Synthesis and Characterization of a New Organocatalytic Biosourced Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Data and Spectroscopic Measurement
2.2. Chromatography
2.3. Usual Procedures
2.4. Tensiometry
2.5. Dynamic Light Scaterring
3. Results and Discussion
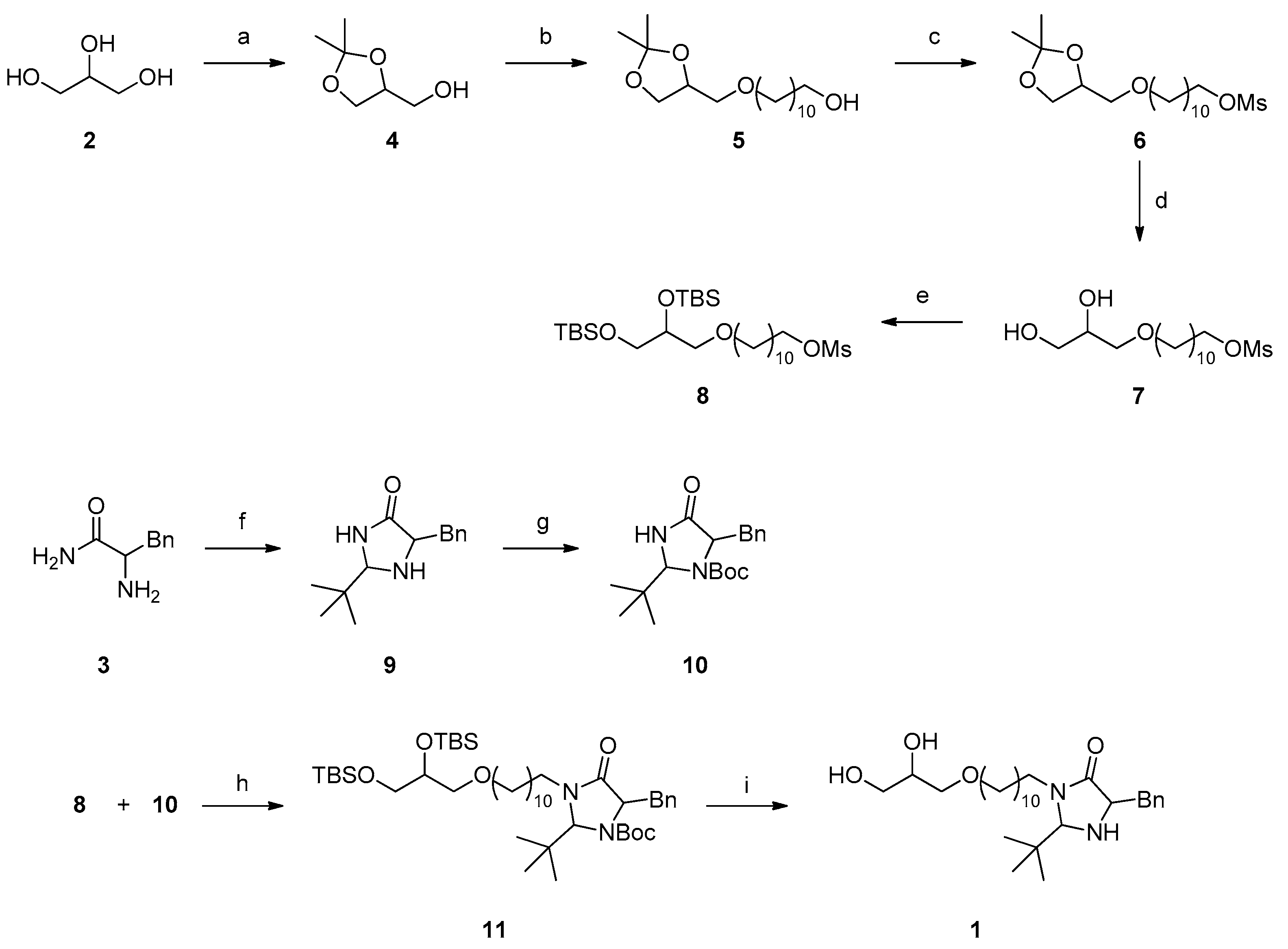
3.1. Synthesis of the Targeted Surfactant
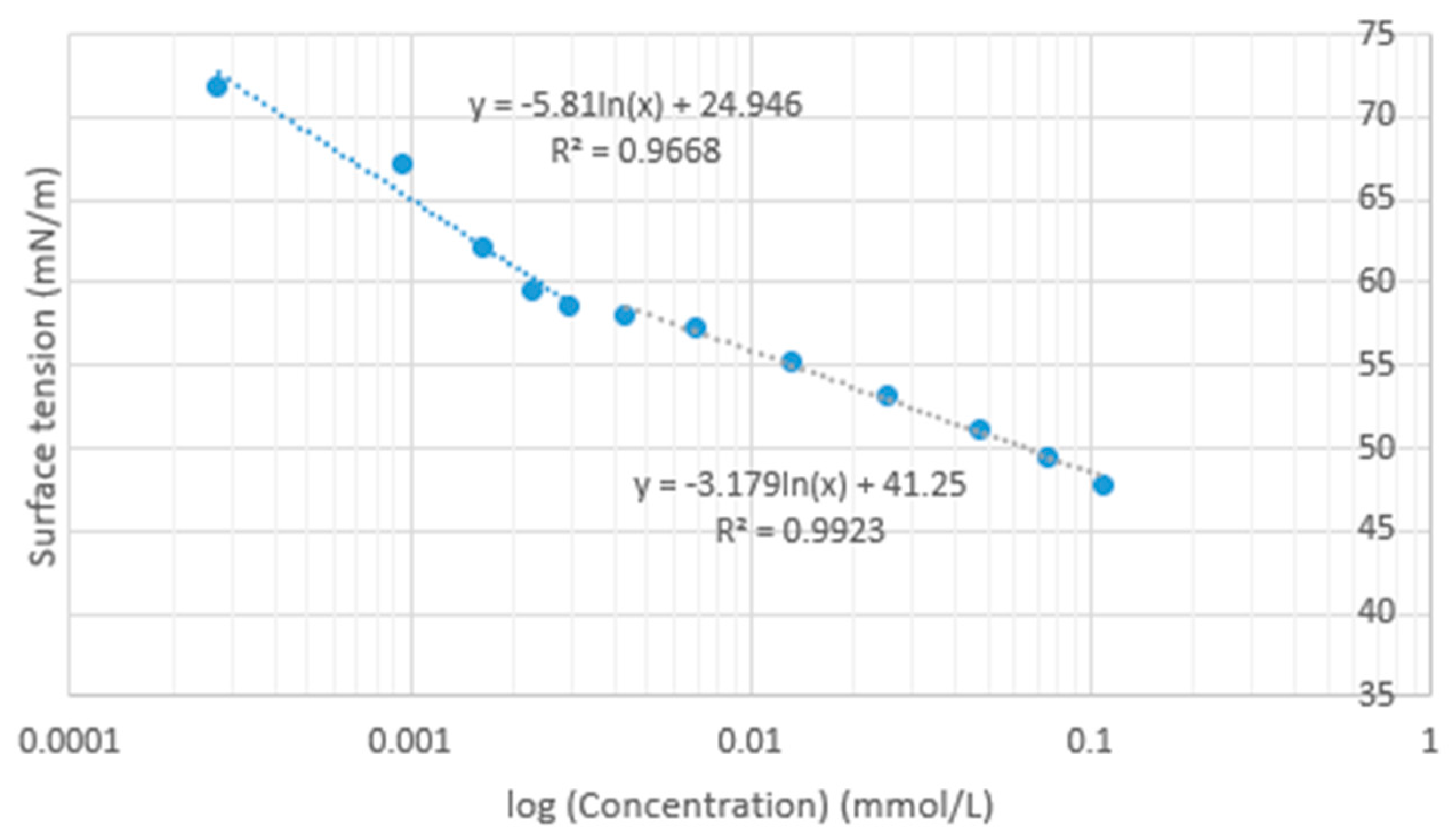
3.2. Effect on Surface Tension
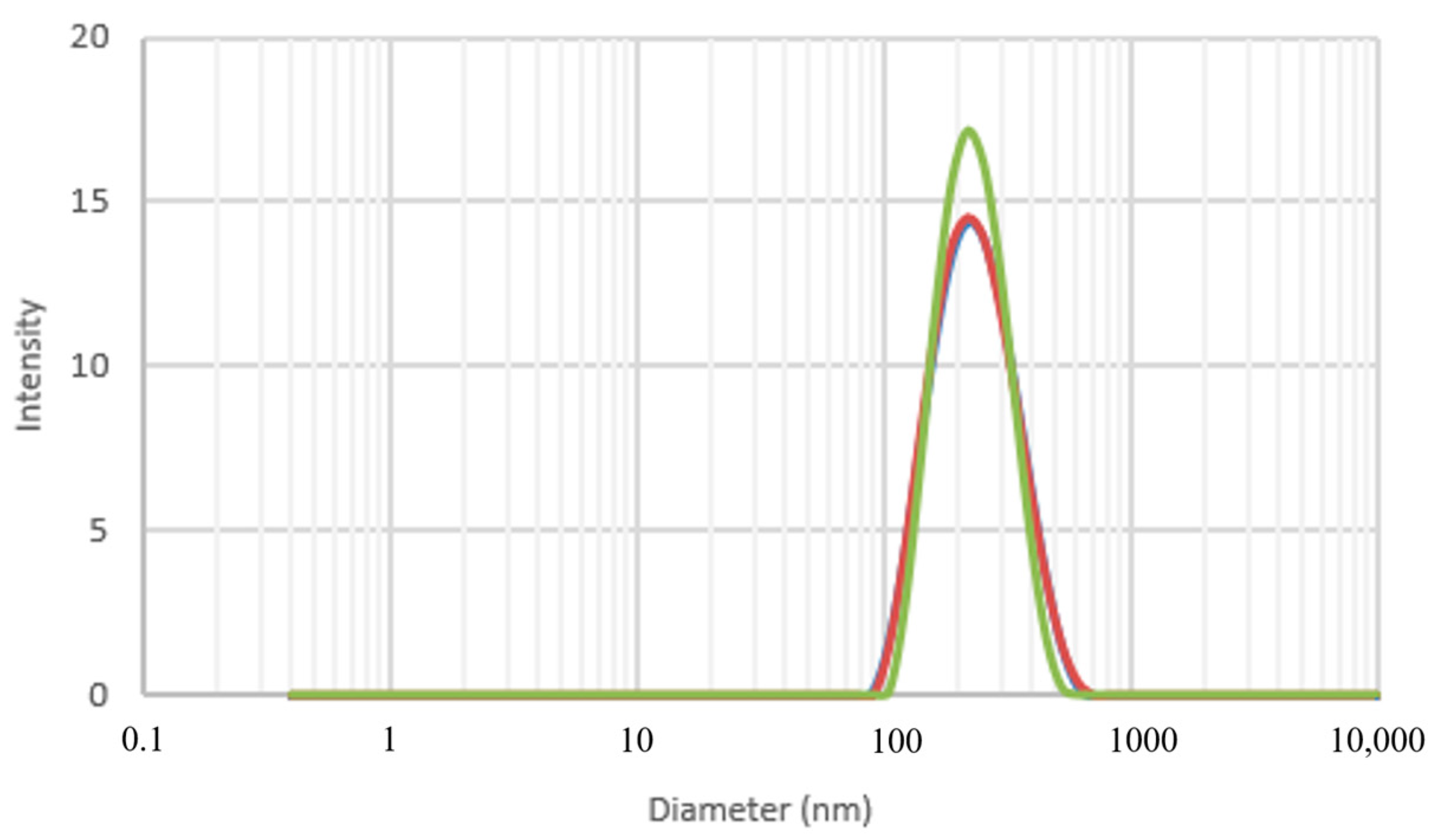
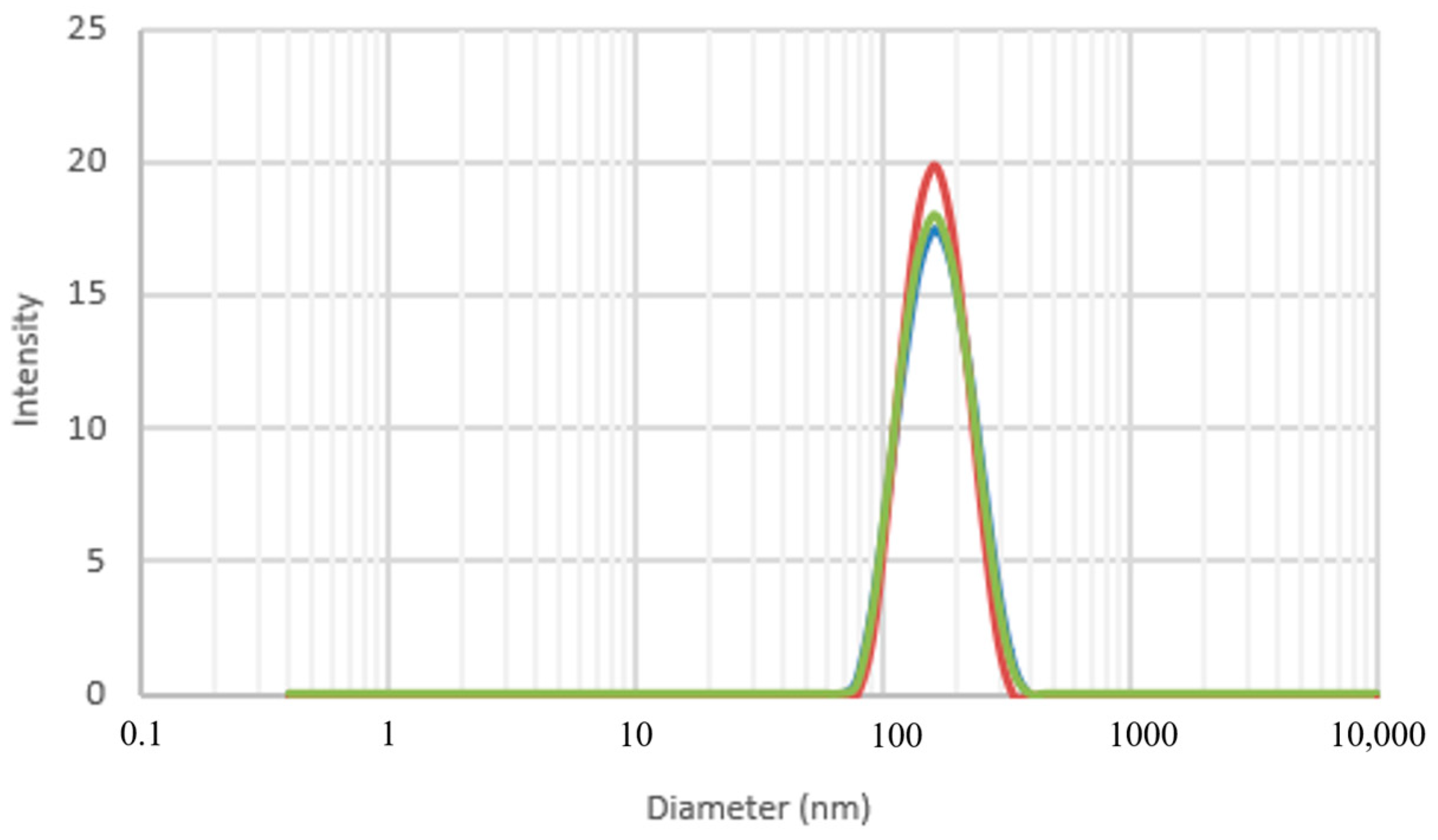
3.3. Dynamic Light Scattering (DLS)
3.4. DFT Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastas, A.P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Lebeuf, R.; Liu, C.-Y.; Pierlot, C.; Nardello-Rataj, V. Synthesis and Surfactant Properties of Nonionic Biosourced Alkylglucuronamides. ACS Sustain. Chem. Eng. 2018, 6, 2758–2766. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K. Chemistry: It’s not easy being green. Nature 2011, 469, 18–20. [Google Scholar] [CrossRef]
- Gupta, M.; Paul, S.; Gupta, R. General aspects of 12 basic principles of green chemistry with applications. Curr. Sci. 2010, 99, 1341–1360. [Google Scholar]
- Pace, V.; Hoyos, P.; Castoldi, L.; Dominguez De Maria, P.; Alcantara, A. 2-Methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Sheldon, R. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Clark, J.; Taverner, S. Alternative Solvents: Shades of Green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Lipshutz, B.; Gallou, F.; Handa, S. Evolution of Solvents in Organic Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef] [PubMed]
- Lindström, U. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef] [PubMed]
- Horak, J. Nový systém výstražných vět k označování rizikových vlastností chemických látek. Chem. Listy 2013, 107, 579–581. [Google Scholar]
- Filly, A.; Fabaino-Tixier, A.S.; Lousi, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C. R. Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- Lipshutz, B.; Aguinaldo, G.; Ghorai, S.; Voigtritter, K. Olefin Cross-Metathesis Reactions at Room Temperature Using the Nonionic Amphiphile “PTS”: Just Add Water. Org. Lett. 2008, 10, 1325–1328. [Google Scholar] [CrossRef]
- Lipshutz, B.; Ghorai, S.; Abela, A.; Moser, R.; Nishikata, T.; Duplais, C.; Krasovskiy, A. TPGS-750-M: A second-generation amphiphile for metal-catalyzed cross-couplings in water at room temperature. J. Org. Chem. 2011, 76, 4379–4391. [Google Scholar] [CrossRef]
- Klumphu, P.; Lipshutz, B. “Nok”: A Phytosterol-Based Amphiphile Enabling Transition-Metal-Catalyzed Couplings in Water at Room Temperature. J. Org. Chem. 2014, 79, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.; Ghorai, S. PQS-2: Ring-closing- and cross-metathesis reactions on lipophilic substrates; in water only at room temperature, with in-flask catalyst recycling. Tetrahedron 2010, 66, 1057–1063. [Google Scholar] [CrossRef]
- Lipshutz, B.; Taft, B. Heck Couplings at Room Temperature in Nanometer Aqueous Micelles. Org. Lett. 2008, 10, 1329–1332. [Google Scholar] [CrossRef]
- Lipshutz, B.; Ghorai, S.; Leong, G.; Benjamin, R. Manipulating Micellar Environments for Enhancing Transition Metal-Catalyzed Cross-Couplings in Water at Room Temperature. J. Org. Chem. 2011, 76, 5061–5073. [Google Scholar] [CrossRef]
- Isley, N.A.; Wang, Y.; Gallou, F.; Handa, S.; Aue, D.H.; Lipshutz, B. A Micellar Catalysis Strategy for Suzuki–Miyaura Cross-Couplings of 2-Pyridyl MIDA Boronates: No Copper, in Water, Very Mild Conditions. ACS Catal. 2017, 7, 8331–8337. [Google Scholar] [CrossRef]
- Lipshutz, B.; Petersen, T.; Abela, A. Room-Temperature Suzuki−Miyaura Couplings in Water Facilitated by Nonionic Amphiphiles. Org. Lett. 2008, 10, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.; Abela, A. Micellar Catalysis of Suzuki−Miyaura Cross-Couplings with Heteroaromatics in Water. Org. Lett. 2008, 10, 5329–5332. [Google Scholar] [CrossRef]
- Isley, N.; Gallou, F.; Lipshutz, B. Transforming Suzuki–Miyaura Cross-Couplings of MIDA Boronates into a Green Technology: No Organic Solvents. J. Am. Chem. Soc. 2013, 135, 17707–17710. [Google Scholar] [CrossRef]
- Lipshutz, B.; Chung, D.; Rich, B. Sonogashira Couplings of Aryl Bromides: Room Temperature, Water Only, No Copper. Org. Lett. 2008, 10, 3793–3796. [Google Scholar] [CrossRef]
- Lipshutz, B.; Ghorai, S. PQS: A New Platform for Micellar Catalysis. RCM Reactions in Water, with Catalyst Recycling. Org. Lett. 2009, 11, 705–708. [Google Scholar] [CrossRef]
- Graber, S.; Kingsbury, J.; Gray, B.; Hoveyda, A. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. [Google Scholar] [CrossRef]
- Oliveira, V.d.G.; Cardoso, M.F.d.C.; Forezi, L.d.S.M. Organocatalysis: A Brief Overview on Its Evolution and Applications. Catalysts 2018, 8, 605. [Google Scholar] [CrossRef]
- Ahrendt, K.; Borths, C.; MacMillan, D. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Lelais, G.; MacMillan, D. Modern Strategies in Organic Catalysis: The Advent and Development of Iminium Activation. Aldrichimica Acta 2006, 39, 79–87. [Google Scholar]
- Wilson, R.; Jen, W.; MacMillan, D. Enantioselective Organocatalytic Intramolecular Diels−Alder Reactions. The Asymmetric Synthesis of Solanapyrone D. J. Am. Chem. Soc. 2005, 127, 11616–11617. [Google Scholar] [CrossRef]
- Paras, N.; MacMillan, D. New Strategies in Organic Catalysis: The First Enantioselective Organocatalytic Friedel−Crafts Alkylation. J. Am. Chem. Soc. 2001, 123, 4370–4371. [Google Scholar] [CrossRef]
- Austin, J.; MacMillan, D. Enantioselective Organocatalytic Indole Alkylations. Design of a New and Highly Effective Chiral Amine for Iminium Catalysis. J. Am. Chem. Soc. 2002, 124, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Paras, N.; MacMillan, D. The Enantioselective Organocatalytic 1,4-Addition of Electron-Rich Benzenes to α,β-Unsaturated Aldehydes. J. Am. Chem. Soc. 2002, 124, 7894–7895. [Google Scholar] [CrossRef] [PubMed]
- Northrup, A.; Mangion, I.; Hettche, F.; Macmillan, D. Enantioselective Organocatalytic Direct Aldol Reactions of α-Oxyaldehydes: Step One in a Two-Step Synthesis of Carbohydrates. Angew. Chem. Int. Ed. 2004, 43, 2152–2154. [Google Scholar] [CrossRef]
- Mangion, I.; Northrup, A.; MacMillan, D. The Importance of Iminium Geometry Control in Enamine Catalysis: Identification of a New Catalyst Architecture for Aldehyde–Aldehyde Couplings. Angew. Chem. Int. Ed. 2004, 43, 6722–6724. [Google Scholar] [CrossRef] [PubMed]
- Hechavarria Fonseca, M.; List, B. Catalytic Asymmetric Intramolecular Michael Reaction of Aldehydes. Angew. Chem. Int. Ed. 2004, 30, 4048–4050. [Google Scholar] [CrossRef]
- Giry, C.; Bertrand, B.; Cecutti, C.; Brossard, C.; Moreau, E.; Thiebaud-Roux, S.; Vaca-Garcia, C.; Vedrenne, E. Green Optimization of the First Steps for the Synthesis of a Novel Surfactant: Towards the Elimination of CMR Solvents and the Drastic Reduction of the Used Solvent Volume. ChemistrySelect 2019, 4, 8621–8625. [Google Scholar] [CrossRef]
- Guerret, O.; Guilonneau, L.; Dufour, S. Soothing Pro-Pheromonal Composition for Mammals. Patent FR 3031104, 1 July 2016. [Google Scholar]
- Tsai, Y.-M.; Jiang, S.-L.; Chen, J.-H.; Chau, L.-Y.; Lin, J.-T. A Practical Formal Synthesis of a Physiologically Active Analogue of Platelet Activating Factor. J. Chin. Chem. Soc. 1988, 35, 429–435. [Google Scholar] [CrossRef]
- Piwowarska, N.; Banala, S.; Overkleeft, H.; Süssmuth, R. Arg-Thz is a minimal substrate for the Nα,Nα-arginyl methyltransferase involved in the biosynthesis of plantazolicin. Chem. Comm. 2013, 49, 10703–10705. [Google Scholar] [CrossRef][Green Version]
- Brenna, D.; Porta, R.; Massolo, E.; Raimondi, L.; Benaglia, M. A New Class of Low-Loading Catalysts for Highly Enantioselective, Metal-Free Imine Reduction of Wide General Applicability. ChemCatChem 2017, 9, 941–945. [Google Scholar] [CrossRef]
- Studer, A.; Hintermann, T.; Seebach, D. Synthesis and First Applications of a New Chiral Auxiliary (tert-butyl 2-(tert-butyl)-5,5-dimethyl-4-oxoimidazolidine-1-carboxylate). Helv. Chim. Acta 1995, 78, 1185–1206. [Google Scholar] [CrossRef]
- Ganesh, N.; Fujikawa, F.; Tan, Y.; Nigudkar, S.; Stine, K.; Demchenko, A. Surface-Tethered Iterative Carbohydrate Synthesis: A Spacer Study. J. Org. Chem. 2013, 78, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Seebach, A.; Dziadulewicz, E.; Behrendt, L.; Cantoreggi, S.; Fitzi, R. Synthesis of Nonproteinogenic (R)- or (S)-Amino Acids Analogues of Phenylalanine, Isotopically Labelled and Cyclic Amino Acids from tert-Butyl 2-(tert-Butyl)-3-methyl-4-oxo-1-imidazolidinecarboxylate (Boc-BMI). Liebigs Ann. Chem. 1989, 12, 1215–1232. [Google Scholar] [CrossRef]
- Bezerra, K.; Gomes, U.; Silva, R.; Sarubbo, L.; Ribeiro, E. The potential application of biosurfactant produced by Pseudomonas aeruginosa TGC01 using crude glycerol on the enzymatic hydrolysis of lignocellulosic material. Biodegradation 2019, 30, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Berchel, M.; Lemiègre, L.; Trépout, S.; Lambert, O.; Jeftic, J.; Benvegnu, T. Synthesis of unsymmetrical saturated or diacetylenic cationic bolaamphiphiles. Tetrahedron Lett. 2008, 79, 7419–7422. [Google Scholar] [CrossRef]
- Obounou Akong, F.; Bouquillon, S. Efficient syntheses of bolaform surfactants from l-rhamnose and/or 3-(4-hydroxyphenyl)propionic acid. Green Chem. 2015, 17, 3290–3300. [Google Scholar] [CrossRef]
- Chorfa, N.; Belkacemi, K.; Arul, J.; Hamoudi, S. Acylation of unprotected lactose with 1,18-octadec-9-enedioyl chloride for the synthesis of monocatenary and bolaform agro-based surfactants. Can. J. Chem. Eng. 2018, 96, 2253–2262. Available online: https://www.cheric.org/research/tech/periodicals/doi.php?art_seq=1663868 (accessed on 11 November 2020). [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988, B 37, 785–789. [Google Scholar] [CrossRef]
- Marenich, A.; Cramer, C.; Truhlar, D. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giry, C.; Bertrand, D.; Pierret, A.; Vedrenne, E.; Lacaze-Dufaure, C.; Fabre, J.-F.; Thiebaud-Roux, S.; Vaca Garcia, C.; Cecutti, C. Synthesis and Characterization of a New Organocatalytic Biosourced Surfactant. Sustain. Chem. 2021, 2, 335-342. https://doi.org/10.3390/suschem2020019
Giry C, Bertrand D, Pierret A, Vedrenne E, Lacaze-Dufaure C, Fabre J-F, Thiebaud-Roux S, Vaca Garcia C, Cecutti C. Synthesis and Characterization of a New Organocatalytic Biosourced Surfactant. Sustainable Chemistry. 2021; 2(2):335-342. https://doi.org/10.3390/suschem2020019
Chicago/Turabian StyleGiry, Clément, David Bertrand, Alexandre Pierret, Emeline Vedrenne, Corinne Lacaze-Dufaure, Jean-François Fabre, Sophie Thiebaud-Roux, Carlos Vaca Garcia, and Christine Cecutti. 2021. "Synthesis and Characterization of a New Organocatalytic Biosourced Surfactant" Sustainable Chemistry 2, no. 2: 335-342. https://doi.org/10.3390/suschem2020019
APA StyleGiry, C., Bertrand, D., Pierret, A., Vedrenne, E., Lacaze-Dufaure, C., Fabre, J.-F., Thiebaud-Roux, S., Vaca Garcia, C., & Cecutti, C. (2021). Synthesis and Characterization of a New Organocatalytic Biosourced Surfactant. Sustainable Chemistry, 2(2), 335-342. https://doi.org/10.3390/suschem2020019





