Essential Oils of Four Virginia Mountain Mint (Pycnanthemum virginianum) Varieties Grown in North Alabama
Abstract
:1. Introduction
2. Results and Discussion
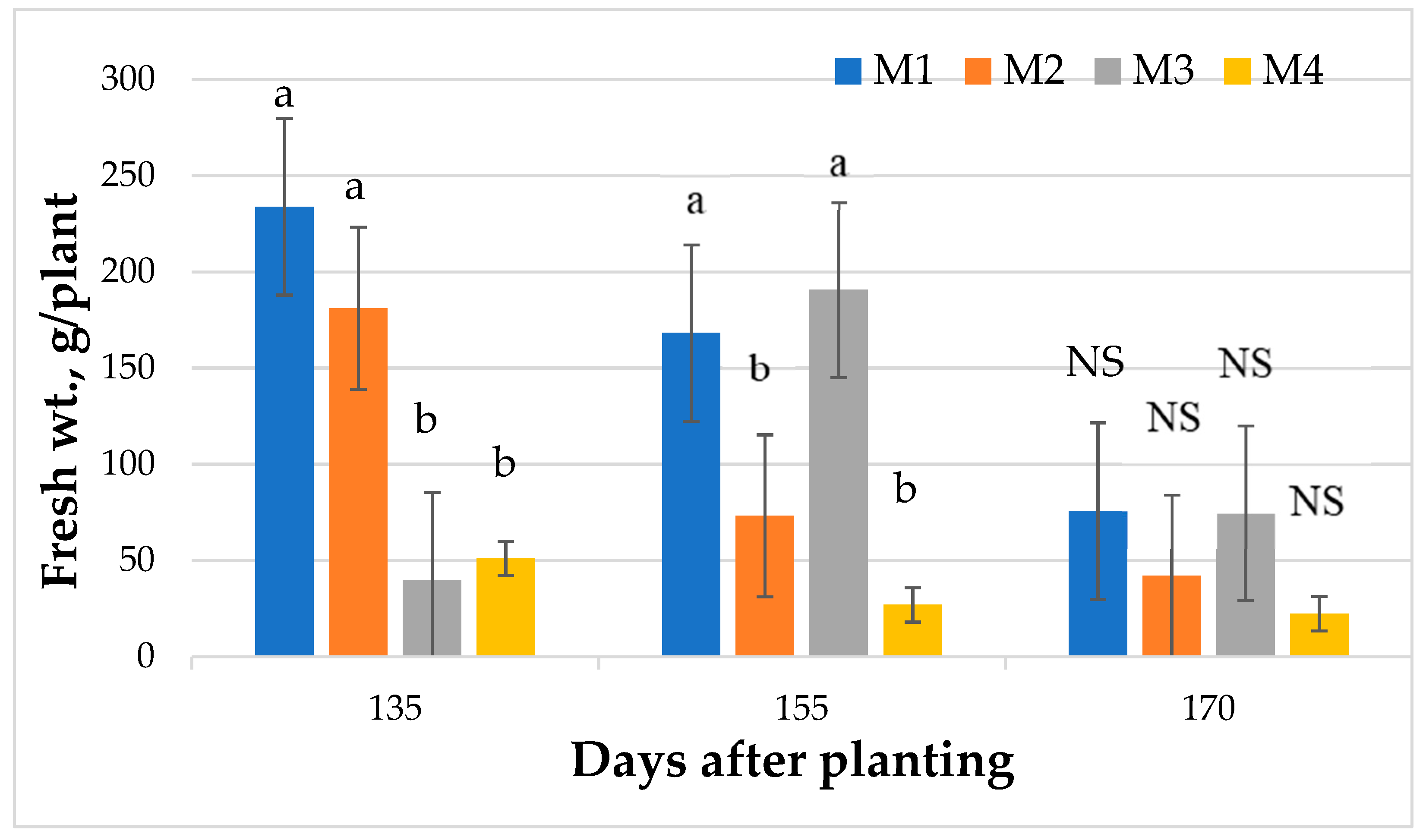
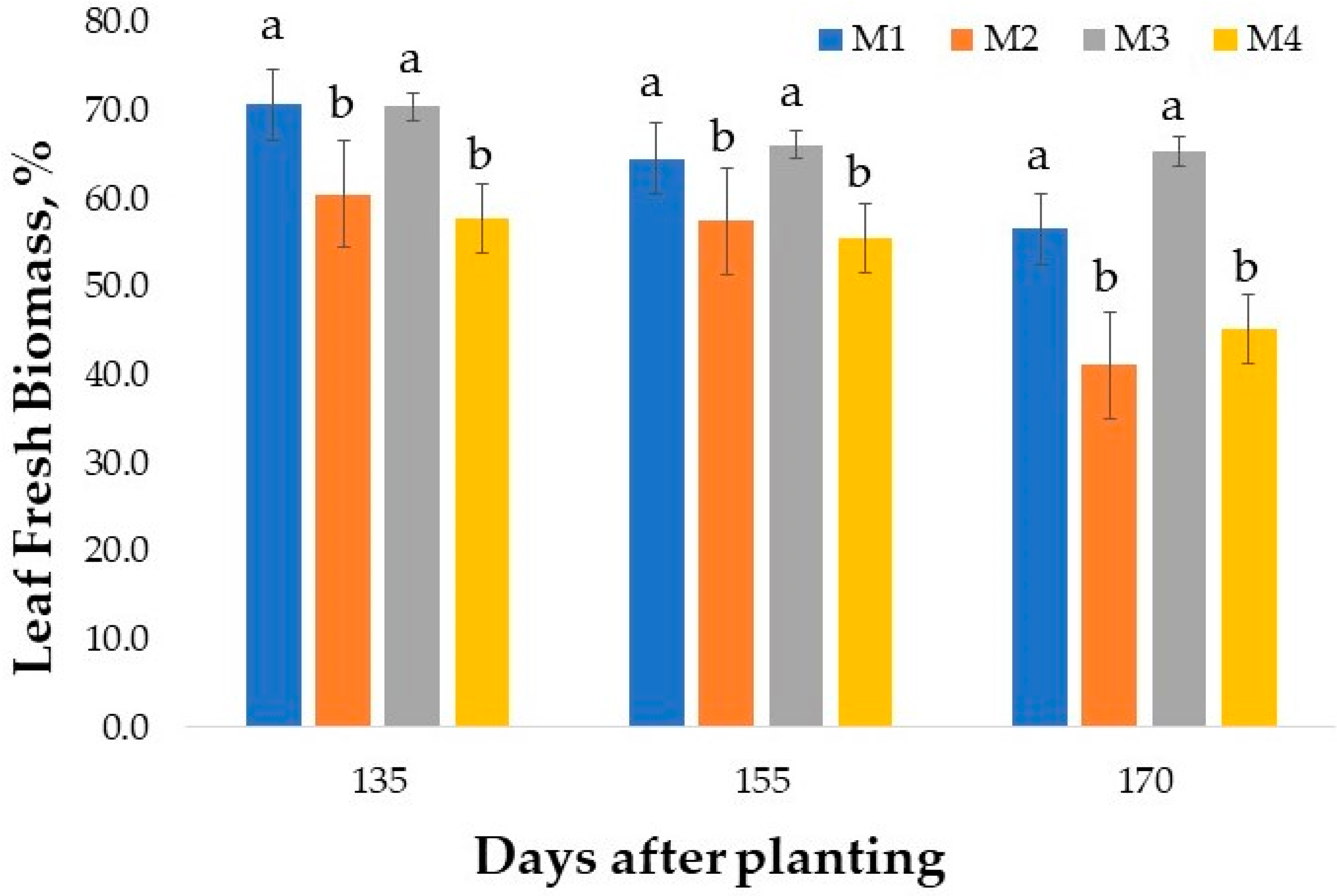
2.1. Fresh Leaf Biomass Yield
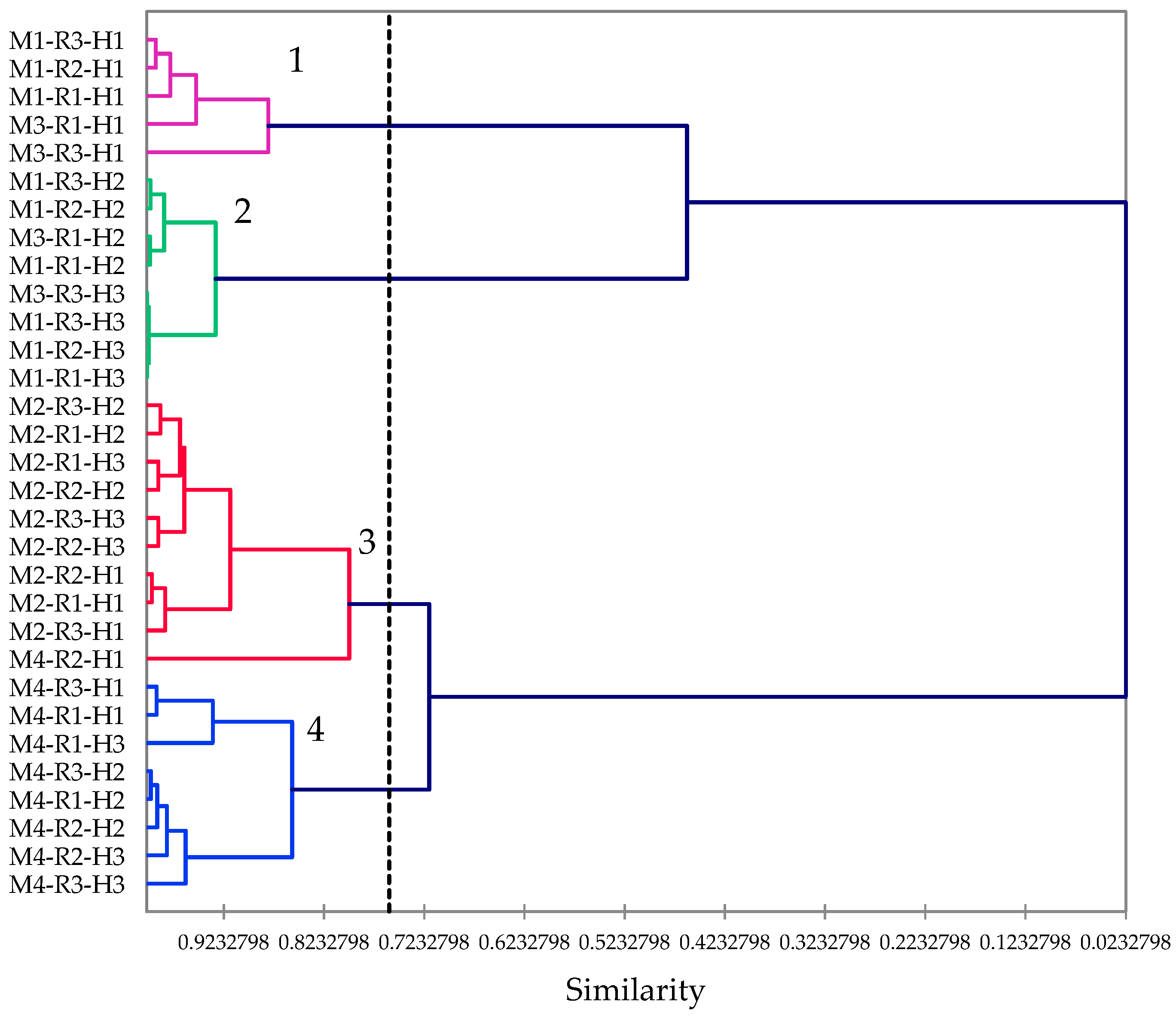
2.2. Chemical Composition of Essential Oils
2.3. Chemical Composition of Essential Oils
3. Materials and Methods
3.1. Cultivation of Pycnanthemum virginianum Varieties
3.2. Hydrodistillation of Pycnanthemum virginianum
3.3. Gas Chromatographic Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moerman, D.E. Native American Ethnobotany; Timber Press, Inc.: Portland, OR, USA, 1998; ISBN 978-0-88192-453-4. [Google Scholar]
- Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants; Houghton Mifflin: Boston, MA, USA, 1990; ISBN 0-395-46722-5. [Google Scholar]
- Bown, D. Encyclopedia of Herbs & Their Uses; Dorling Kindersley: London, UK, 1995; ISBN 978-0789401847. [Google Scholar]
- Ravid, U.; Putievsky, E.; Katzir, I. Enantiomeric distribution of piperitone in essential oils of some Mentha spp., Calamintha incána (Sm.) Heldr. and Artemisia judaica L. Flavour Fragr. J. 1994, 9, 85–87. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betz, S.; Knapke, D.; Schlosser, K.; Stravinsky, D.; Wells, L. The Herb Society of America’s Notable Native 2016—Mountain Mints Pycnanthemum Michx. Available online: www.ncherbsociety.org/uploads/8/4/4/3/84437124/pycnanthemum_nn_2016.pdf (accessed on 31 March 2021).
- Grand View Research. Mint Essential Oils Market Size, Share & Trends Analysis Report by Product (Cornmint, Peppermint, Spearmint, Dementholized Peppermint), by Application, by Usage (Direct, Indirect), and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/mint-essential-oils-market (accessed on 22 June 2021).
- Sims, C.A.; Juliani, H.R.; Mentreddy, S.R.; Simon, J.E. Essential oils in Holy basil (Ocimum tenuiflorum L.) as influenced by planting dates and harvest times in north Alabama. J. Med. Act. Plants 2014, 2, 33–41. [Google Scholar] [CrossRef]
- Brar, S.K.; Gill, B.S.; Brar, A.S.; Kaur, T. Planting date and straw mulch affect biomass yield, oil yield and oil quality of Japanese mint (Mentha arvensis L.) harvested at successive intervals. J. Essent. Oil Bear. Plants 2014, 17, 676–695. [Google Scholar] [CrossRef]
- Solomon, A.M.; Beemnet, M.K. Row spacing and harvesting age affect agronomic characteristics and essential oil yield of Japanese mint (Mentha arvensis L.). Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 74–76. [Google Scholar]
- Kassahun, B.M.; Teixeira da Silva, J.A.; Mekonnen, S.A. Agronomic characters, leaf and essential oil yield of peppermint (Mentha piperiata L.) as influenced by harvesting age and row spacing. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 49–53. [Google Scholar]
- Domingues, P.M.; Santos, L. Essential oil of pennyroyal (Mentha pulegium): Composition and applications as alternatives to pesticides—New tendencies. Ind. Crops Prod. 2019, 139, 111534. [Google Scholar] [CrossRef]
- Croteau, R.; Venkatachalam, K.V. Metabolism of monoterpenes: Demonstration that (+)-cis-isopulegone, not piperitenone, is the key intermediate in the conversion of (–)-isopiperitenone to (+)-pulegone in peppermint (Mentha piperita). Arch. Biochem. Biophys. 1986, 249, 306–315. [Google Scholar] [CrossRef]
- Fuchs, S.; Beck, T.; Sandvoss, M.; Mosandl, A. Biogenetic studies in Mentha × piperita. 2. Stereoselectivity in the bioconversion of pulegone into menthone and isomenthone. J. Agric. Food Chem. 1999, 47, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Asllani, U.; Toska, V. Chemical composition of Albanian thyme oil (Thymus vulgaris L.). J. Essent. Oil Res. 2003, 15, 165–167. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.K.; Satyal, P.; Setzer, W.N. The volatile phytochemistry of seven Native American aromatic medicinal plants. Plants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, D.; Paz, D.; Dellacassa, E.; Davies, P.; Vila, R.; Cañigueral, S. Essential oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Braz. Arch. Biol. Technol. 2002, 45, 519–524. [Google Scholar] [CrossRef]
- DeCarlo, A.; Johnson, S.; Okeke-Agulu, K.I.; Dosoky, N.S.; Wax, S.J.; Owolabi, M.S.; Setzer, W.N. Compositional analysis of the essential oil of Boswellia dalzielii frankincense from West Africa reveals two major chemotypes. Phytochemistry 2019, 164, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
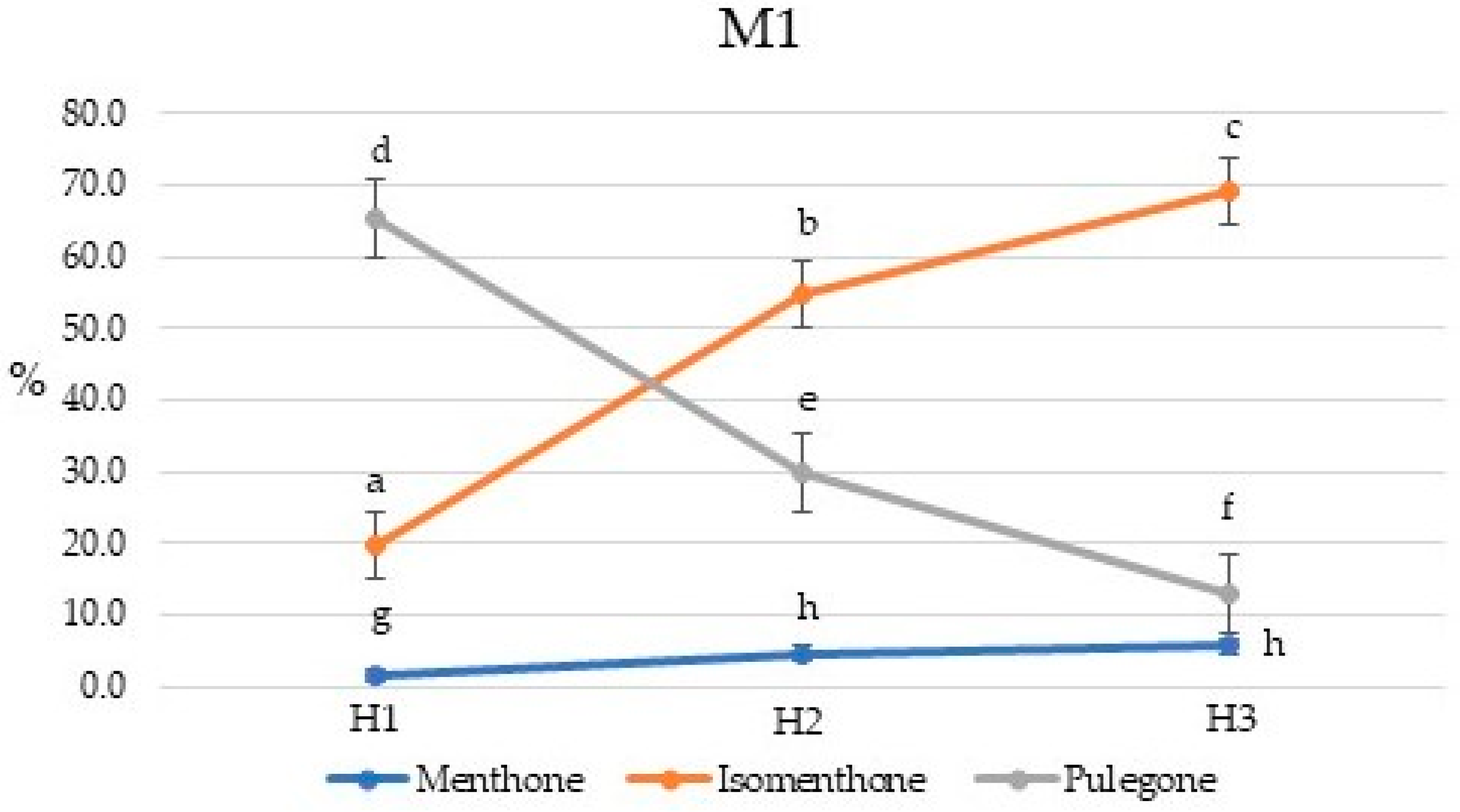
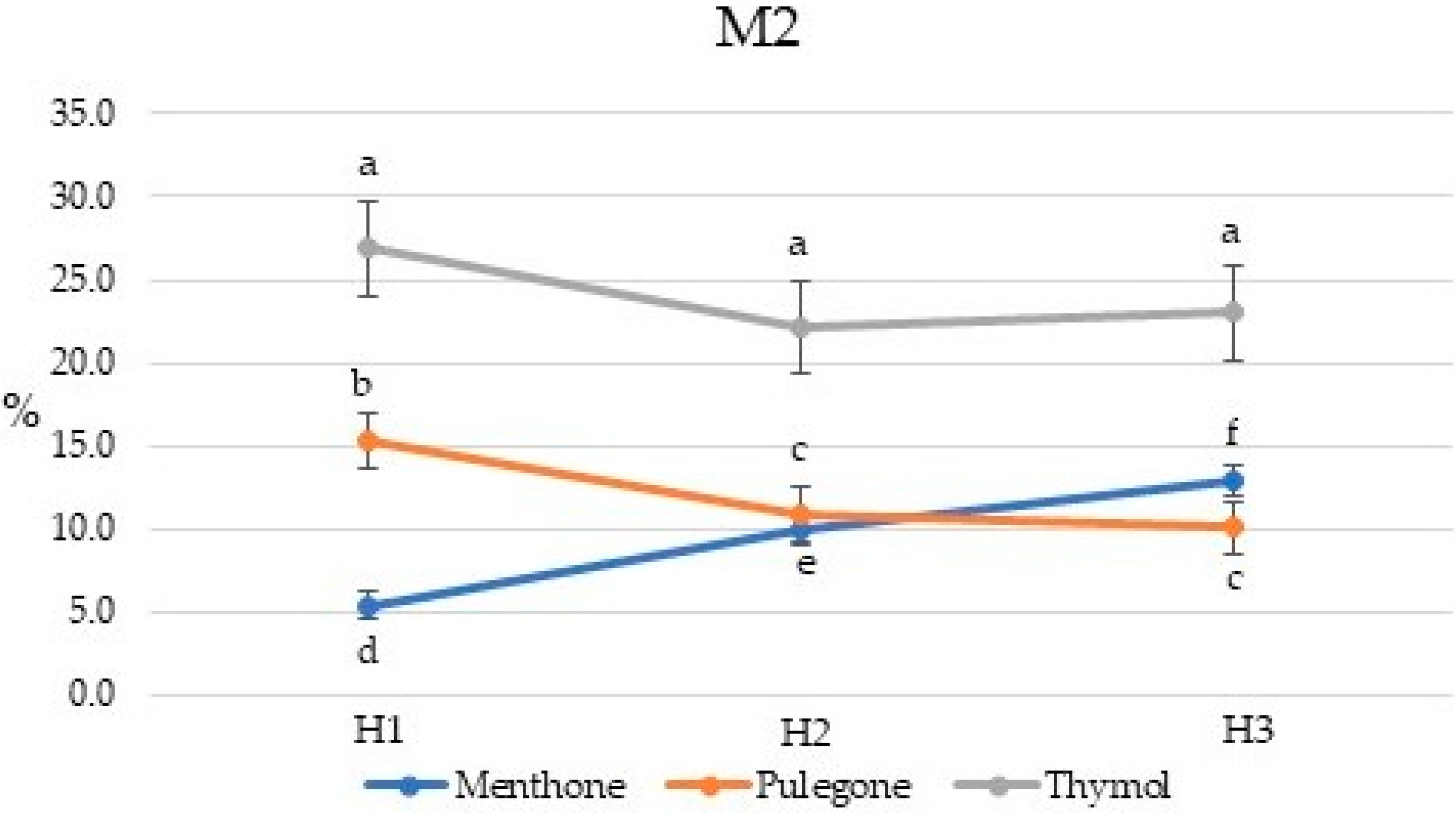
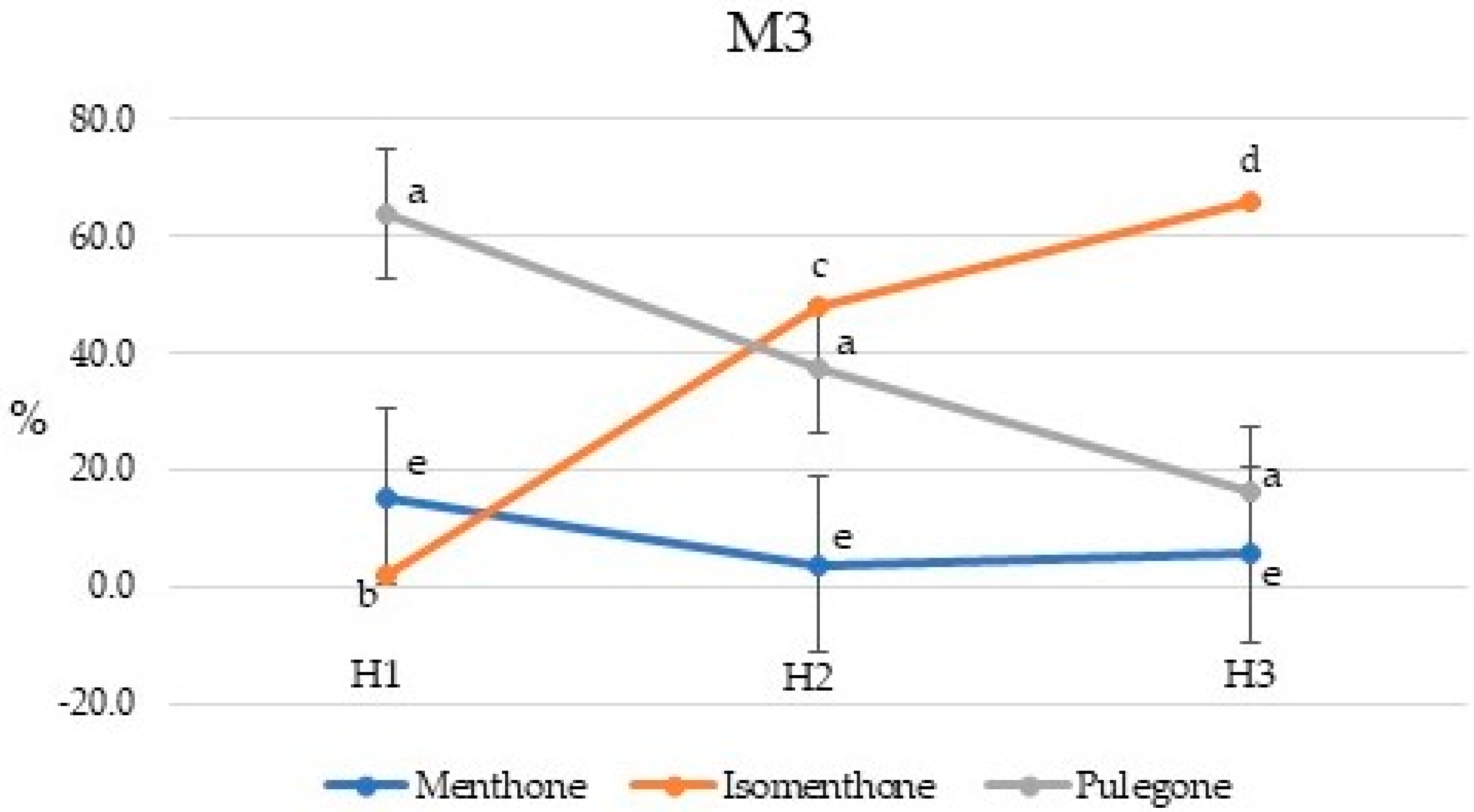
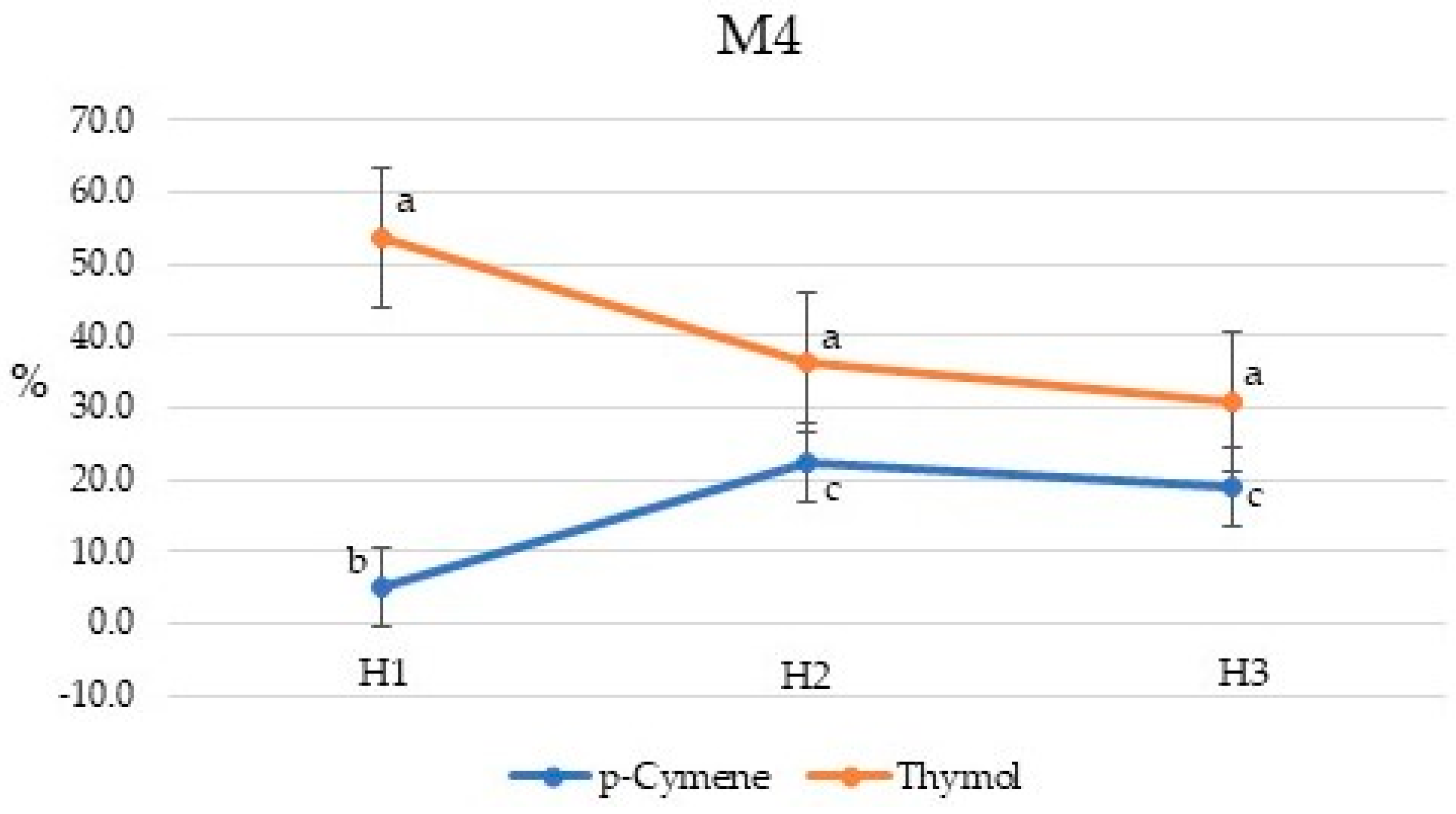
| Compound | Percent Composition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1H1 b | M1H2 b | M1H3 b | M2H1 b | M2H2 b | M2H3 b | M3H1 c | M3H2 d | M3H3 d | M4H1 b | M4H2 b | M4H3 b | |
| 1-Octen-3-ol | 2.0 ± 0.2 | 2.0 ± 0.2 | 1.5 ± 0.2 | 2.6 ± 0.1 | 2.0 ± 0.4 | 2.2 ± 0.7 | 3.5 ± 0.4 | 1.8 | 1.9 | 3.2 ± 0.5 | 5.5 ± 0.7 | 5.8 ± 1.5 |
| Myrcene | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.0 | 3.1 ± 1.1 | 2.6 ± 0.9 | 1.9 ± 0.7 | 0.1 ± 0.1 | 0.4 | 0.4 | 1.3 ± 1.0 | 0.8 ± 0.5 | 0.5 ± 0.1 |
| p-Cymene | 0.1 ± 0.0 | tr | tr | 7.1 ± 0.8 | 8.7 ± 2.1 | 7.4 ± 0.7 | 0.2 ± 0.1 | tr | 0.3 | 5.0 ± 2.7 | 22.5 ± 2.4 | 18.9 ± 8.8 |
| Limonene | 1.9 ± 0.2 | 1.3 ± 0.1 | 1.1 ± 0.2 | 5.8 ± 1.2 | 5.5 ± 1.4 | 4.1 ± 1.0 | 1.0 ± 0.7 | 1.5 | 1.2 | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.5 ± 0.1 |
| γ-Terpinene | tr | tr | tr | 2.4 ± 0.3 | 1.7 ± 0.6 | 0.9 ± 0.2 | nd | nd | tr | 3.9 ± 2.4 | 1.7 ± 0.5 | 1.2 ± 0.3 |
| cis-Sabinene hydrate | tr | tr | tr | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | tr | tr | tr | 1.0 ± 0.2 | 2.5 ± 0.8 | 2.7 ± 1.0 |
| Menthone | 1.5 ± 0.6 | 4.7 ± 0.6 | 5.6 ± 0.9 | 5.4 ± 0.9 | 9.9 ± 0.1 | 12.9 ± 1.2 | 15.3 ± 15.1 | 3.8 | 5.6 | 0.3 ± 0.3 | 0.1 ± 0.0 | 0.9 ± 0.7 |
| Isomenthone | 19.9 ± 6.9 | 54.7 ± 3.8 | 69.3 ± 1.4 | 0.1 ± 0.0 | 0.4 ± 0.4 | 1.8 ± 1.3 | 1.8 ± 0.1 | 48.0 | 65.8 | 2.7 ± 3.2 | 0.4 ± 0.6 | 6.3 ± 5.9 |
| trans-Isopulegone | 1.6 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.0 | 3.6 ± 0.2 | 4.1 ± 0.4 | 4.1 ± 0.8 | 1.6 ± 0.2 | 1.2 | 1.1 | 0.2 ± 0.4 | nd | 0.2 ± 0.3 |
| cis-Piperitenol | nd | nd | nd | 2.6 ± 0.2 | 2.3 ± 0.3 | 2.2 ± 0.4 | nd | nd | 0.1 | tr | nd | 0.1 ± 0.1 |
| Pulegone | 65.3 ± 8.4 | 29.8 ± 3.9 | 13.0 ± 1.8 | 15.3 ± 1.8 | 10.8 ± 1.4 | 10.1 ± 1.6 | 63.8 ± 11.2 | 37.3 | 16.1 | 11.8 ± 15.4 | 0.6 ± 0.9 | 2.3 ± 1.8 |
| Thymol | 0.4 ± 0.1 | 0.2 ± 0.2 | tr | 26.9 ± 4.1 | 22.1 ± 2.3 | 23.0 ± 1.3 | 0.3 ± 0.4 | tr | 0.6 | 53.8 ± 15.2 | 36.5 ± 5.4 | 30.9 ± 5.3 |
| Carvacrol | tr | tr | tr | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.1 | tr | nd | tr | 1.3 ± 0.4 | 1.1 ± 0.3 | 0.7 ± 0.1 |
| Unidentified (RI 1345) | 0.1 ± 0.0 | 0.1 ± 0.0 | tr | 7.5 ± 0.7 | 10.7 ± 0.8 | 10.7 ± 2.4 | 0.1 ± 0.1 | nd | 0.3 | 0.2 ± 0.2 | nd | 0.6 ± 0.6 |
| (E)-β-Caryophyllene | 1.2 ± 0.2 | 0.6 ± 0.5 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 1.1 | 1.0 | 1.1 | 1.8 ± 0.2 | 1.7 ± 0.4 | 1.6 ± 0.7 |
| Germacrene D | 1.2 ± 0.4 | 0.7 ± 0.5 | 1.1 ± 0.0 | 1.8 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.1 | 0.2 ± 0.3 | 0.7 | 1.0 | 1.4 ± 0.5 | 0.8 ± 0.0 | 1.0 ± 0.6 |
| Compound | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| α-Thujene | 54:46 | 72:28 | --- | 76:24 |
| α-Pinene | 24:76 | 58:42 | 23:77 | 72:28 |
| Camphene | 100:0 | 100:0 | --- | 100:0 |
| Sabinene | 30:70 | 0:100 | 28:72 | variable b |
| β-Pinene | 46:54 | 54:46 | 46:54 | 31:69 |
| α-Phellandrene | --- | 95:5 | --- | 96:4 |
| δ-3-Carene | --- | 100:0 | --- | 100:0 |
| α-Terpinene | --- | 100:0 | --- | 100:0 |
| Limonene | 6:94 | 0:100 | 7:93 | variable c |
| β-Phellandrene | --- | 0:100 | --- | 0:100 |
| cis-Sabinene hydrate | --- | 95:5 | --- | 99:1 |
| Linalool | --- | 100:0 | --- | variable d |
| trans-Sabinene hydrate | --- | 78:22 | --- | 82:18 |
| Menthone | 0:100 | 0:100 | 0:100 | 0:100 |
| Isomenthone | 100:0 | 100:0 | 100:0 | 100:0 |
| Borneol | --- | 0:100 | --- | 0:100 |
| Terpinen-4-ol | --- | 65:35 | --- | 66:34 |
| α-Terpineol | 9:91 | 32:68 | 10:90 | variable e |
| Pulegone | 100:0 | 100:0 | 100:0 | 100:0 |
| Piperitone | 94:6 | --- | 89:11 | --- |
| δ-Elemene | --- | --- | --- | variable f |
| α-Copaene | --- | --- | --- | 100:0 |
| trans-β-Elemene | 15:85 | 20:80 | 16:84 | 6:94 |
| (E)-β-Caryophyllene | 100:0 | 100:0 | 100:0 | 100:0 |
| Germacrene D | 91:9 | 94:6 | 91:9 | 80:20 |
| δ-Cadinene | 0:100 | 0:100 | 0:100 | 0:100 |
| Sample a | Plant Mass (g) | Essential Oil Yield (mg) | % Yield | Essential Oil Color |
|---|---|---|---|---|
| M1H1R1 | 104.08 | 1127.6 | 1.083 | pale yellow |
| M1H1R2 | 105.70 | 1164.6 | 1.102 | pale yellow |
| M1H1R3 | 122.45 | 1548.9 | 1.265 | pale yellow |
| M2H1R1 | 82.71 | 704.9 | 0.852 | yellow |
| M2H1R2 | 70.22 | 580.0 | 0.826 | yellow |
| M2H1R3 | 75.80 | 791.6 | 1.044 | yellow |
| M3H1R1 | 40.13 | 302.3 | 0.753 | pale yellow |
| M3H1R2 | 0.00 | plant died | --- | --- |
| M3H1R3 | 74.15 | 572.1 | 0.772 | pale yellow |
| M4H1R1 | 30.67 | 301.2 | 0.982 | yellow |
| M4H1R2 | 35.31 | 345.8 | 0.979 | yellow |
| M4H1R3 | 73.80 | 825.5 | 1.119 | orange |
| M1H2R1 | 139.76 | 1330.7 | 0.952 | pale yellow |
| M1H2R2 | 133.69 | 1093.3 | 0.818 | pale yellow |
| M1H2R3 | 167.15 | 1862.5 | 1.114 | pale yellow |
| M2H2R1 | 74.47 | 596.6 | 0.801 | yellow |
| M2H2R2 | 59.86 | 463.1 | 0.774 | yellow |
| M2H2R3 | 132.61 | 1003.9 | 0.757 | yellow |
| M3H2R1 | 182.68 | 2157.9 | 1.181 | pale yellow |
| M3H2R2 | 0.00 | plant died | --- | --- |
| M3H2R3 | 0.00 | plant died | --- | --- |
| M4H2R1 | 8.10 | 92.6 | 1.143 | orange |
| M4H2R2 | 60.66 | 252.3 | 0.416 | orange |
| M4H2R3 | 32.81 | 208.2 | 0.635 | orange |
| M1H3R1 | 100.14 | 582.0 | 0.581 | pale yellow |
| M1H3R2 | 110.22 | 897.3 | 0.814 | pale yellow |
| M1H3R3 | 59.71 | 556.9 | 0.933 | pale yellow |
| M2H3R1 | 39.93 | 298.4 | 0.747 | yellow |
| M2H3R2 | 53.23 | 510.8 | 0.960 | yellow |
| M2H3R3 | 70.02 | 495.8 | 0.708 | yellow |
| M3H3R1 | 0.00 | plant died | --- | --- |
| M3H3R2 | 0.00 | plant died | --- | --- |
| M3H3R3 | 110.07 | 945.5 | 0.859 | yellow |
| M4H3R1 | 53.98 | 320.2 | 0.593 | orange |
| M4H3R2 | 29.91 | 108.9 | 0.364 | orange |
| M4H3R3 | 19.84 | 143.9 | 0.725 | orange |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setzer, W.N.; Duong, L.; Pham, T.; Poudel, A.; Nguyen, C.; Mentreddy, S.R. Essential Oils of Four Virginia Mountain Mint (Pycnanthemum virginianum) Varieties Grown in North Alabama. Plants 2021, 10, 1397. https://doi.org/10.3390/plants10071397
Setzer WN, Duong L, Pham T, Poudel A, Nguyen C, Mentreddy SR. Essential Oils of Four Virginia Mountain Mint (Pycnanthemum virginianum) Varieties Grown in North Alabama. Plants. 2021; 10(7):1397. https://doi.org/10.3390/plants10071397
Chicago/Turabian StyleSetzer, William N., Lam Duong, Trang Pham, Ambika Poudel, Cuong Nguyen, and Srinivasa Rao Mentreddy. 2021. "Essential Oils of Four Virginia Mountain Mint (Pycnanthemum virginianum) Varieties Grown in North Alabama" Plants 10, no. 7: 1397. https://doi.org/10.3390/plants10071397