Bay, Philippines using ultra high performance liquid chromatography tandem mass spectrometry, and assessment of human dietary exposure. J. Hazard. Mater. 2011, 192, 1739–1745. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Trace analysis of endocrine disrupting compounds in environmental water samples by use of solid-phase extraction and gas chromatography with mass spectrometry detection. J. Chromatogr. A 2014, 1360, 248–257. [Google Scholar] [CrossRef]
- Renz, L.; Volz, C.; Michanowicz, D.; Ferrar, K.; Christian, C.; Lenzner, D.; El-Hefnawy, T. A study of parabens and bisphenol A in surface water and fish brain tissue from the Greater Pittsburgh Area. Ecotoxicology 2013, 22, 632–641. [Google Scholar] [CrossRef]
- Canosa, P.; Rodríguez, I.; Rubi, E.; Negreira, N.; Cela, R. Formation of halogenated by-products of parabens in chlorinated water. Anal. Chim. Acta 2006, 575, 106–113. [Google Scholar] [CrossRef]
- Albero, B.; Pérez, R.A.; Sánchez-Brunete, C.; Tadeo, J.L. Occurrence and analysis of parabens in municipal sewage sludge from wastewater treatment plants in Madrid (Spain). J. Hazard. Mater. 2012, 239, 48–55. [Google Scholar] [CrossRef]
- Pouillot, A.; Polla, B.; Polla, A. Conservateurs en cosmétologie: Mise au point sur les Parabènes. J. De Médecine Esthétique Et De Chir. Dermatol. 2006, 33, 187–190. [Google Scholar]
- Eriksson, E.; Andersen, H.R.; Ledin, A. Substance flow analysis of parabens in Denmark complemented with a survey of presence and frequency in various commodities. J. Hazard. Mater. 2008, 156, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kannan, K. Characteristic profiles of benzonphenone-3 and its derivatives in urine of children and adults from the United States and China. Environ. Sci. Technol. 2013, 47, 12532–12538. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Sasaki, N.; Elangovan, M.; Diamond, G.; Kannan, K. Elevated accumulation of parabens and their metabolites in marine mammals from the United States coastal waters. Environ. Sci. Technol. 2015, 49, 12071–12079. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.-L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity a review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Teng, C.; Goodwin, B.; Shockley, K.; Xia, M.; Huang, R.; Norris, J.; Merrick, B.A.; Jetten, A.M.; Austin, C.P.; Tice, R.R. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem. Biol. Interact. 2013, 203, 556–564. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Kolatorova, L.; Duskova, M.; Vitku, J.; Starka, L. Prenatal exposure to bisphenols and parabens and impacts on human physiology. Physiol. Res. 2017, 66, S305–S315. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, L. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016, 103, 343–351. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Guo, Y.; Moon, H.-B.; Nakata, H.; Wu, Q.; Kannan, K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 2012, 46, 9138–9145. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.; Moon, H.-B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Ballesteros, O.; Zafra-Gómez, A.; Ballesteros, L.; Navalón, A. UHPLC–MS/MS method for the determination of bisphenol A and its chlorinated derivatives, bisphenol S, parabens, and benzophenones in human urine samples. Anal. Bioanal. Chem. 2014, 406, 3773–3785. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Gerona, R.R.; Kannan, K.; Taylor, J.A.; van Breemen, R.B.; Dickenson, C.A.; Liao, C.; Yuan, Y.; Newbold, R.R.; Padmanabhan, V. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ. Health 2014, 13, 25. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.-Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of US adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Castillo, M.; Riu, J.; Ventura, F.; Boleda, R.; Scheding, R.; Schröder, H.F.; Nistor, C.; Emneus, J.; Eichhorn, P.; Knepper, T.P. Inter-laboratory comparison of liquid chromatographic techniques and enzyme-linked immunosorbent assay for the determination of surfactants in wastewaters. J. Chromatogr. A 2000, 889, 195–209. [Google Scholar] [CrossRef]
- Magoarou, P. Proceedings of Workshop on Problems Around Sludge, Stresa, Italy, 18–19 November 1999. EUR 19657 EN. 2000. Available online: https://ec.europa.eu/environment/archives/waste/sludge/pdf/workshoppart1.pdf (accessed on 10 May 2021).
- Martínez-Zapata, M.; Aristizábal, C.; Peñuela, G. Photodegradation of the endocrine-disrupting chemicals 4n-nonylphenol and triclosan by simulated solar UV irradiation in aqueous solutions with Fe (III) and in the absence/presence of humic acids. J. Photochem. Photobiol. A Chem. 2013, 251, 41–49. [Google Scholar] [CrossRef]
- Cheng, G.; Sun, M.; Lu, J.; Ge, X.; Zhang, H.; Xu, X.; Lou, L.; Lin, Q. Role of biochar in biodegradation of nonylphenol in sediment: Increasing microbial activity versus decreasing bioavailability. Sci. Rep. 2017, 7, 4726. [Google Scholar] [CrossRef]
- Abargues, M.; Giménez, J.; Ferrer, J.; Bouzas, A.; Seco, A. Endocrine disrupter compounds removal in wastewater using microalgae: Degradation kinetics assessment. Chem. Eng. J. 2018, 334, 313–321. [Google Scholar] [CrossRef]
- Hess, S.C. Interferentes hormonais no ambiente: Um risco à saúde pública. Eng. Ambient. Espírito St. Do Pinhal 2010, 7, 311–329. [Google Scholar]
- Benjamin, S.; Pradeep, S.; Josh, M.S.; Kumar, S.; Masai, E. A monograph on the remediation of hazardous phthalates. J. Hazard. Mater. 2015, 298, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Tayubi, I.A.; Ahmad, E.; Ganaie, M.A.; Bajouh, O.S.; AlBasri, S.F.; Abdulkarim, I.M.; Beg, M.A. Computational insights into the molecular interactions of environmental xenoestrogens 4-tert-octylphenol, 4-nonylphenol, bisphenol A (BPA), and BPA metabolite, 4-methyl-2, 4-bis (4-hydroxyphenyl) pent-1-ene (MBP) with human sex hormone-binding globulin. Ecotoxicol. Environ. Saf. 2017, 135, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Turki, R.F.; Abuzenadah, A.M.; Damanhouri, G.A.; Beg, M.A. Endocrine disruption: Computational perspectives on human sex hormone-binding globulin and phthalate plasticizers. PLoS ONE 2016, 11, e0151444. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Polinski, K.J.; Dabelea, D.; Hamman, R.F.; Adgate, J.L.; Calafat, A.M.; Ye, X.; Starling, A.P. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ. Res. 2018, 162, 308–317. [Google Scholar] [CrossRef]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of endocrine-disrupting chemicals on the ovary. Biol. Reprod. 2015, 93, 20. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–479. [Google Scholar] [CrossRef]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.-I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Berlioz-Barbier, A.; Vauchez, A.; Wiest, L.; Baudot, R.; Vulliet, E.; Cren-Olivé, C. Multi-residue analysis of emerging pollutants in sediment using QuEChERS-based extraction followed by LC-MS/MS analysis. Anal. Bioanal. Chem. 2014, 406, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Kannan, K. Phthalates and parabens in personal care products from China: Concentrations and human exposure. Arch. Environ. Contam. Toxicol. 2014, 66, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total Environ. 2008, 397, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, B.R.; Shanmugam, G.; Velu, G.; Rengarajan, B.; Larsson, D.J. GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 2011, 186, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kameda, Y.; Yamamoto, H.; Nakada, N.; Tamura, I.; Miyazaki, M.; Masunaga, S. Occurrence of preservatives and antimicrobials in Japanese rivers. Chemosphere 2014, 107, 393–399. [Google Scholar] [CrossRef]
- Jonkers, N.; Kohler, H.-P.E.; Dammshäuser, A.; Giger, W. Mass flows of endocrine disruptors in the Glatt River during varying weather conditions. Environ. Pollut. 2009, 157, 714–723. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Evaluation of the occurrence and biodegradation of parabens and halogenated by-products in wastewater by accurate-mass liquid chromatography-quadrupole-time-of-flight-mass spectrometry (LC-QTOF-MS). Water Res. 2011, 45, 6770–6780. [Google Scholar] [CrossRef]
- Peng, X.; Ou, W.; Wang, C.; Wang, Z.; Huang, Q.; Jin, J.; Tan, J. Occurrence and ecological potential of pharmaceuticals and personal care products in groundwater and reservoirs in the vicinity of municipal landfills in China. Sci. Total Environ. 2014, 490, 889–898. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Zhan, Y.; Lu, H.; Tsang, D.C.; Liu, W.; Wang, X.; Li, X.; Zhu, L. Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Sci. Total Environ. 2016, 544, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Kung, T.A.; Lee, S.H.; Yang, T.C.; Wang, W.H. Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan. Sci. Total Environ. 2018, 635, 1302–1307. [Google Scholar] [CrossRef]
- Emnet, P.; Gaw, S.; Northcott, G.; Storey, B.; Graham, L. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ. Res. 2015, 136, 331–342. [Google Scholar] [CrossRef]
- Xue, J.; Liu, W.; Kannan, K. Bisphenols, benzophenones, and bisphenol A diglycidyl ethers in textiles and infant clothing. Environ. Sci. Technol. 2017, 51, 5279–5286. [Google Scholar] [CrossRef]
- Ma, W.-L.; Zhao, X.; Zhang, Z.-F.; Xu, T.-F.; Zhu, F.-J.; Li, Y.-F. Concentrations and fate of parabens and their metabolites in two typical wastewater treatment plants in northeastern China. Sci. Total Environ. 2018, 644, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Delgado, B.; Pino, V.; Anderson, J.L.; Ayala, J.H.; Afonso, A.M.; Gonzalez, V. An in-situ extraction–preconcentration method using ionic liquid-based surfactants for the determination of organic contaminants contained in marine sediments. Talanta 2012, 99, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Viglino, L.; Prévost, M.; Sauvé, S. High throughput analysis of solid-bound endocrine disruptors by LDTD-APCI-MS/MS. J. Environ. Monit. 2011, 13, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Lee, J.-E.; Choe, W.; Kim, T.; Lee, J.-Y.; Kho, Y.; Choi, K.; Zoh, K.-D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, J.; Xi, N.; Guo, W.; Sun, J. Parabens and their metabolite in surface water and sediment from the Yellow River and the Huai River in Henan Province: Spatial distribution, seasonal variation and risk assessment. Ecotoxicol. Environ. Saf. 2019, 172, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Kannan, K. Accumulation profiles of parabens and their metabolites in fish, black bear, and birds, including bald eagles and albatrosses. Environ. Int. 2016, 94, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xiong, S.; Ou, W.; Wang, Z.; Tan, J.; Jin, J.; Tang, C.; Liu, J.; Fan, Y. Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J. Hazard. Mater. 2017, 323, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, L.-H.; Liu, G.-Q.; Shi, L.; Guo, Y. Occurrence and ecological risk assessment of eight endocrine-disrupting chemicals in urban river water and sediments of South China. Arch. Environ. Contam. Toxicol. 2018, 75, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Karthikraj, R.; Vasu, A.K.; Balakrishna, K.; Sinha, R.K.; Kannan, K. Occurrence and fate of parabens and their metabolites in five sewage treatment plants in India. Sci. Total Environ. 2017, 593, 592–598. [Google Scholar] [CrossRef]
- Moon, H.B.; Choi, H.G.; Lee, P.Y.; Ok, G. Congener-specific characterization and sources of polychlorinated dibenzo-p-dioxins, dibenzofurans and dioxin-like polychlorinated biphenyls in marine sediments from industrialized bays of Korea. Environ. Toxicol. Chem. Int. J. 2008, 27, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-B.; Kannan, K.; Choi, M.; Choi, H.-G. Polybrominated diphenyl ethers (PBDEs) in marine sediments from industrialized bays of Korea. Mar. Pollut. Bull. 2007, 54, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Albero, B.; Miguel, E.; Sánchez-Brunete, C. Determination of parabens and endocrine-disrupting alkylphenols in soil by gas chromatography–mass spectrometry following matrix solid-phase dispersion or in-column microwave-assisted extraction: A comparative study. Anal. Bioanal. Chem. 2012, 402, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.C.; Möder, M.; Laespada, M.F. Stir bar sorptive extraction of parabens, triclosan and methyl triclosan from soil, sediment and sludge with in situ derivatization and determination by gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 3837–3844. [Google Scholar] [CrossRef]
- Villaverde-de-Sáa, E.; Rodil, R.; Quintana, J.B.; Cela, R. Matrix solid-phase dispersion combined to liquid chromatography–tandem mass spectrometry for the determination of paraben preservatives in mollusks. J. Chromatogr. A 2016, 1459, 57–66. [Google Scholar] [CrossRef]
- Lu, S.; Wang, N.; Ma, S.; Hu, X.; Kang, L.; Yu, Y. Parabens and triclosan in shellfish from Shenzhen coastal waters: Bioindication of pollution and human health risks. Environ. Pollut. 2019, 246, 257–263. [Google Scholar] [CrossRef]
- Liao, C.; Lee, S.; Moon, H.-B.; Yamashita, N.; Kannan, K. Parabens in sediment and sewage sludge from the United States, Japan, and Korea: Spatial distribution and temporal trends. Environ. Sci. Technol. 2013, 47, 10895–10902. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Maulvault, A.L.; Tediosi, A.; Fernández-Tejedor, M.; Van den Heuvel, F.; Kotterman, M.; Marques, A.; Barceló, D. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ. Res. 2015, 143, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Gorga, M.; Petrovic, M.; González-Alonso, S.; Barceló, D.; Valcárcel, Y. Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci. Total Environ. 2014, 466, 939–951. [Google Scholar] [CrossRef]
- Gorga, M.; Insa, S.; Petrovic, M.; Barceló, D. Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers. Sci. Total Environ. 2015, 503, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Tamura, I.; Hirata, Y.; Kato, J.; Kagota, K.; Katsuki, S.; Yamamoto, A.; Kagami, Y.; Tatarazako, N. Aquatic toxicity and ecological risk assessment of seven parabens: Individual and additive approach. Sci. Total Environ. 2011, 410, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Takemura, Y.; Makino, M. Paraben-chlorinated derivatives in river waters. Environ. Chem. Lett. 2012, 10, 401–406. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, W.; Zheng, Y.; Xiong, J.; Gao, C.; Hu, S. Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China. Ecotoxicol. Environ. Saf. 2019, 180, 43–52. [Google Scholar] [CrossRef]
- Liu, W.-R.; Yang, Y.-Y.; Liu, Y.-S.; Zhao, J.-L.; Zhang, Q.-Q.; Yao, L.; Zhang, M.; Jiang, Y.-X.; Wei, X.-D.; Ying, G.-G. Biocides in the river system of a highly urbanized region: A systematic investigation involving runoff input. Sci. Total Environ. 2018, 624, 1023–1030. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Q.; Gao, R.; Hou, H.; Tan, W.; He, X.; Zhang, H.; Yu, M.; Ma, L.; Xi, B. Contamination of phthalate esters (PAEs) in typical wastewater-irrigated agricultural soils in Hebei, North China. PLoS ONE 2015, 10, e0137998. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zhao, J.-L.; Liu, Y.-S.; Chen, Z.-F.; Yang, Y.-Y.; Zhang, Q.-Q.; Ying, G.-G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Bishop, A.M.; Needham, L.L. Urinary concentrations of four parabens in the US population: NHANES 2005–2006. Environ. Health Perspect. 2010, 118, 679–685. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jørgensen, N.; Andersson, A.-M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef]
- Dualde, P.; Pardo, O.; Fernández, S.F.; Pastor, A.; Yusà, V. Determination of four parabens and bisphenols A, F and S in human breast milk using QuEChERS and liquid chromatography coupled to mass spectrometry. J. Chromatogr. B 2019, 1114, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015, 54, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Jiménez-Díaz, I.; Díaz, C.; Pérez, J.; Iribarne-Durán, L.M.; Serrano-López, L.; Arrebola, J.P.; Fernández, M.F.; Olea, N. Determination of endocrine-disrupting chemicals in human milk by dispersive liquid–liquid microextraction. Bioanalysis 2016, 8, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Iribarne-Durán, L.; Mustieles, V.; Jiménez-Díaz, I.; Fernández, M.; Olea, N. QuEChERS and ultra-high performance liquid chromatography–tandem mass spectrometry method for the determination of parabens and ultraviolet filters in human milk samples. J. Chromatogr. A 2018, 1546, 1–9. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Determination of free and conjugated forms of endocrine-disrupting chemicals in human biological fluids by GC− MS. Bioanalysis 2016, 8, 1145–1158. [Google Scholar] [CrossRef]
- Van Overmeire, I.; Vrijens, K.; Nawrot, T.; Van Nieuwenhuyse, A.; Van Loco, J.; Reyns, T. Simultaneous determination of parabens, bisphenols and alkylphenols in human placenta by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2019, 1121, 96–102. [Google Scholar] [CrossRef]
- Valle-Sistac, J.; Molins-Delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Díaz-Cruz, M.S. Determination of parabens and benzophenone-type UV filters in human placenta. First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016, 88, 243–249. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Z.; Luo, Z.; Li, H.; Chen, G. Endocrine disrupting chemicals in wild freshwater fishes: Species, tissues, sizes and human health risks. Environ. Pollut. 2019, 244, 462–468. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Deng, X.; Gong, X.; Song, X.; Zhang, H.; Tian, X.; Zhang, X. Survey of bisphenol A contamination in Laizhou Bay. Prog. Fish. Sci. 2013, 34, 16–20. [Google Scholar]
- Basheer, C.; Lee, H.K.; Tan, K.S. Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Mar. Pollut. Bull. 2004, 48, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, Z.; Mai, B.; Chen, F.; Chen, S.; Tan, J.; Yu, Y.; Tang, C.; Li, K.; Zhang, G. Temporal trends of nonylphenol and bisphenol A contamination in the Pearl River Estuary and the adjacent South China Sea recorded by dated sedimentary cores. Sci. Total Environ. 2007, 384, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.H.; Wee, S.Y.; Kamarulzaman, N.H.; Aris, A.Z. Quantification of multi-classes of endocrine-disrupting compounds in estuarine water. Environ. Pollut. 2019, 249, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Ryu, J.; Oh, J.; Choi, B.-G.; Snyder, S.A. Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Sci. Total Environ. 2010, 408, 636–643. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence and risk assessment of multiclass endocrine disrupting compounds in an urban tropical river and a proposed risk management and monitoring framework. Sci. Total Environ. 2019, 671, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Santhi, V.; Sakai, N.; Ahmad, E.; Mustafa, A. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci. Total Environ. 2012, 427, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.H.; Wee, S.Y.; Aris, A.Z. Bisphenol A and alkylphenols concentrations in selected mariculture fish species from Pulau Kukup, Johor, Malaysia. Mar. Pollut. Bull. 2018, 127, 536–540. [Google Scholar] [CrossRef]
- Pignotti, E.; Dinelli, E. Distribution and partition of endocrine disrupting compounds in water and sediment: Case study of the Romagna area (North Italy). J. Geochem. Explor. 2018, 195, 66–77. [Google Scholar] [CrossRef]
- Ying, G.-G.; Kookana, R.S.; Kumar, A.; Mortimer, M. Occurrence and implications of estrogens and xenoestrogens in sewage effluents and receiving waters from South East Queensland. Sci. Total Environ. 2009, 407, 5147–5155. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Liu, F.; Yin, S.; Bao, Z.; Liu, H. Spatial distribution and migration of nonylphenol in groundwater following long-term wastewater irrigation. J. Contam. Hydrol. 2015, 177, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Ran, Y.; Chen, D.; Yang, Y.; Zeng, E.Y. Association of endocrine-disrupting chemicals with total organic carbon in riverine water and suspended particulate matter from the Pearl River, China. Environ. Toxicol. Chem. 2012, 31, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.; Andreu, V.; Picó, Y. Multi-residue determination of 47 organic compounds in water, soil, sediment and fish—Turia River as case study. J. Pharm. Biomed. Anal. 2017, 146, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Li, G.; Wang, Z.; Yan, C. Levels of estrogenic compounds in Xiamen Bay sediment, China. Mar. Pollut. Bull. 2009, 58, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Klosterhaus, S.L.; Grace, R.; Hamilton, M.C.; Yee, D. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ. Int. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Andreu, V.; Ferrer, E.; Rubio, J.L.; Font, G.; Picó, Y. Quantitative determination of octylphenol, nonylphenol, alkylphenol ethoxylates and alcohol ethoxylates by pressurized liquid extraction and liquid chromatography–mass spectrometry in soils treated with sewage sludges. Sci. Total Environ. 2007, 378, 124–129. [Google Scholar] [CrossRef]
- Grześkowiak, T.; Czarczyńska-Goślińska, B.; Zgoła-Grześkowiak, A. Current approaches in sample preparation for trace analysis of selected endocrine-disrupting compounds: Focus on polychlorinated biphenyls, alkylphenols, and parabens. Trac Trends Anal. Chem. 2016, 75, 209–226. [Google Scholar] [CrossRef]
- Oberdörster, E.; Cheek, A.O. Gender benders at the beach: Endocrine disruption in marine and estuarine organisms. Environ. Toxicol. Chem. Int. J. 2001, 20, 23–36. [Google Scholar] [CrossRef]
- Errico, S.; Nicolucci, C.; Migliaccio, M.; Micale, V.; Mita, D.G.; Diano, N. Analysis and occurrence of some phenol endocrine disruptors in two marine sites of the northern coast of Sicily (Italy). Mar. Pollut. Bull. 2017, 120, 68–74. [Google Scholar] [CrossRef]
- Ros, O.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Prieto, A. Determination of endocrine disrupting compounds in fish liver, brain, and muscle using focused ultrasound solid–liquid extraction and dispersive solid phase extraction as clean-up strategy. Anal. Bioanal. Chem. 2016, 408, 5689–5700. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, K.; Liu, J.; Fan, Y.; Tang, C.; Xiong, S. Body size–dependent bioaccumulation, tissue distribution, and trophic and maternal transfer of phenolic endocrine-disrupting contaminants in a freshwater ecosystem. Environ. Toxicol. Chem. 2018, 37, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Z.; Yao, L.; Wang, L.; Liu, W.-R.; Zhao, J.-L.; He, L.-Y.; Ying, G.-G. Bioaccumulation, metabolism, and risk assessment of phenolic endocrine disrupting chemicals in specific tissues of wild fish. Chemosphere 2019, 226, 607–615. [Google Scholar] [CrossRef]
- Wang, B.; Dong, F.; Chen, S.; Chen, M.; Bai, Y.; Tan, J.; Li, F.; Wang, Q. Phenolic endocrine disrupting chemicals in an urban receiving river (Panlong river) of Yunnan–Guizhou plateau: Occurrence, bioaccumulation and sources. Ecotoxicol. Environ. Saf. 2016, 128, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro-González, N.; Turnes-Carou, I.; Viñas, L.; Besada, V.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Occurrence of alkylphenols and bisphenol A in wild mussel samples from the Spanish Atlantic coast and Bay of Biscay. Mar. Pollut. Bull. 2016, 106, 360–365. [Google Scholar] [CrossRef] [PubMed]
- López-Roldán, R.; de Alda, M.L.; Gros, M.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Advanced monitoring of pharmaceuticals and estrogens in the Llobregat River basin (Spain) by liquid chromatography–triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography–time of flight-mass spectrometry. Chemosphere 2010, 80, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Brix, R.; Postigo, C.; González, S.; Villagrasa, M.; Navarro, A.; Kuster, M.; de Alda, M.J.L.; Barceló, D. Analysis and occurrence of alkylphenolic compounds and estrogens in a European river basin and an evaluation of their importance as priority pollutants. Anal. Bioanal. Chem. 2010, 396, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Pelayo, S.; López-Roldán, R.; González, S.; Casado, M.; Raldúa, D.; Cortina, J.L.; Piña, B. A zebrafish scale assay to monitor dioxin-like activity in surface water samples. Anal. Bioanal. Chem. 2011, 401, 1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Song, X.-F.; Kondoh, A.; Xia, J.; Tang, C.-Y. Behavior, mass inventories and modeling evaluation of xenobiotic endocrine-disrupting chemicals along an urban receiving wastewater river in Henan Province, China. Water Res. 2011, 45, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.; Chen, X.; Rao, K.M.; Lu, S.Y.; Ma, S.T.; Jiang, P.; Zheng, D.; Xu, S.Q.; Zheng, H.Y. Increased urinary 8-hydroxy-2′-deoxyguanosine levels in workers exposed to di-(2-ethylhexyl) phthalate in a waste plastic recycling site in China. Environ. Sci. Pollut. Res. 2011, 18, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.; Aquino, S.; Coutrim, M.; Silva, J.; Afonso, R. Determination of endocrine-disrupting compounds in waters from Rio das Velhas, Brazil, by liquid chromatography/high resolution mass spectrometry (ESI-LC-IT-TOF/MS). Environ. Technol. 2011, 32, 1409–1417. [Google Scholar] [CrossRef]
- Lou, L.; Cheng, G.; Yang, Q.; Xu, X.; Hu, B.; Chen, Y. Development of a novel solid-phase extraction element for the detection of nonylphenol in the surface water of Hangzhou. J. Environ. Monit. 2012, 14, 517–523. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Li, Q.; Li, G.; Guo, Q.; Yan, C. Estrogenic compounds and estrogenicity in surface water, sediments, and organisms from Yundang Lagoon in Xiamen, China. Arch. Environ. Contam. Toxicol. 2011, 61, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Moreno-Merino, L.; Matellanes, R.; Catalá, M.; Gorga, M.; Petrovic, M.; de Alda, M.L.; Barceló, D.; Silva, A.; Durán, J. Presence of endocrine disruptors in freshwater in the northern Antarctic Peninsula region. Environ. Res. 2016, 147, 179–192. [Google Scholar] [CrossRef]
- Wang, L.; Ying, G.-G.; Chen, F.; Zhang, L.-J.; Zhao, J.-L.; Lai, H.-J.; Chen, Z.-F.; Tao, R. Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools. Environ. Pollut. 2012, 165, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tu, Y.; Li, H.; Qiu, B.; Liu, Y.; Yang, Z. Endocrine-disrupting compounds in the Xiangjiang River of China: Spatio-temporal distribution, source apportionment, and risk assessment. Ecotoxicol. Environ. Saf. 2019, 167, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Xu, L.; Yang, Y.; Chen, D.-Y.; Ran, Y. Sequential ASE extraction of alkylphenols from sediments: Occurrence and environmental implications. J. Hazard. Mater. 2011, 192, 643–650. [Google Scholar] [CrossRef]
- Montagner, C.C.; Jardim, W.F. Spatial and seasonal variations of pharmaceuticals and endocrine disruptors in the Atibaia River, São Paulo State (Brazil). J. Braz. Chem. Soc. 2011, 22, 1452–1462. [Google Scholar] [CrossRef]
- Li, X.; Ying, G.-G.; Su, H.-C.; Yang, X.-B.; Wang, L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010, 36, 557–562. [Google Scholar] [CrossRef]
- Fan, J.-J.; Wang, S.; Tang, J.-P.; Zhao, J.-L.; Wang, L.; Wang, J.-X.; Liu, S.-L.; Li, F.; Long, S.-X.; Yang, Y. Bioaccumulation of endocrine disrupting compounds in fish with different feeding habits along the largest subtropical river, China. Environ. Pollut. 2019, 247, 999–1008. [Google Scholar] [CrossRef]
- Ros, O.; Izaguirre, J.K.; Olivares, M.; Bizarro, C.; Ortiz-Zarragoitia, M.; Cajaraville, M.P.; Etxebarria, N.; Prieto, A.; Vallejo, A. Determination of endocrine disrupting compounds and their metabolites in fish bile. Sci. Total Environ. 2015, 536, 261–267. [Google Scholar] [CrossRef]
- Chen, F.; Ying, G.-G.; Kong, L.-X.; Wang, L.; Zhao, J.-L.; Zhou, L.-J.; Zhang, L.-J. Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ. Pollut. 2011, 159, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci. Total Environ. 2017, 584, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, S.; Xu, H.; Zhang, Q.; Zhang, S.; Shi, L.; Yao, C.; Liu, Y.; Cheng, J. Distribution and bioaccumulation of endocrine disrupting chemicals in water, sediment and fishes in a shallow Chinese freshwater lake: Implications for ecological and human health risks. Ecotoxicol. Environ. Saf. 2017, 140, 222–229. [Google Scholar]
- Yang, J.; Li, H.; Ran, Y.; Chan, K. Distribution and bioconcentration of endocrine disrupting chemicals in surface water and fish bile of the Pearl River Delta, South China. Chemosphere 2014, 107, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Miege, C.; Peretti, A.; Labadie, P.; Budzinski, H.; Le Bizec, B.; Vorkamp, K.; Tronczyński, J.; Persat, H.; Coquery, M.; Babut, M. Occurrence of priority and emerging organic compounds in fishes from the Rhone River (France). Anal. Bioanal. Chem. 2012, 404, 2721–2735. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.; Bakhtiari, A.R.; Sari, A.E.; Bahramifar, N.; Rahbarizadeh, F. Occurrence of endocrine disruption chemicals (bisphenol a, 4-nonylphenol, and octylphenol) in muscle and liver of, Cyprinus carpino common, from Anzali Wetland, Iran. Bull. Environ. Contam. Toxicol. 2013, 90, 578–584. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Huang, B.; Lin, C.; Wang, Y.; Pan, X. Distribution and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in wild fish species from Dianchi Lake, China. Environ. Pollut. 2011, 159, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, J.; Gong, Z.; Zhang, N.; Duan, H. Occurrence, spatial distribution, historical trend and ecological risk of phthalate esters in the Jiulong River, Southeast China. Sci. Total Environ. 2017, 580, 388–397. [Google Scholar] [CrossRef]
- Sun, J.; Huang, J.; Zhang, A.; Liu, W.; Cheng, W. Occurrence of phthalate esters in sediments in Qiantang River, China and inference with urbanization and river flow regime. J. Hazard. Mater. 2013, 248, 142–149. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Wang, X.; Zhou, J.; Fan, B.; Li, W.; Liu, Z. Exposure and ecological risk of phthalate esters in the Taihu Lake basin, China. Ecotoxicol. Environ. Saf. 2019, 171, 564–570. [Google Scholar] [CrossRef]
- Zhang, W.; Han, Y.; Lu, L.; Zhao, T. An analysis of water environmental pollution in the Taige Canal watershed. China Rural Water Hydropower 2012, 9, 47–50. [Google Scholar]
- He, W.; Qin, N.; Kong, X.; Liu, W.; He, Q.; Ouyang, H.; Yang, C.; Jiang, Y.; Wang, Q.; Yang, B. Spatio-temporal distributions and the ecological and health risks of phthalate esters (PAEs) in the surface water of a large, shallow Chinese lake. Sci. Total Environ. 2013, 461, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Wu, W.; Mo, Z.; Wang, Z. Persistent organic pollutants in water and surface sediments of Taihu Lake, China and risk assessment. Chemosphere 2003, 50, 557–562. [Google Scholar] [CrossRef]
- Jiang, J.; Mu, D.; Ding, M.; Zhang, S.; Zhang, H.; Hu, J. Simultaneous determination of primary and secondary phthalate monoesters in the Taihu Lake: Exploration of sources. Chemosphere 2018, 202, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Li, S.; Zhang, J.; Di, H.; Li, F.; Feng, T. The human health assessment to phthalate acid esters (PAEs) and potential probability prediction by chromophoric dissolved organic matter EEM-FRI fluorescence in Erlong Lake. Int. J. Environ. Res. Public Health 2018, 15, 1109. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Khim, J.; Chung, S.; Seo, D.; Son, Y. Occurrence of micropollutants in four major rivers in Korea. Sci. Total Environ. 2014, 491, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, B.-T.; Teng, Y. Distribution of phthalate acid esters in lakes of Beijing and its relationship with anthropogenic activities. Sci. Total Environ. 2014, 476, 107–113. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; He, W.; Xu, F. The occurrence, composition and partitioning of phthalate esters (PAEs) in the water-suspended particulate matter (SPM) system of Lake Chaohu, China. Sci. Total Environ. 2019, 661, 285–293. [Google Scholar] [CrossRef]
- Bianucci, L.; Balaguru, K.; Smith, R.W.; Leung, L.R.; Moriarty, J.M. Contribution of hurricane-induced sediment resuspension to coastal oxygen dynamics. Sci. Rep. 2018, 8, 15740. [Google Scholar] [CrossRef]
- Tran, B.C.; Teil, M.-J.; Blanchard, M.; Alliot, F.; Chevreuil, M. Fate of phthalates and BPA in agricultural and non-agricultural soils of the Paris area (France). Environ. Sci. Pollut. Res. 2015, 22, 11118–11126. [Google Scholar] [CrossRef]
- Škrbić, B.D.; Ji, Y.; Đurišić-Mladenović, N.; Zhao, J. Occurence of the phthalate esters in soil and street dust samples from the Novi Sad city area, Serbia, and the influence on the children’s and adults’ exposure. J. Hazard. Mater. 2016, 312, 272–279. [Google Scholar] [CrossRef]
- Guo, D.; Wu, Y. Determination of phthalic acid esters of soil in south of Xinjiang cotton fields. Arid Environ. Monit. 2011, 25, 76–79. [Google Scholar]
- Wu, W.; Hu, J.; Wang, J.; Chen, X.; Yao, N.; Tao, J.; Zhou, Y.-K. Analysis of phthalate esters in soils near an electronics manufacturing facility and from a non-industrialized area by gas purge microsyringe extraction and gas chromatography. Sci. Total Environ. 2015, 508, 445–451. [Google Scholar] [CrossRef]
- Lü, H.; Mo, C.-H.; Zhao, H.-M.; Xiang, L.; Katsoyiannis, A.; Li, Y.-W.; Cai, Q.-Y.; Wong, M.-H. Soil contamination and sources of phthalates and its health risk in China: A review. Environ. Res. 2018, 164, 417–429. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, G.; Sun, L.; Zhou, Z.; Zhang, S.; Sui, H. Preliminary study on phthalic acid esters pollution of typical plastic mulched crops soils. Env. Monit China 2013, 29, 60–63. [Google Scholar]
- Niu, L.; Xu, Y.; Xu, C.; Yun, L.; Liu, W. Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ. Pollut. 2014, 195, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Yang, L.; Bu, Q.; Liu, R. Levels, distribution, and health risk of phthalate esters in urban soils of Beijing, China. J. Environ. Qual. 2011, 40, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.A.; Okedeyi, O.O.; Yusuf, K.A. Flame ionization gas chromatographic determination of phthalate esters in water, surface sediments and fish species in the Ogun river catchments, Ketu, Lagos, Nigeria. Environ. Monit. Assess. 2011, 172, 561–569. [Google Scholar] [CrossRef]
- Huang, P.-C.; Tien, C.-J.; Sun, Y.-M.; Hsieh, C.-Y.; Lee, C.-C. Occurrence of phthalates in sediment and biota: Relationship to aquatic factors and the biota-sediment accumulation factor. Chemosphere 2008, 73, 539–544. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wen, Z.; Ren, N. Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere 2014, 95, 24–32. [Google Scholar] [CrossRef]
- Paluselli, A.; Aminot, Y.; Galgani, F.; Net, S.; Sempere, R. Occurrence of phthalate acid esters (PAEs) in the northwestern Mediterranean Sea and the Rhone River. Prog. Oceanogr. 2018, 163, 221–231. [Google Scholar] [CrossRef]
- Mousa, A.; Basheer, C.; Al-Arfaj, A.R. Application of electro-enhanced solid-phase microextraction for determination of phthalate esters and bisphenol A in blood and seawater samples. Talanta 2013, 115, 308–313. [Google Scholar] [CrossRef]
- Liu, H.; Cui, K.; Zeng, F.; Chen, L.; Cheng, Y.; Li, H.; Li, S.; Zhou, X.; Zhu, F.; Ouyang, G. Occurrence and distribution of phthalate esters in riverine sediments from the Pearl River Delta region, South China. Mar. Pollut. Bull. 2014, 83, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Sharma, V.P.; Tripathi, R.; Kumar, R.; Patel, D.K.; Mathur, P.K. Occurrence of phthalic acid esters in Gomti River Sediment, India. Environ. Monit. Assess. 2010, 169, 397–406. [Google Scholar] [CrossRef]
- Tavares, R.S.; Martins, F.C.; Oliveira, P.J.; Ramalho-Santos, J.; Peixoto, F.P. Parabens in male infertility—Is there a mitochondrial connection? Reprod. Toxicol. 2009, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, S.; Suzuki, T.; Hitomi, T.; Yoshino, T.; Matsukuma, S.; Tsuji, T. Effects of methyl paraben on skin keratinocytes. J. Appl. Toxicol. Int. J. 2007, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Mortensen, G.K.; Andersson, A.-M.; Kongshoj, B.; Skakkebaek, N.E.; Wulf, H.C. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 2007, 41, 5564–5570. [Google Scholar] [CrossRef]
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect. 2011, 119, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Koeppe, E.S.; Ferguson, K.K.; Colacino, J.A.; Meeker, J.D. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci. Total Environ. 2013, 445, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tao, L.J.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC–MS/MS method for measuring environmental phenols and parabens in serum. Talanta 2008, 76, 865–871. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Vela-Soria, F.; Zafra-Gómez, A.; Navalón, A.; Ballesteros, O.; Navea, N.; Fernández, M.; Olea, N.; Vílchez, J. A new liquid chromatography–tandem mass spectrometry method for determination of parabens in human placental tissue samples. Talanta 2011, 84, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.B.; Feo, M.L.; Corcellas, C.; Gago-Ferrero, P.; Bertozzi, C.P.; Marigo, J.; Flach, L.; Meirelles, A.C.O.; Carvalho, V.L.; Azevedo, A.F. Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut. 2015, 207, 391–402. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Ballesteros, O.; Camino-Sánchez, F.; Zafra-Gómez, A.; Ballesteros, L.; Navalón, A. Matrix solid phase dispersion for the extraction of selected endocrine disrupting chemicals from human placental tissue prior to UHPLC-MS/MS analysis. Microchem. J. 2015, 118, 32–39. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Jiménez-Díaz, I.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Ballesteros, O.; Fernández, M.; Olea, N.; Navalón, A. A multiclass method for endocrine disrupting chemical residue analysis in human placental tissue samples by UHPLC–MS/MS. Anal. Methods 2011, 3, 2073–2081. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Jiménez-Díaz, I.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Vílchez, J.; Fernández, M.; Olea, N. Determination of benzophenones in human placental tissue samples by liquid chromatography–tandem mass spectrometry. Talanta 2011, 85, 1848–1855. [Google Scholar] [CrossRef]
- Dewalque, L.; Pirard, C.; Charlier, C. Measurement of urinary biomarkers of parabens, benzophenone-3, and phthalates in a Belgian population. Biomed Res. Int. 2014, 2014, 649314. [Google Scholar] [CrossRef]
- UNION, P. Regulation (EC) No 1223/2009 of the european parliament and of the council. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Sun, L.; Zhu, D.; Liu, Q.; Jiao, J.; Li, J.; Qi, M. The estrogenic effects of benzylparaben at low doses based on uterotrophic assay in immature SD rats. Food Chem. Toxicol. 2013, 53, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Molina-Molina, J.-M.; Iribarne-Durán, L.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Mustieles, V.; Arrebola, J.P.; Fernández, M.F.; Artacho-Cordón, F.; Olea, N. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ. Int. 2019, 127, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Qin, X.; Wu, Q.; Zhang, Y.; Ma, J.; Kannan, K. Benzophenone-type UV filters in urine and blood from children, adults, and pregnant women in China: Partitioning between blood and urine as well as maternal and fetal cord blood. Sci. Total Environ. 2013, 461, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Ryu, H.-Y.; Kim, H.-K.; Min, C.S.; Lee, J.H.; Kim, E.; Nam, B.H.; Park, J.H.; Jung, J.Y.; Jang, D.D. Maternal and fetal exposure to bisphenol A in Korea. Reprod. Toxicol. 2008, 25, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Pinney, S.E.; Mesaros, C.A.; Snyder, N.W.; Busch, C.M.; Xiao, R.; Aijaz, S.; Ijaz, N.; Blair, I.A.; Manson, J.M. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod. Toxicol. 2017, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 224–242. [Google Scholar] [CrossRef]
- Fisher, M.; MacPherson, S.; Braun, J.M.; Hauser, R.; Walker, M.; Feeley, M.; Mallick, R.; Bérubé, R.; Arbuckle, T.E. Paraben concentrations in maternal urine and breast milk and its association with personal care product use. Environ. Sci. Technol. 2017, 51, 4009–4017. [Google Scholar] [CrossRef]
- Fotouhi, M.; Seidi, S.; Shanehsaz, M.; Naseri, M.T. Magnetically assisted matrix solid phase dispersion for extraction of parabens from breast milks. J. Chromatogr. A 2017, 1504, 17–26. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, R.; Zafra-Gómez, A.; Camino-Sánchez, F.; Ballesteros, O.; Navalón, A. Gas chromatography and ultra high performance liquid chromatography tandem mass spectrometry methods for the determination of selected endocrine disrupting chemicals in human breast milk after stir-bar sorptive extraction. J. Chromatogr. A 2014, 1349, 69–79. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal. Chim. Acta 2008, 622, 150–156. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Park, J.; Moon, H.-B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.-J.; Eun, S.-H.; Kim, G.-H. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother–neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Sabaredzovic, A.; Papadopoulou, E.; Cequier, E.; Thomsen, C. Levels, variability and determinants of environmental phenols in pairs of Norwegian mothers and children. Environ. Int. 2018, 114, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, L.; Duskova, M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Henare, K.; Thorstensen, E.B.; Ponnampalam, A.P.; Mitchell, M.D. Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 2010, 202, 393.e1–393.e7. [Google Scholar] [CrossRef]
- Takahashi, O.; Oishi, S. Disposition of orally administered 2, 2-Bis (4-hydroxyphenyl) propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ. Health Perspect. 2000, 108, 931–935. [Google Scholar] [CrossRef]
- Wan, Y.; Choi, K.; Kim, S.; Ji, K.; Chang, H.; Wiseman, S.; Jones, P.D.; Khim, J.S.; Park, S.; Park, J. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: Placental transfer and potential risks. Environ. Sci. Technol. 2010, 44, 5233–5239. [Google Scholar] [CrossRef]
- Kosarac, I.; Kubwabo, C.; Lalonde, K.; Foster, W. A novel method for the quantitative determination of free and conjugated bisphenol A in human maternal and umbilical cord blood serum using a two-step solid phase extraction and gas chromatography/tandem mass spectrometry. J. Chromatogr. B 2012, 898, 90–94. [Google Scholar] [CrossRef]
- Minatoya, M.; Araki, A.; Nakajima, S.; Sasaki, S.; Miyashita, C.; Yamazaki, K.; Yamamoto, J.; Matumura, T.; Kishi, R. Cord blood BPA level and child neurodevelopment and behavioral problems: The Hokkaido Study on Environment and Children’s Health. Sci. Total Environ. 2017, 607, 351–356. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Twaddle, N.C.; Churchwell, M.I.; Doerge, D.R. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem. Toxicol. 2016, 92, 129–142. [Google Scholar] [CrossRef]
- Kuruto-Niwa, R.; Tateoka, Y.; Usuki, Y.; Nozawa, R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere 2007, 66, 1160–1164. [Google Scholar] [CrossRef]
- Braun, J.M.; Yolton, K.; Stacy, S.L.; Erar, B.; Papandonatos, G.D.; Bellinger, D.C.; Lanphear, B.P.; Chen, A. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 2017, 62, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Bourguignon, N.; Lux-Lantos, V.; Libertun, C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ. Health Perspect. 2010, 118, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.E.; Ferguson, K.K.; Mukherjee, B.; McElrath, T.F.; Meeker, J.D. Urinary bisphenol A levels during pregnancy and risk of preterm birth. Environ. Health Perspect. 2015, 123, 895–901. [Google Scholar] [CrossRef]
- Li, A.J.; Kannan, K. Elevated concentrations of bisphenols, benzophenones, and antimicrobials in pantyhose collected from six countries. Environ. Sci. Technol. 2018, 52, 10812–10819. [Google Scholar] [CrossRef]
- Molina-Molina, J.-M.; Amaya, E.; Grimaldi, M.; Sáenz, J.-M.; Real, M.; Fernandez, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- DeMatteo, R.; Keith, M.M.; Brophy, J.T.; Wordsworth, A.; Watterson, A.E.; Beck, M.; Rochon, A.; Michael, F.; Jyoti, G.; Magali, P. Chemical exposures of women workers in the plastics industry with particular reference to breast cancer and reproductive hazards. New Solut. J. Environ. Occup. Health Policy 2013, 22, 427–448. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Multigner, L.; Beausoleil, C.; Rousselle, C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018, 475, 74–91. [Google Scholar] [CrossRef]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef]
- Bamai, Y.A.; Araki, A.; Nomura, T.; Kawai, T.; Tsuboi, T.; Kobayashi, S.; Miyashita, C.; Takeda, M.; Shimizu, H.; Kishi, R. Association of filaggrin gene mutations and childhood eczema and wheeze with phthalates and phosphorus flame retardants in house dust: The Hokkaido study on Environment and Children’s Health. Environ. Int. 2018, 121, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lin, Z.; Liao, C.; Zhang, J.; Liu, W.; Wang, X.; Cai, J.; Zou, Z.; Wang, H.; Norback, D. Urinary phthalate metabolites in relation to childhood asthmatic and allergic symptoms in Shanghai. Environ. Int. 2018, 121, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Ebrahimpour, K.; Parastar, S.; Shoshtari-Yeganeh, B.; Hashemi, M.; Mansourian, M.; Poursafa, P.; Fallah, Z.; Rafiei, N.; Kelishadi, R. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere 2018, 211, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, D.; Chi, Z.; Li, W.; Shan, Y. Study on the interaction between typical phthalic acid esters (PAEs) and human haemoglobin (hHb) by molecular docking. Environ. Toxicol. Pharmacol. 2017, 53, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Spanier, A.J.; Sathyanarayana, S.; Attina, T.M.; Blustein, J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics 2013, 132, e646–e655. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Huang, Z.; Chen, L.; Feng, C.; Li, B.; Li, T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS ONE 2014, 9, e92465. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Papadopoulos, V. Prenatal phthalate exposure: Epigenetic changes leading to lifelong impact on steroid formation. Andrology 2016, 4, 573–584. [Google Scholar] [CrossRef]
- Hannon, P.R.; Flaws, J.A. The effects of phthalates on the ovary. Front. Endocrinol. 2015, 6, 8. [Google Scholar] [CrossRef]
- Dodge, L.; Williams, P.; Williams, M.; Missmer, S.; Souter, I.; Calafat, A.; Hauser, R.; Team, E.S. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod. Toxicol. 2015, 58, 184–193. [Google Scholar] [CrossRef]
- Hauser, R.; Gaskins, A.J.; Souter, I.; Smith, K.W.; Dodge, L.E.; Ehrlich, S.; Meeker, J.D.; Calafat, A.M.; Williams, P.L.; Team, E.S. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the EARTH study. Environ. Health Perspect. 2016, 124, 831–839. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Coskun, S.; Al-Doush, I.; Abduljabbar, M.; Al-Rouqi, R.; Al-Rajudi, T.; Al-Hassan, S. Couples exposure to phthalates and its influence on in vitro fertilization outcomes. Chemosphere 2019, 226, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, S.; Baek, Y.-W.; Choi, K.; Lee, K.; Kim, S.; Do Yu, S.; Choi, K. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012–2014). Int. J. Hyg. Environ. Health 2017, 220, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.A.; Saravanabhavan, G.; Werry, K.; Khoury, C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int. J. Hyg. Environ. Health 2017, 220, 13–28. [Google Scholar] [CrossRef]
- Machtinger, R.; Gaskins, A.J.; Racowsky, C.; Mansur, A.; Adir, M.; Baccarelli, A.A.; Calafat, A.M.; Hauser, R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 2018, 111, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bu, Z.; Mmereki, D.; Wang, J.; Dong, C. Exposure to commonly-used phthalates and the associated health risks in indoor environment of urban China. Sci. Total Environ. 2019, 658, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Gruber, L.; Seckin, E.; Raab, U.; Zimmermann, S.; Kiranoglu, M.; Schlummer, M.; Schwegler, U.; Smolic, S.; Völkel, W. Phthalates and their metabolites in breast milk—results from the Bavarian Monitoring of Breast Milk (BAMBI). Environ. Int. 2011, 37, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Correia-Sá, L.; Kasper-Sonnenberg, M.; Pälmke, C.; Schütze, A.; Norberto, S.; Calhau, C.; Domingues, V.F.; Koch, H.M. Obesity or diet? Levels and determinants of phthalate body burden–A case study on Portuguese children. Int. J. Hyg. Environ. Health 2018, 221, 519–530. [Google Scholar] [CrossRef]
- Garí, M.; Koch, H.M.; Pälmke, C.; Jankowska, A.; Wesołowska, E.; Hanke, W.; Nowak, D.; Bose-O’Reilly, S.; Polańska, K. Determinants of phthalate exposure and risk assessment in children from Poland. Environ. Int. 2019, 127, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Attina, T.M.; Hauser, R.; Sathyanarayana, S.; Hunt, P.A.; Bourguignon, J.-P.; Myers, J.P.; DiGangi, J.; Zoeller, R.T.; Trasande, L. Exposure to endocrine-disrupting chemicals in the USA: A population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016, 4, 996–1003. [Google Scholar] [CrossRef]
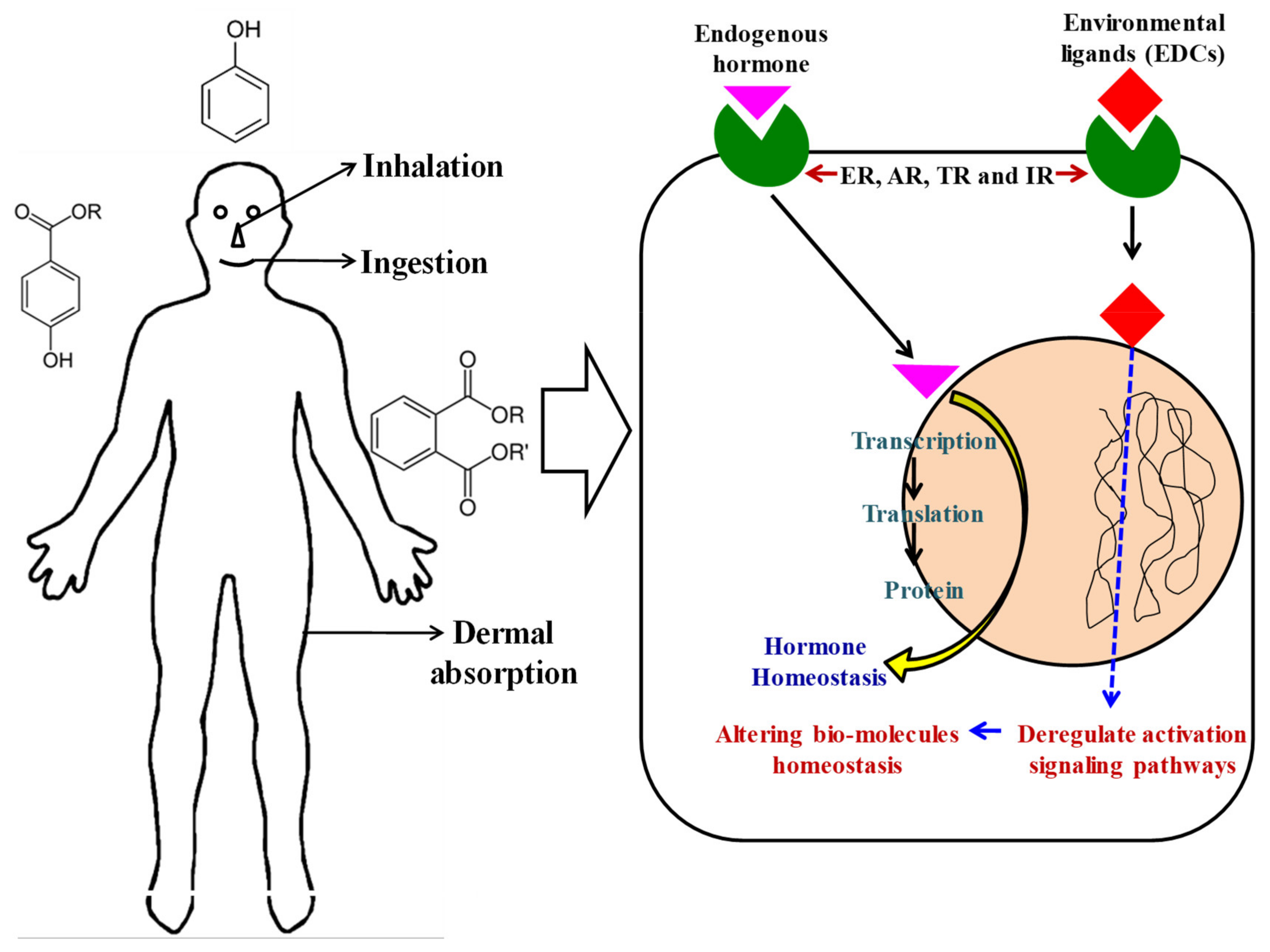


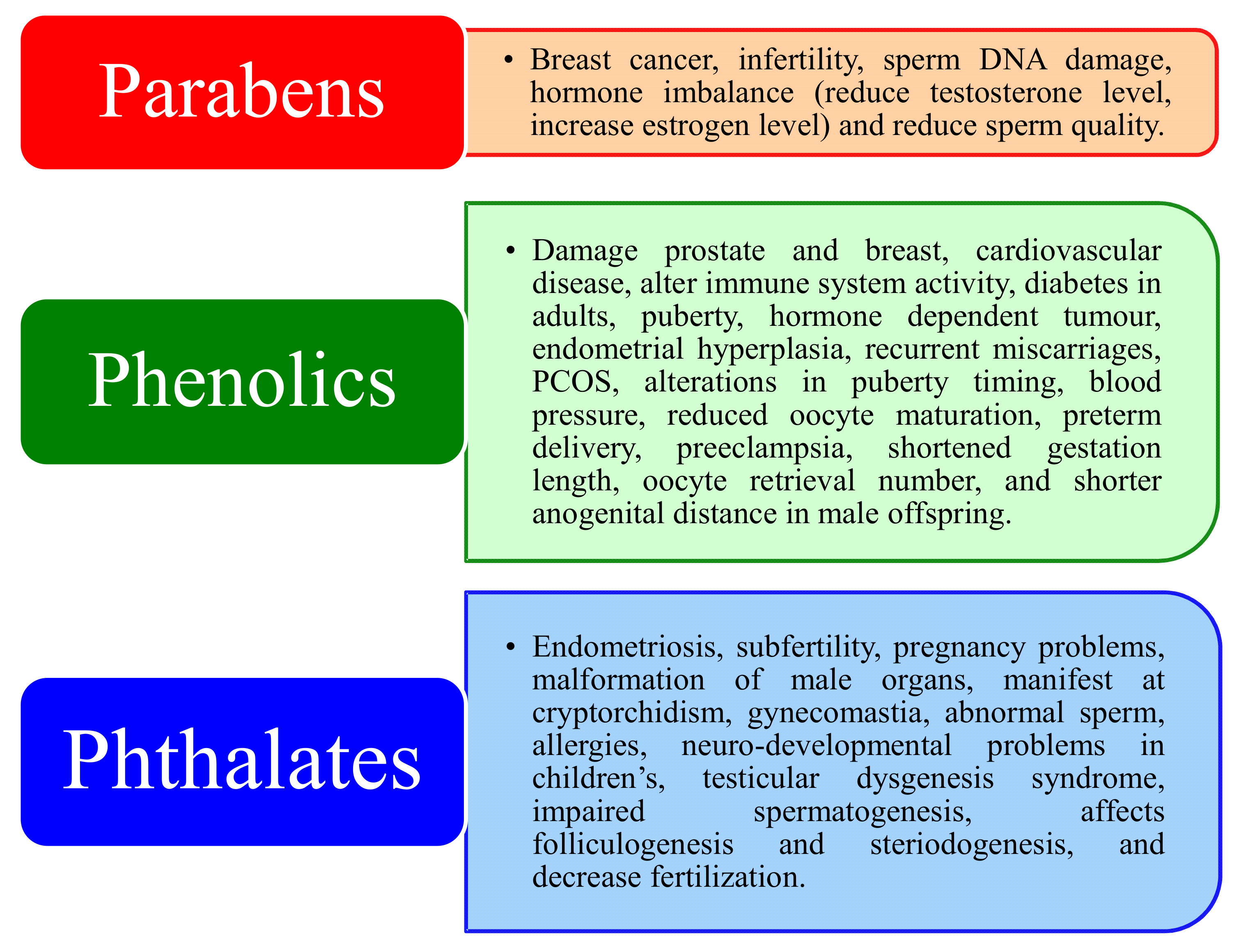
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangeetha, S.; Vimalkumar, K.; Loganathan, B.G. Environmental Contamination and Human Exposure to Select Endocrine-Disrupting Chemicals: A Review. Sustain. Chem. 2021, 2, 343-380. https://doi.org/10.3390/suschem2020020
Sangeetha S, Vimalkumar K, Loganathan BG. Environmental Contamination and Human Exposure to Select Endocrine-Disrupting Chemicals: A Review. Sustainable Chemistry. 2021; 2(2):343-380. https://doi.org/10.3390/suschem2020020
Chicago/Turabian StyleSangeetha, Seethappan, Krishnamoorthi Vimalkumar, and Bommanna G. Loganathan. 2021. "Environmental Contamination and Human Exposure to Select Endocrine-Disrupting Chemicals: A Review" Sustainable Chemistry 2, no. 2: 343-380. https://doi.org/10.3390/suschem2020020
APA StyleSangeetha, S., Vimalkumar, K., & Loganathan, B. G. (2021). Environmental Contamination and Human Exposure to Select Endocrine-Disrupting Chemicals: A Review. Sustainable Chemistry, 2(2), 343-380. https://doi.org/10.3390/suschem2020020







