A Preliminary Laboratory Evaluation of Artificial Aggregates from Alkali-Activated Basalt Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursors
2.1.1. Basalt
2.1.2. Metakaolin
2.2. Activators
2.3. Research Plan
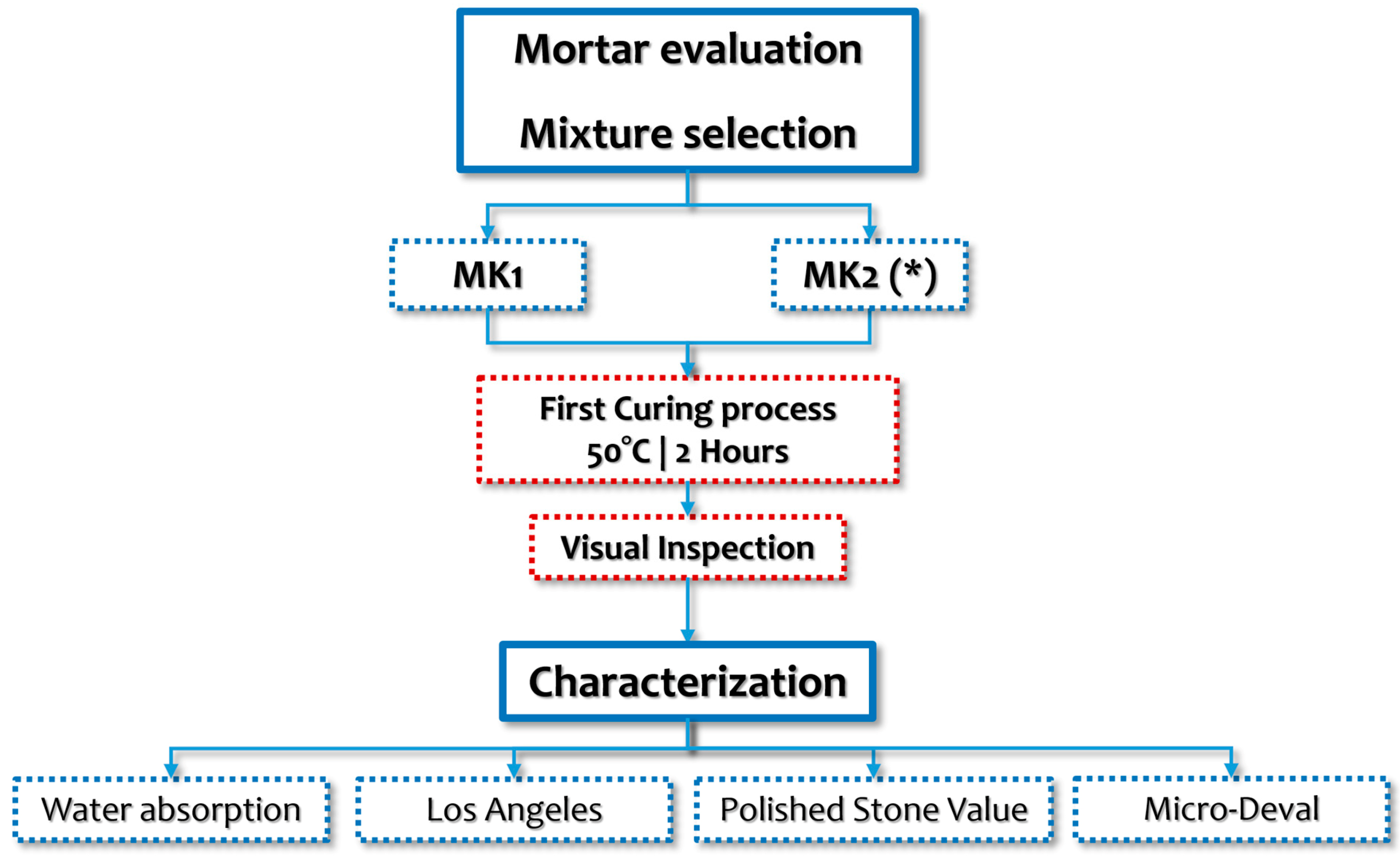
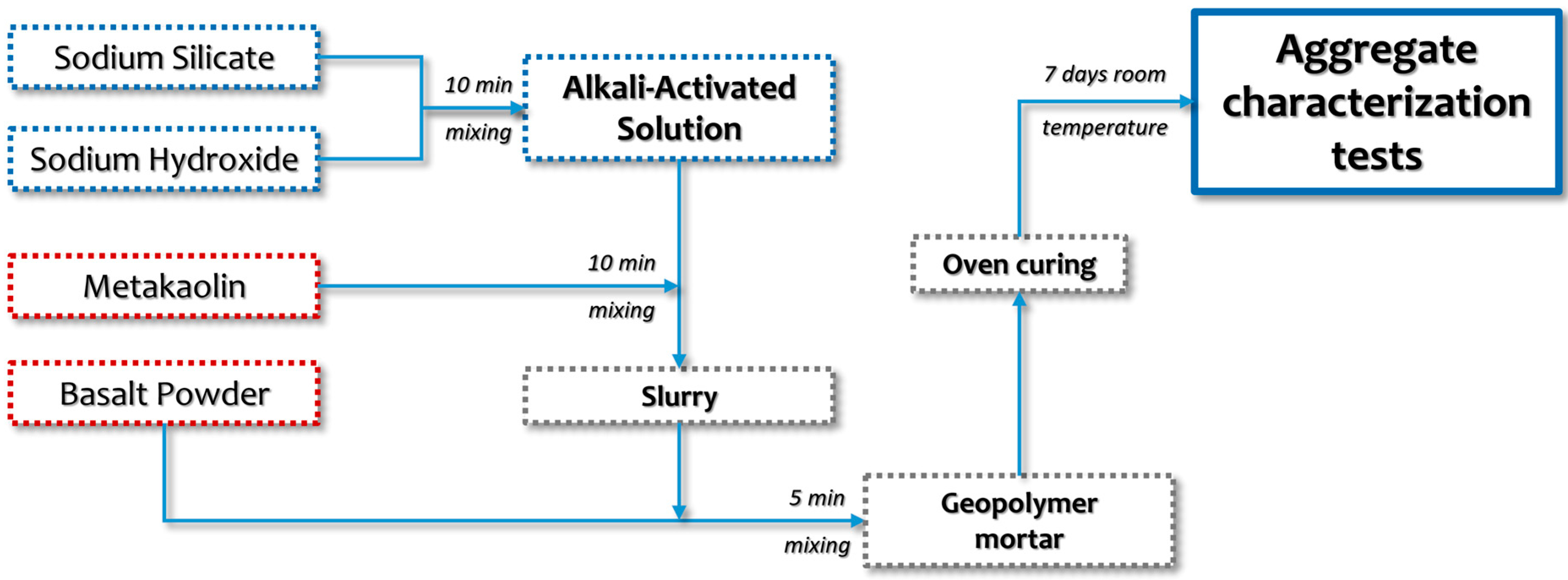
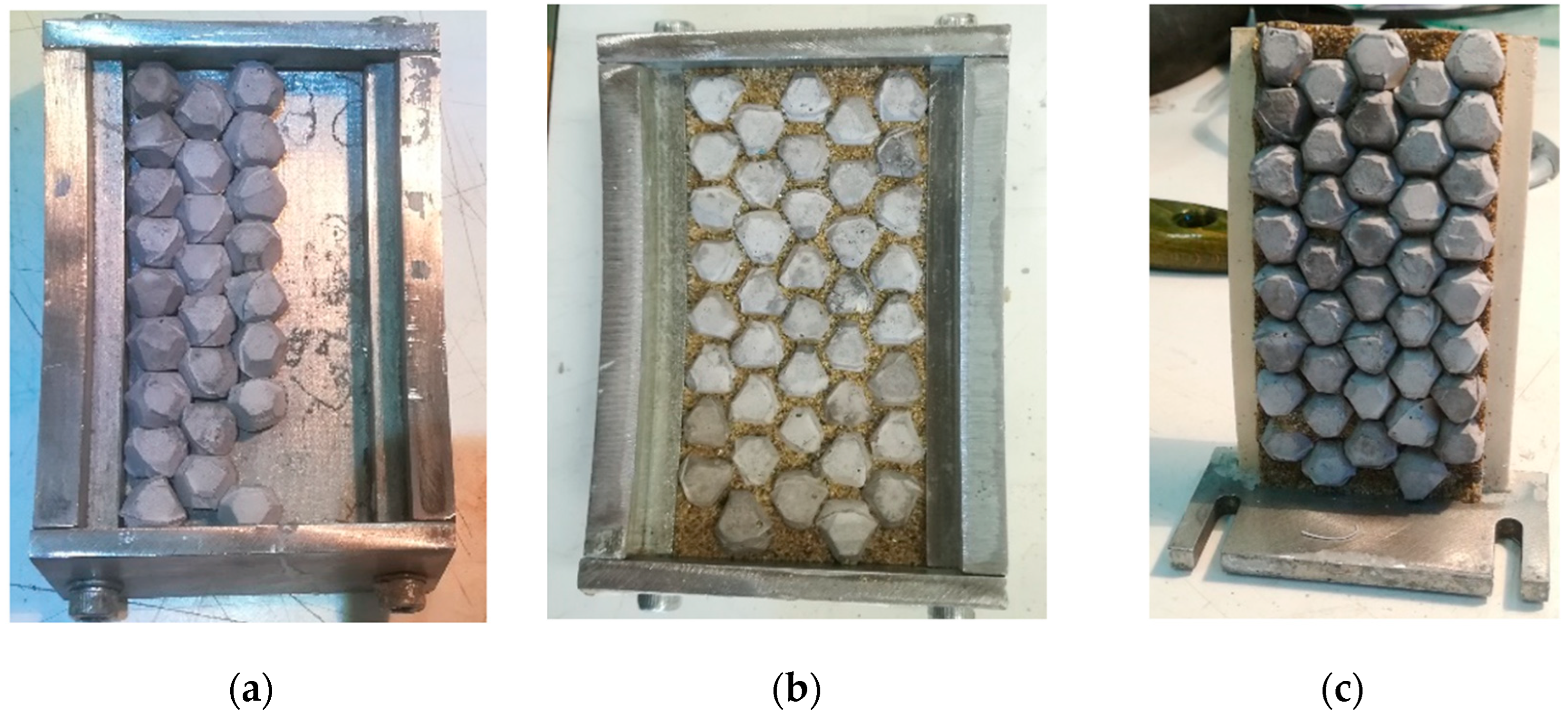
2.4. Artificial Aggregate Production
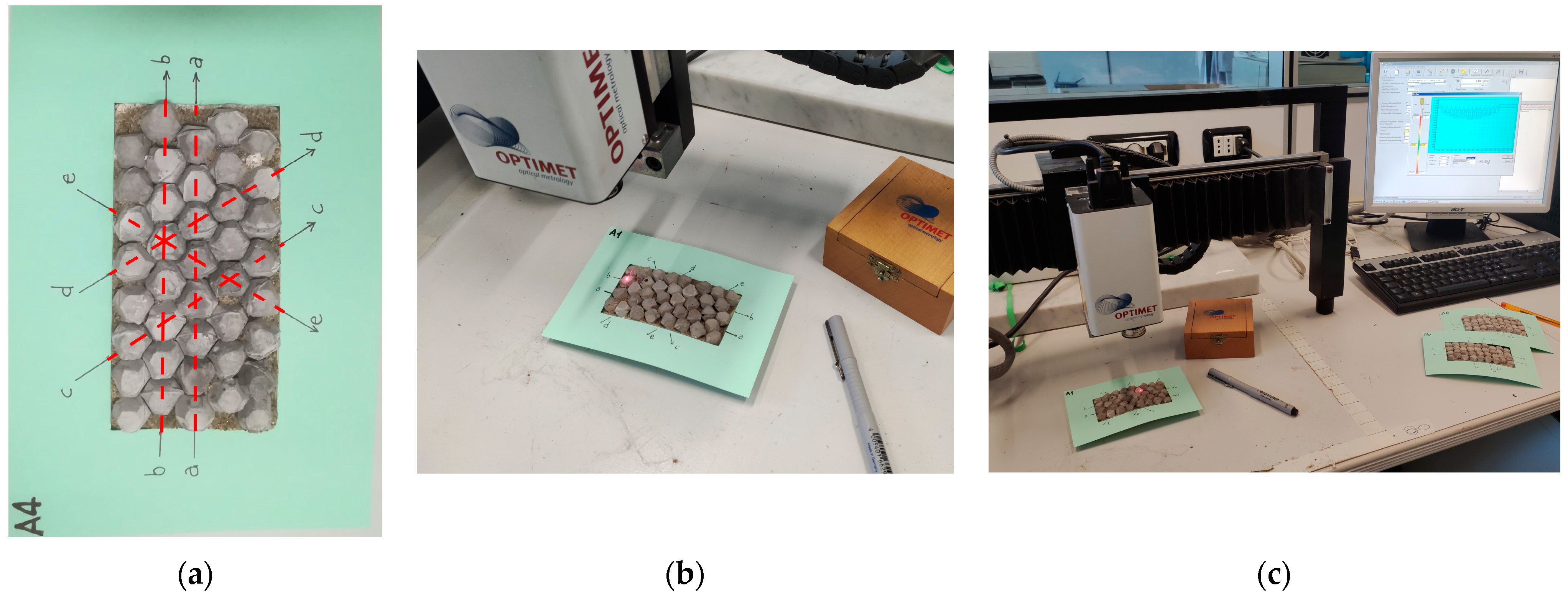
2.5. Artificial Aggregate Evaluation Methods
- S is the mean value for the aggregate test specimens (Aggregate B-MK1);
- X is the mean PSV for the source of the control stone (in this case, 49);
- C is the mean value for the control stone specimens (basalt).
- Ra: arithmetical mean deviation roughness of the profile, which represents an average of the profile deviations from a center line;
- Rq: root mean squared roughness;
- Rz: average peak-to-valley height, which is based on the five highest peaks and the lowest valleys over the entire length of the evaluation segment [47]
| Roughness Indicator | Ra | Rq | Rz |
|---|---|---|---|
| Formula | |||
| Graphical Explanation | 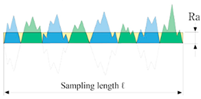 | 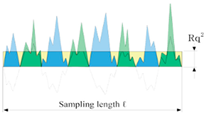 |  |
3. Results
3.1. Alkali-Activated Materials’ Chemical Characterization
3.2. Artificial Aggregate Characterization
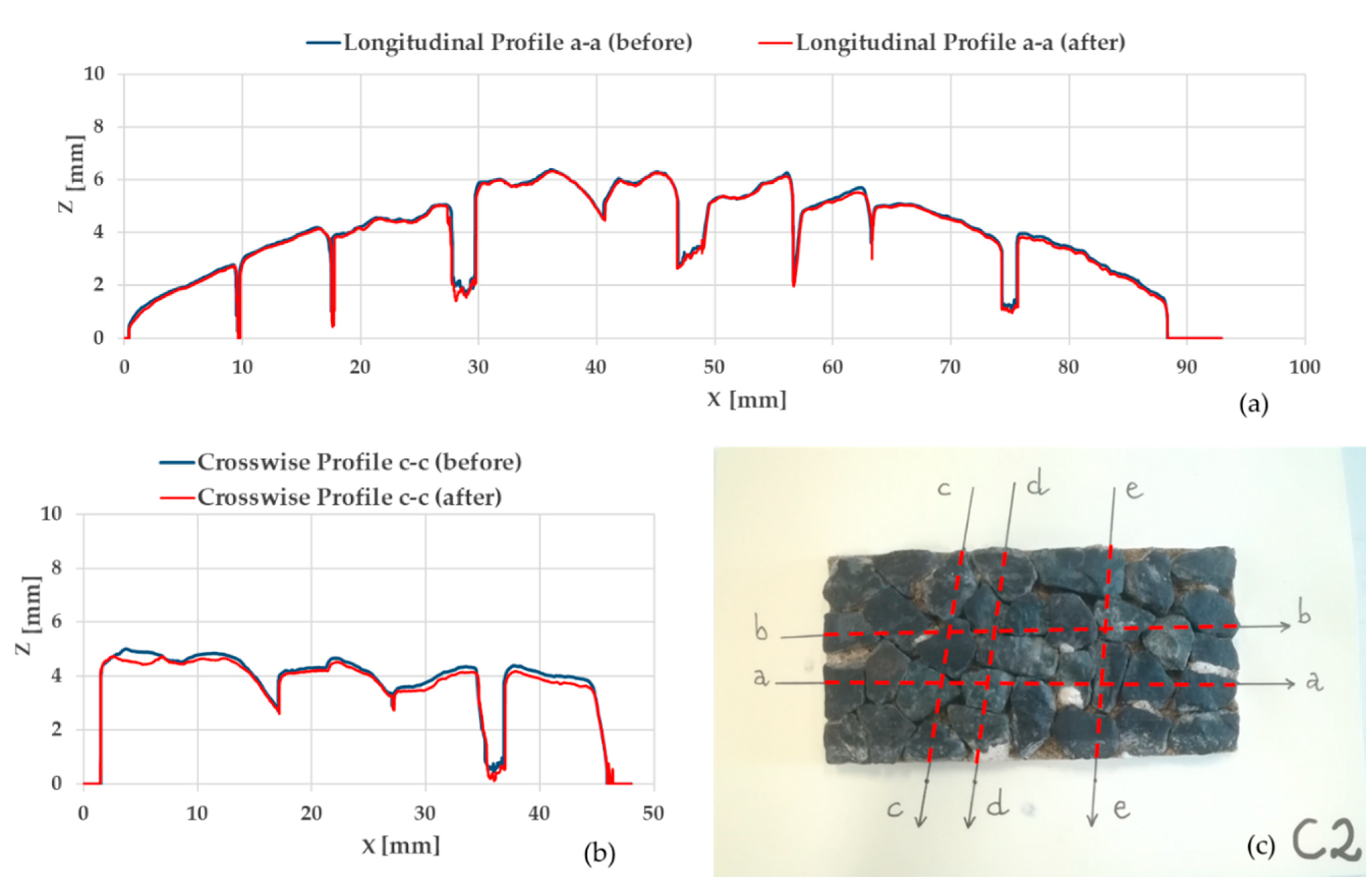
3.3. Artificial Aggregate Micro-Texture Analysis
4. Conclusions
- It was noted that the selected metakaolin played a very important role, as the MK2 one had better geopolymerization results.
- The basalt powder proved to be a suitable material.
- The AEA with MK2 had a higher resistance to wear than the one with MK1.
- The post-curing heat treatment improves the material characteristics.
- The PSV and BPN results are quite promising, showing that it might be used in replacement of natural aggregate or other artificial aggregates.
- The process must be improved in order to increase the overall quality of the AEA, especially in terms of fragmentation results.
Author Contributions
Funding
Conflicts of Interest
References
- Langer, W. 9—Sustainability of aggregates in construction. In Sustainability of Construction Materials, 2nd ed.; Khatib, J.M., Ed.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2016; pp. 181–207. ISBN 978-0-08-100995-6. [Google Scholar]
- The Freedonia Group. Global Demand for Construction Aggregates to Exceed 48 Billion Metric Tons in 2015. Available online: https://www.freedoniagroup.com/World-Construction-Aggregates.html (accessed on 1 May 2021).
- Akhtar, A.; Sarmah, A.K. Construction and demolition waste generation and properties of recycled aggregate concrete: A global perspective. J. Clean. Prod. 2018, 186, 262–281. [Google Scholar] [CrossRef]
- Zabalza Bribián, I.; Valero Capilla, A.; Aranda Usón, A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Chen, W.; Jin, R.; Xu, Y.; Wanatowski, D.; Li, B.; Yan, L.; Pan, Z.; Yang, Y. Adopting recycled aggregates as sustainable construction materials: A review of the scientific literature. Constr. Build. Mater. 2019, 218, 483–496. [Google Scholar] [CrossRef]
- EN 13242:2015; Aggregates for Unbound and Hydraulically Bound Materials for Use in Civil Engineering Work and Road Construction. European Committee for Standardization (CEN): Brussels, Belgium, 2015.
- Bai, G.; Zhu, C.; Liu, C.; Liu, B. An evaluation of the recycled aggregate characteristics and the recycled aggregate concrete mechanical properties. Constr. Build. Mater. 2020, 240, 117978. [Google Scholar] [CrossRef]
- Aslam, M.S.; Huang, B.; Cui, L. Review of construction and demolition waste management in China and USA. J. Environ. Manag. 2020, 264, 110445. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Bhattacharyya, S.K.; Minocha, A.K.; Deoliya, R.; Maiti, S. Recycled aggregate from C&D waste & its use in concrete—A breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar]
- Ren, P.; Ling, T.-C.; Mo, K.H. Recent advances in artificial aggregate production. J. Clean. Prod. 2021, 291, 125215. [Google Scholar] [CrossRef]
- Colangelo, F.; Messina, F.; Cioffi, R. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. J. Hazard. Mater. 2015, 299, 181–191. [Google Scholar] [CrossRef]
- Tajra, F.; Abd Elrahman, M.; Lehmann, C.; Stephan, D. Properties of lightweight concrete made with core-shell structured lightweight aggregate. Constr. Build. Mater. 2019, 205, 39–51. [Google Scholar] [CrossRef]
- Tajra, F.; Elrahman, M.A.; Stephan, D. The production and properties of cold-bonded aggregate and its applications in concrete: A review. Constr. Build. Mater. 2019, 225, 29–43. [Google Scholar] [CrossRef]
- Balapour, M.; Zhao, W.; Garboczi, E.J.; Oo, N.Y.; Spatari, S.; Hsuan, Y.G.; Billen, P.; Farnam, Y. Potential use of lightweight aggregate (LWA) produced from bottom coal ash for internal curing of concrete systems. Cem. Concr. Compos. 2020, 105, 103428. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Dinkha, Y.Z.; Haido, J.H.; Ali, M.H.; Tayeh, B.A. Engineering properties of sustainable green concrete incorporating eco-friendly aggregate of crumb rubber: A review. J. Clean. Prod. 2021, 324, 129251. [Google Scholar] [CrossRef]
- Saikia, N.; de Brito, J. Use of plastic waste as aggregate in cement mortar and concrete preparation: A review. Constr. Build. Mater. 2012, 34, 385–401. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An overview on the reuse of waste glasses in alkali-activated materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Vlček, J. Effects of Kaolin Additives in Fly Ash on Sintering and Properties of Mullite Ceramics. Minerals 2021, 11, 887. [Google Scholar] [CrossRef]
- Yue, M.; Yue, Q.Y.; Qi, Y.F. Effect of Sintering Temperature on the Properties of Sludge Ceramics and Fly-Ash Ceramics. In Advanced Materials Research; Trans Tech Publications Ltd: Bäch, Switzerland, 2011; Volume 150, pp. 1068–1072. [Google Scholar]
- Tang, P.; Xuan, D.; Poon, C.S.; Tsang, D.C.W. Valorization of concrete slurry waste (CSW) and fine incineration bottom ash (IBA) into cold bonded lightweight aggregates (CBLAs): Feasibility and influence of binder types. J. Hazard. Mater. 2019, 368, 689–697. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Morone, M.; Costa, G.; Georgakopoulos, E.; Manovic, V.; Stendardo, S.; Baciocchi, R. Granulation–Carbonation Treatment of Alkali Activated Steel Slag for Secondary Aggregates Production. Waste Biomass Valorization 2017, 8, 1381–1391. [Google Scholar] [CrossRef]
- Bui, L.A.; Hwang, C.; Chen, C.; Lin, K.; Hsieh, M. Manufacture and performance of cold bonded lightweight aggregate using alkaline activators for high performance concrete. Constr. Build. Mater. 2012, 35, 1056–1062. [Google Scholar] [CrossRef]
- Gopalakrishna, B.; Dinakar, P. Mix design development of fly ash-GGBS based recycled aggregate geopolymer concrete. J. Build. Eng. 2022, 63, 105551. [Google Scholar] [CrossRef]
- Saloni; Parveen; Lim, Y.Y.; Pham, T.M. Effective utilisation of ultrafine slag to improve mechanical and durability properties of recycled aggregates geopolymer concrete. Clean. Eng. Technol. 2021, 5, 100330. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, Y.; Wang, C.; Li, Y. Behavior of square geopolymer recycled brick aggregate concrete filled steel tubular stub columns under axial compression. Constr. Build. Mater. 2022, 363, 129823. [Google Scholar] [CrossRef]
- Wongkvanklom, A.; Posi, P.; Kampala, A.; Kaewngao, T.; Chindaprasirt, P. Beneficial utilization of recycled asphaltic concrete aggregate in high calcium fly ash geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00615. [Google Scholar] [CrossRef]
- Fang, Y.; Ahmad, M.R.; Lao, J.C.; Qian, L.P.; Dai, J.D. Development of artificial geopolymer aggregates with thermal energy storage capacity. Cem. Concr. Compos. 2022, 135, 104834. [Google Scholar] [CrossRef]
- Zhou, X.; Kastiukas, G.; Lantieri, C.; Tataranni, P.; Vaiana, R.; Sangiorgi, C. Mechanical and thermal performance of macro-encapsulated phase change materials for pavement application. Materials 2018, 11, 1398. [Google Scholar] [CrossRef]
- Ismaiel Saraya, M.E.-S.; El-Fadaly, E. Preliminary Study of Alkali Activation of Basalt: Effect of NaOH Concentration on Geopolymerization of Basalt. J. Mater. Sci. Chem. Eng. 2017, 5, 58–76. [Google Scholar] [CrossRef]
- Callai, S.C.; Tataranni, P.; Sangiorgi, C. Preliminary evaluation of geopolymer mix design applying the design of experiments method. Infrastructures 2021, 6, 35. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Application, 4th ed.; Institut Geopolymere: Saint-Quentin, France, 2015. [Google Scholar]
- Liu, Z.; Cai, C.S.; Liu, F.; Fan, F. Feasibility Study of Loess Stabilization with Fly Ash–Based Geopolymer. J. Mater. Civ. Eng. 2016, 28, 04016003. [Google Scholar] [CrossRef]
- Moro, D.; Fabbri, R.; Romano, J.; Ulian, G.; Calafato, A.; Solouki, A.; Sangiorgi, C.; Valdrè, G. Thermal, X-ray Diffraction and Oedometric Analyses of Silt-Waste/NaOH-Activated Metakaolin Geopolymer Composite. J. Compos. Sci. 2021, 5, 269. [Google Scholar] [CrossRef]
- Solouki, A.; Viscomi, G.; Lamperti, R.; Tataranni, P. Quarry waste as precursors in geopolymers for civil engineering applications: A decade in review. Materials 2020, 13, 3146. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, Y.; Chen, T.; Bao, S.; Liu, T.; Huang, J. Geopolymerisation of a silica-rich tailing. Miner. Eng. 2011, 24, 1710–1712. [Google Scholar] [CrossRef]
- Rill, E.; Lowry, D.R.; Kriven, W.M. Properties of basalt fiber reinforced geopolymer composites. Ceram. Eng. Sci. Proc. 2010, 31, 57–67. [Google Scholar] [CrossRef]
- Tataranni, P.; Besemer, G.M.; Bortolotti, V.; Sangiorgi, C. Preliminary research on the physical and mechanical properties of alternative lightweight aggregates produced by alkali-activation of waste powders. Materials 2018, 10, 1255. [Google Scholar] [CrossRef]
- Provis, J.L.; Rees, C. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Aboulayt, A.; Riahi, M.; Ouazzani Touhami, M.; Hannache, H.; Gomina, M.; Moussa, R. Properties of metakaolin based geopolymer incorporating calcium carbonate. Adv. Powder Technol. 2017, 28, 2393–2401. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Lizion, J.; Landi, E. Metakaolin-based geopolymer beads: Production methods and characterization. J. Clean. Prod. 2020, 244, 118844. [Google Scholar] [CrossRef]
- Copetti Callai, S.; Sangiorgi, C. A review on acoustic and skid resistance solutions for road pavements. Infrastructures 2021, 6, 41. [Google Scholar] [CrossRef]
- Artoni, R.; Cazacliu, B.; Hamard, E.; Cothenet, A.; Parhanos, R.S. Resistance to fragmentation of recycled concrete aggregates. Mater. Struct. Constr. 2017, 50, 11. [Google Scholar] [CrossRef]
- Dunford, A. Establishing a New Supply of UK PSV Control Stone Including Results of Supplementary Experiments; Published Project Report PPR603; Transport Research Laboratory: Crothon, UK, 2013; p. 20. [Google Scholar]
- Vaiana, R.; Balzano, F.; Iuele, T.; Gallelli, V. Microtexture performance of EAF slags used as aggregate in asphalt mixes: A comparative study with surface properties of natural stones. Appl. Sci. 2019, 9, 3197. [Google Scholar] [CrossRef]
- Woodward, D.; Friel, S. Predicting the wear of high friction surfacing aggregate. Coatings 2017, 7, 71. [Google Scholar] [CrossRef]
- Czinder, B.; Vásárhelyi, B.; Török, Á. Long-term abrasion of rocks assessed by micro-Deval tests and estimation of the abrasion process of rock types based on strength parameters. Eng. Geol. 2021, 282, 105996. [Google Scholar] [CrossRef]
- Korkanç, M.; Tuǧrul, A. Evaluation of selected basalts from Nǐde, Turkey, as source of concrete aggregate. Eng. Geol. 2004, 75, 291–307. [Google Scholar] [CrossRef]
- Li, S.; Xiong, R.; Dong, X.; Sheng, Y.; Guan, B.; Zong, Y.; Xie, C.; Zhai, J.; Li, C. Effect of chemical composition of calcined bauxite aggregates on mechanical and physical properties for high friction surface course. Constr. Build. Mater. 2021, 302, 124390. [Google Scholar] [CrossRef]
- Manaf, M.B.H.A.; Abdul Razak, R.; Muhamad, K.; Abdul Rahim, M.; Ahmad, M.M.; Hao, T.P. A study on the potential of geopolymer artificial aggregate as substitute for granite and limestone aggregate. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012034. [Google Scholar] [CrossRef]
- Li, S.; Xiong, R.; Yu, D.; Zhao, G.; Cong, P.; Jiang, Y. Friction Surface Treatment Selection: Aggregate Properties, Surface Characteristics, Alternative Treatments, and Safety Effects; Purdue University: West Lafayette, IN, USA, 2017; ISBN 9781622604784. [Google Scholar]
- Mohajerani, A.; Suter, D.; Jeffrey-Bailey, T.; Song, T.; Arulrajah, A.; Horpibulsuk, S.; Law, D. Recycling waste materials in geopolymer concrete. Clean Technol. Environ. Policy 2019, 21, 493–515. [Google Scholar] [CrossRef]
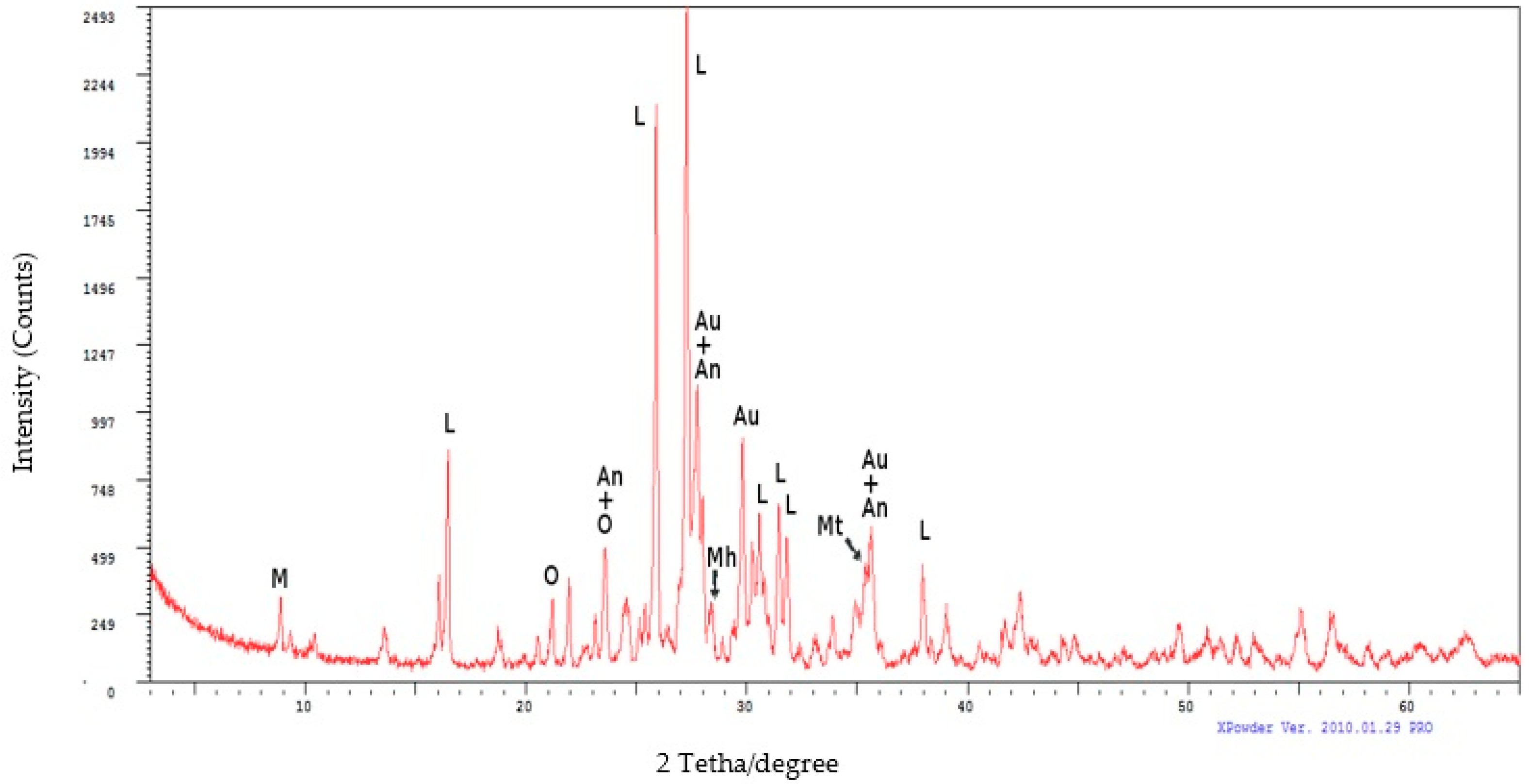




| Name | Composition | Percentage (%) |
|---|---|---|
| Leucite (L) | (K(AlSi2O6)) | 44 |
| Augite (Au) | ((Ca,Mg,Fe)2Si2O6) | 22 |
| Anorthite (An) | (Ca(Al2Si2O8)) | 11 |
| Orthoclase (O) | (K(AlSi3O8)) | 5 |
| Muscovite (M) | (KAl2(Si3Al)O10(OH)2) | 5 |
| Magnesiohornblendeferroan (Mh) | (Ca2(Mg4Fe3+) (Si7Al)O22(OH)2) | 4 |
| Magnetite (Mt) | (Fe2+Fe3+O2) | 1 |
| Aggregate | Water Content (%) | Specific Mass (g/cm3) | Los Angeles (%) | PSV | Micro-Deval (%) | Reference |
|---|---|---|---|---|---|---|
| Aggregate A | 19.1 | 2.120 | - | - | 35 * (27) | - |
| Aggregate B | 21.0 | 2.017 | 37 | 59 | 70 | - |
| Basalt | <2.5 | 2.700 | 14–20 | 53 | 14 | [48,49,50] |
| Calcined Bauxite | 6.8 | 2.629 | 10–17 | 50–70 | 5 | [48,51,52] |
| Fly ash AA | 5.5 | 2.140 | 27 | - | - | [53] |
| Steel slag | 1.1–9.0 | 2.96–3.59 | 14–15 | 25–55 | 6–10 | [47,54] |
| ID Sample: | Data | Before Polishing (µm) | After Polishing (µm) | Variation (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ra,bp | Rq,bp | Rz,bp | Ra,ap | Rq,ap | Rz,ap | ΔRa | ΔRq | ΔRz | ||
| Aggregate B-MK1 (B) | Longitudinal sections (mean) | 12.6 | 16.8 | 50.9 | 19.6 | 24.4 | 69.1 | +88.9 | +78.0 | +60.1 |
| Crosswise sections (mean) | 9.5 | 12.6 | 40.9 | 25.4 | 30.9 | 79.1 | +173.4 | +151.6 | +94.5 | |
| Mean | 10.7 | 14.3 | 45.0 | 23.1 | 28.3 | 75.1 | +127.7 | +111.1 | +75.4 | |
| Control Basalt (C) | Longitudinal sections (mean) | 24.8 | 30.6 | 75.7 | 26.3 | 32.5 | 84.7 | +6.1 | +6.5 | +14.3 |
| Crosswise sections (mean) | 22.3 | 27.5 | 67.9 | 27.0 | 32.9 | 80.0 | +21.2 | +20.0 | +17.9 | |
| Mean | 23.3 | 28.7 | 71.0 | 26.7 | 32.8 | 81.9 | +14.7 | +14.0 | +15.8 | |
| ID Sample: Aggregate B-MK1 (B) | ΔA Variation (%) | St. Dev (%) |
| Longitudinal sections (mean) | −16.9 | ±4.5 |
| Crosswise sections (mean) | −17.4 | ±2.1 |
| ID Sample: Control Basalt (C) | ΔA Variation (%) | St. Dev (%) |
| Longitudinal sections (mean) | −3.7 | ±1.4 |
| Crosswise sections (mean) | −4.1 | ±1.0 |
| ID Sample | PTVb | PTVa | Variation (%) | PSV |
|---|---|---|---|---|
| Aggregate B-MK1 (B) | 62 | 56 | −9.9 | 59.0 |
| Control Basalt (C) | 56 | 46 | −18.3 | 49.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copetti Callai, S.; Tataranni, P.; De Rose, M.; Natali Murri, A.; Vaiana, R.; Sangiorgi, C. A Preliminary Laboratory Evaluation of Artificial Aggregates from Alkali-Activated Basalt Powder. Sustainability 2022, 14, 16653. https://doi.org/10.3390/su142416653
Copetti Callai S, Tataranni P, De Rose M, Natali Murri A, Vaiana R, Sangiorgi C. A Preliminary Laboratory Evaluation of Artificial Aggregates from Alkali-Activated Basalt Powder. Sustainability. 2022; 14(24):16653. https://doi.org/10.3390/su142416653
Chicago/Turabian StyleCopetti Callai, Sergio, Piergiorgio Tataranni, Manuel De Rose, Annalisa Natali Murri, Rosolino Vaiana, and Cesare Sangiorgi. 2022. "A Preliminary Laboratory Evaluation of Artificial Aggregates from Alkali-Activated Basalt Powder" Sustainability 14, no. 24: 16653. https://doi.org/10.3390/su142416653
APA StyleCopetti Callai, S., Tataranni, P., De Rose, M., Natali Murri, A., Vaiana, R., & Sangiorgi, C. (2022). A Preliminary Laboratory Evaluation of Artificial Aggregates from Alkali-Activated Basalt Powder. Sustainability, 14(24), 16653. https://doi.org/10.3390/su142416653











