Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Culture of hUCMSCs in PBS-Vertical Wheel (PBS-VW) Bioreactors
2.2.2. Atomic Force Microscopy
2.2.3. Preparation of Optimized CBD-Loaded Extracellular Vesicles and Characterization
2.2.4. Release Studies
2.2.5. Animals
2.2.6. DRG Primary Cultures
2.2.7. Behavioral Parameters
Thermal and Mechanical Hyperalgesia
- a.
- Plantar Test (Hargreaves Method)
- b.
- Hot immersion test
- c.
- Electronic Vonfrey Test and Randall Selitto Test
2.2.8. Biochemical and Molecular Parameters
Estimation of ATP Levels
Estimation of NAD+ Levels and NADH Levels
JC1 Assay
Western Blotting
Neurite Outgrowth Assay
Immunocytochemistry
2.2.9. Statistical Analysis
3. Results
3.1. Isolation and Characterization of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extra Cellular Vesicles (EVs)
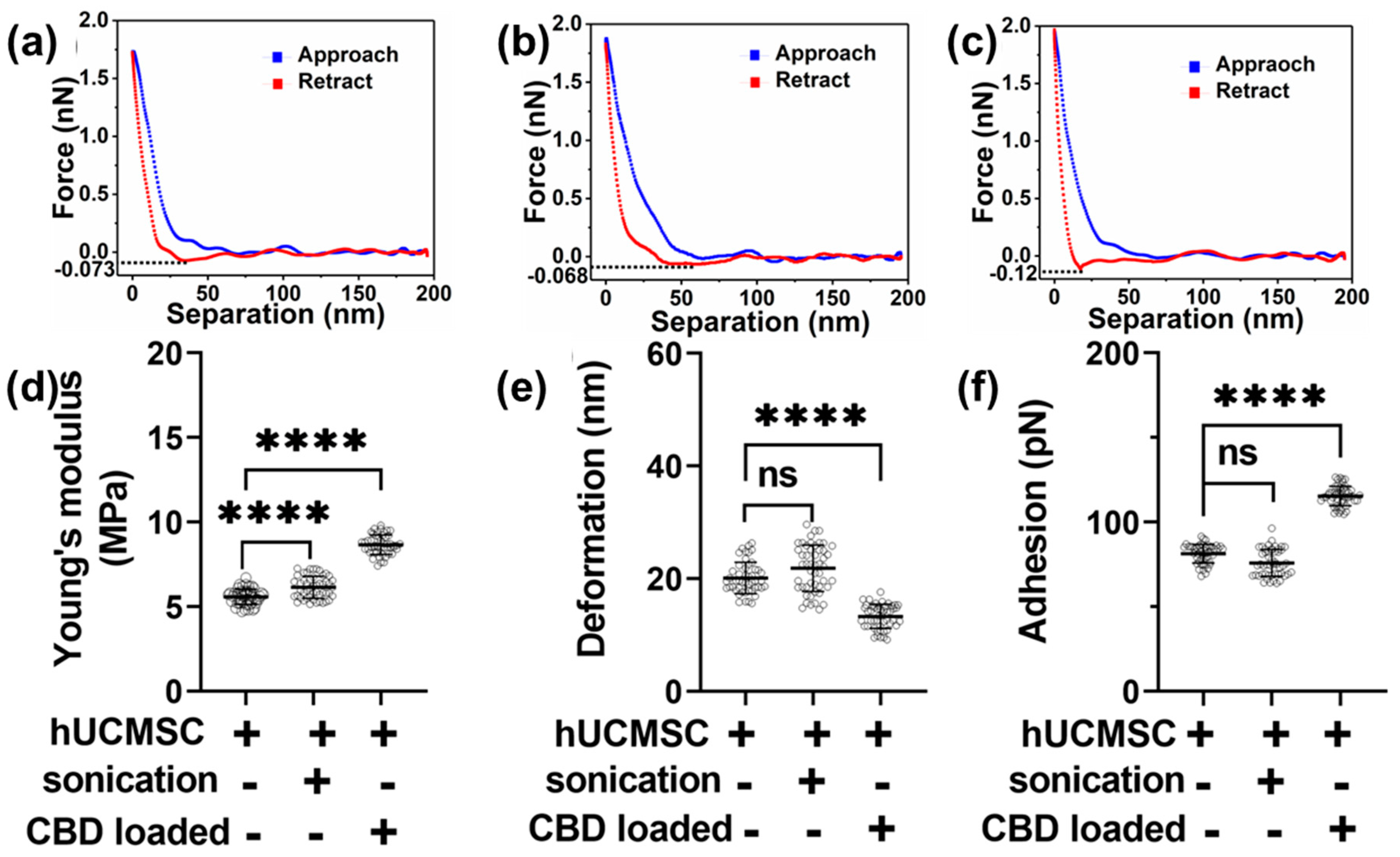
3.2. Morphology and Nanomechanical Attributes Characterization of Human Umbilical Cord Mesenchymal Stem Cell-Derived EVs for Various Treatments
3.3. In Vitro Drug Release from CBD-Loaded EVs
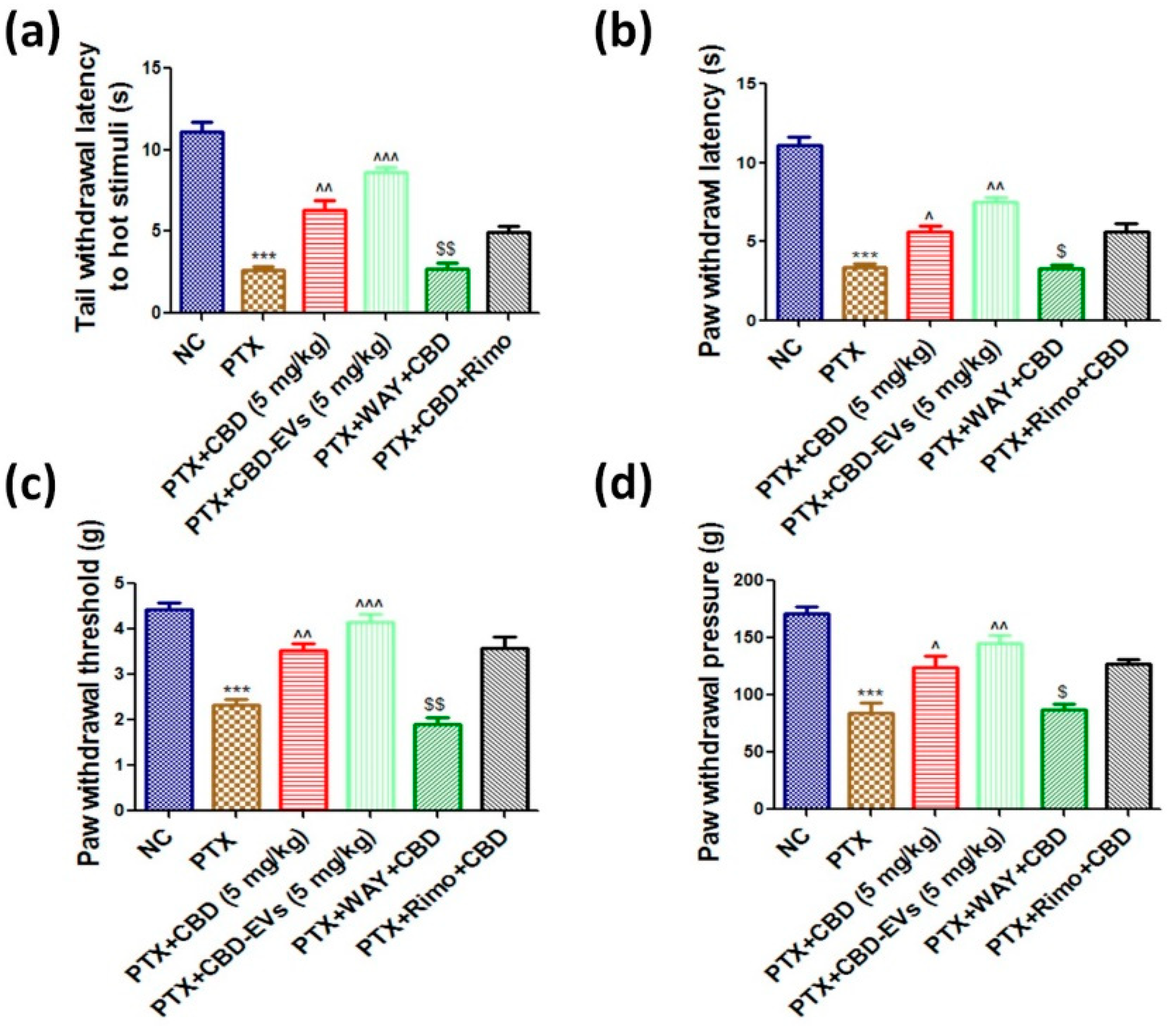
3.4. Effect of CBD and CBD-Loaded EVs on Neurobehavior of PTX-Treated Mice
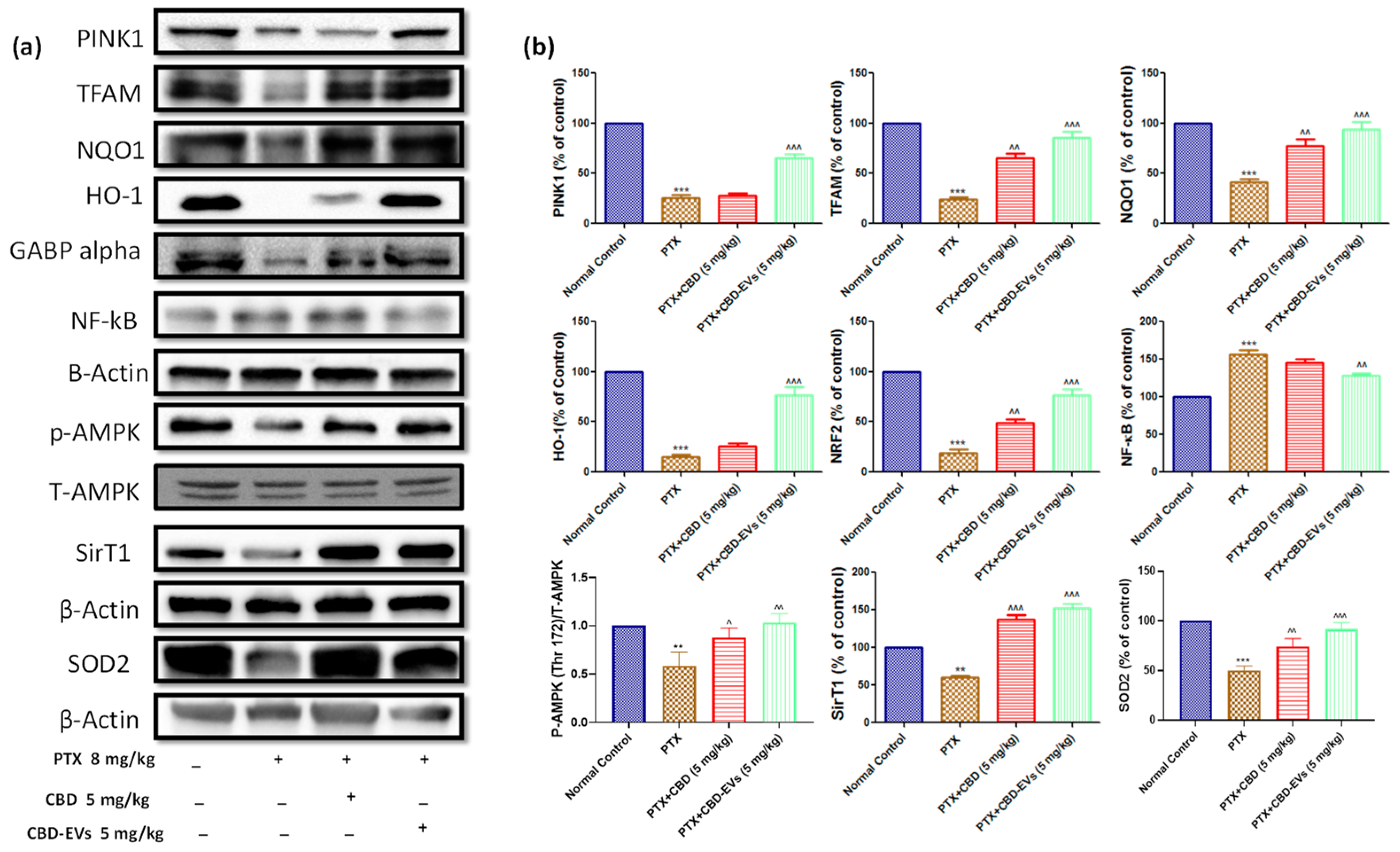
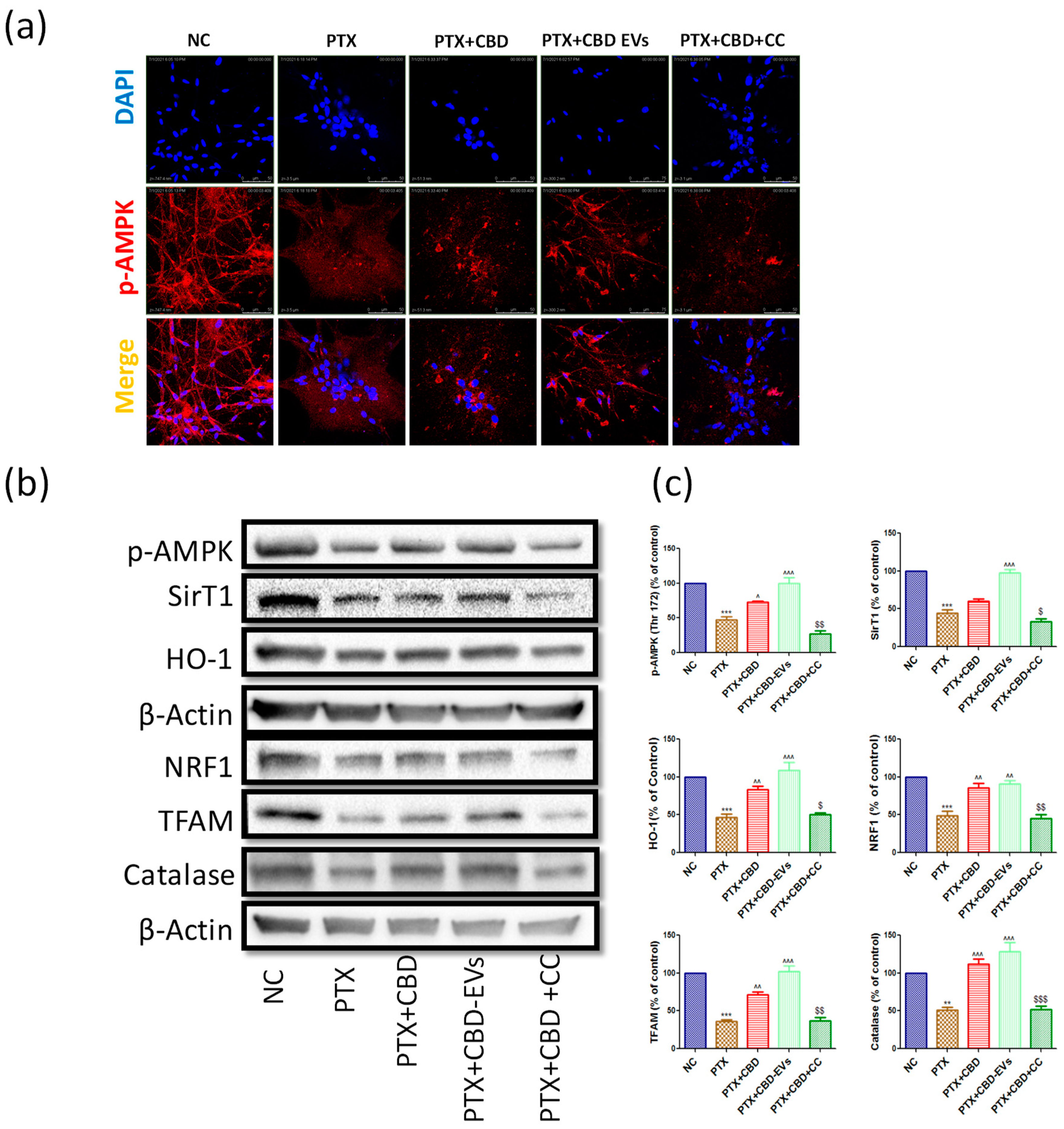
3.5. Effect of CBD and CBD-Loaded EVs on AMPK Pathway
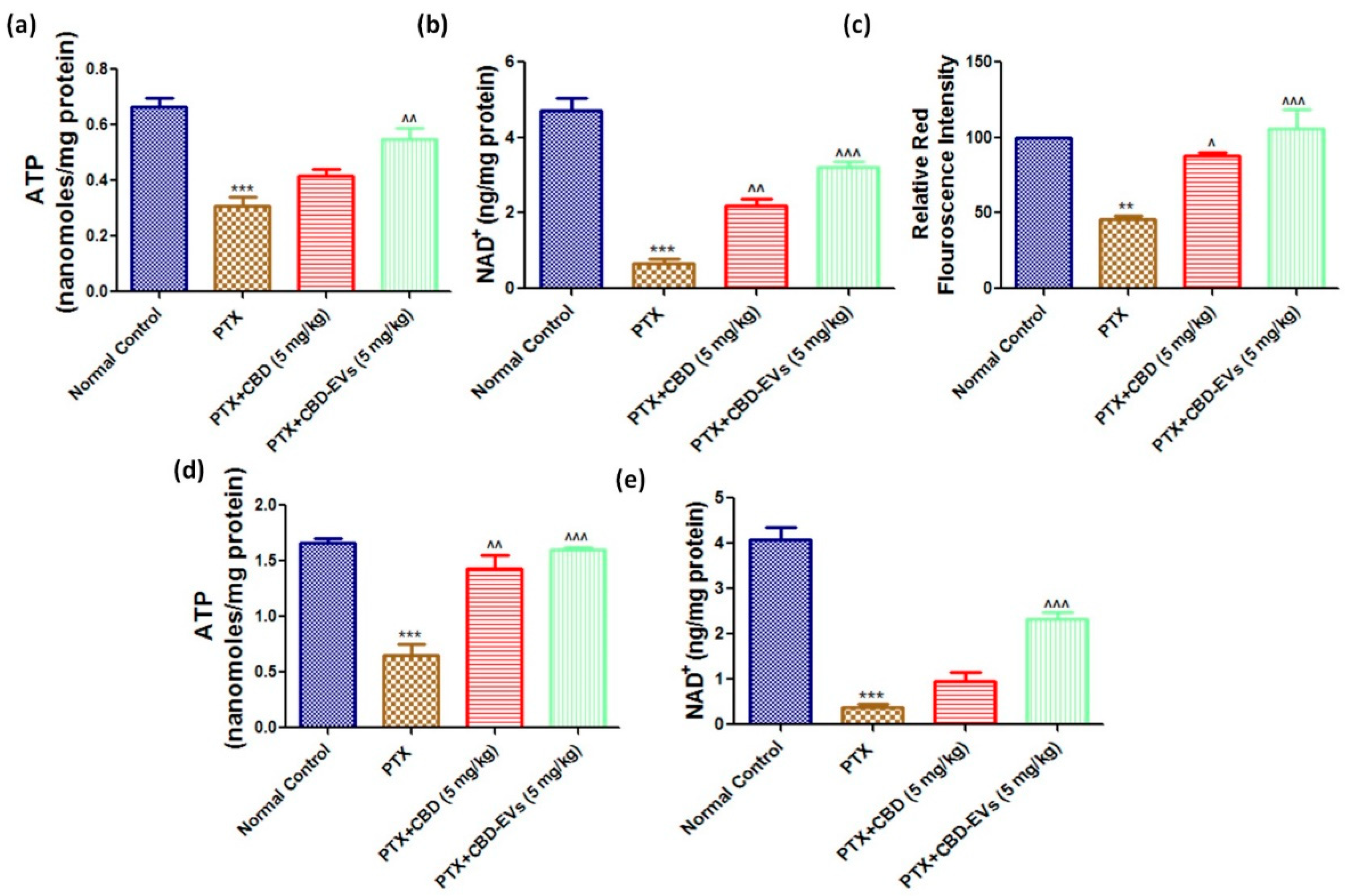
3.6. Effect of CBD and CBD-Loaded EVs on Mitochondrial Function in DRG and Spinal Homogenates of PTX-Treated Mice
3.7. AMPK Dependent Neuroprotective Effects of CBD in PTX-Treated Primary Rat DRG Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nehate, C.; Jain, S.; Saneja, A.; Khare, V.; Alam, N.; Dubey, R.; Gupta, P. Paclitaxel Formulations: Challenges and Novel Delivery Options. Curr. Drug Deliv. 2014, 11, 666–686. [Google Scholar] [CrossRef]
- Patel, A.R.; Chougule, M.B.; Ian, T.; Patlolla, R.; Wang, G.; Singh, M. Efficacy of Aerosolized Celecoxib Encapsulated Nanostructured Lipid Carrier in Non-small Cell Lung Cancer in Combination with Docetaxel. Pharm. Res. 2013, 30, 1435–1446. [Google Scholar] [CrossRef]
- Ferdous, A.J.; Stembridge, N.Y.; Singh, M. Role of monensin PLGA polymer nanoparticles and liposomes as potentiator of ricin A immunotoxins in vitro. J. Control. Release 1998, 50, 71–78. [Google Scholar] [CrossRef]
- Rugo, H.S.; Barry, W.T.; Moreno-Aspitia, A.; Lyss, A.P.; Cirrincione, C.; Leung, E.; Mayer, E.L.; Naughton, M.; Toppmeyer, D.; Carey, L.A.; et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J. Clin. Oncol. 2015, 33, 2361. [Google Scholar]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Postma, T.J.; Vermorken, J.B.; Liefting AJ, M.; Pinedo, H.M.; Heimans, J.J. Paclitaxel-induced neuropathy. Ann. Oncol. 1995, 6, 489–494. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Ramalho, Í.M.D.M.; Pereira, D.T.; Galvão, G.B.L.; Freire, D.T.; Amaral-Machado, L.; Alencar, E.D.N.; Egito, E.S.T.D. Current trends on cannabidiol delivery systems: Where are we and where are we going? Expert Opin. Drug Deliv. 2021, 18, 1577–1587. [Google Scholar] [CrossRef]
- Ren, J.; Liu, N.; Sun, N.; Zhang, K.; Yu, L. Mesenchymal Stem Cells and their Exosomes: Promising Therapeutics for Chronic Pain. Curr. Stem Cell Res. Ther. 2019, 14, 644–653. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Chen, K.; Wang, X.; Wang, Z.; Lian, H.; Lin, Y.; Rong, X.; Chu, M.; Lin, J.; et al. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J. Cell. Mol. Med. 2020, 24, 7515–7530. [Google Scholar] [CrossRef]
- Madhavi, Y.; Gaikwad, N.; Yerra, V.G.; Kalvala, A.K.; Nanduri, S.; Kumar, A. Targeting AMPK in Diabetes and Diabetic Complications: Energy Homeostasis, Autophagy and Mitochondrial Health. Curr. Med. Chem. 2019, 26, 5207–5229. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Kommineni, N.; Meckes, D.; Sachdeva, M. Exosome Vehicles as Nano-Drug Delivery Materials for Chemotherapeutic Drugs. In Critical Reviews™ in Therapeutic Drug Carrier Systems; Begell House: Danbury, CT, USA, 2021. [Google Scholar]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Schindler, C.; Collinson, A.; Matthews, C.; Pointon, A.; Jenkinson, L.; Minter, R.R.; Vaughan, T.J.; Tigue, N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 2019, 14, e0214545. [Google Scholar] [CrossRef]
- Liu, B.; Kong, Y.; Shi, W.; Kuss, M.; Liao, K.; Hu, G.; Xiao, P.; Sankarasubramanian, J.; Guda, C.; Wang, X.; et al. Exosomes derived from differentiated human ADMSC with the Schwann cell phenotype modulate peripheral nerve-related cellular functions. Bioact. Mater. 2021, 14, 61–75. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef]
- Polter, A.M.; Li, X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell. Signal. 2010, 22, 1406–1412. [Google Scholar] [CrossRef]
- Jain, S.S.; Paglialunga, S.; Vigna, C.; Ludzki, A.; Herbst, E.A.; Lally, J.S.; Schrauwen, P.; Hoeks, J.; Tupling, A.R.; Bonen, A.; et al. High-Fat Diet–Induced Mitochondrial Biogenesis Is Regulated by Mitochondrial-Derived Reactive Oxygen Species Activation of CaMKII. Diabetes 2014, 63, 1907–1913. [Google Scholar] [CrossRef]
- Hock, M.B.; Kralli, A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Yerra, V.G.; Kumar, A. LONP1 induction by SRT1720 attenuates mitochondrial dysfunction against high glucose induced neurotoxicity in PC12 cells. Toxicol. Vitr. 2020, 62, 104695. [Google Scholar] [CrossRef]
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Khan, I.; Gundu, C.; Kumar, A. An Overview on ATP Dependent and Independent Proteases Including an Anterograde to Retrograde Control on Mitochondrial Function; Focus on Diabetes and Diabetic Complications. Curr. Pharm. Des. 2019, 25, 2584–2594. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Bagde, A.; Arthur, P.; Surapaneni, S.K.; Ramesh, N.; Nathani, A.; Singh, M. Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-induced neuropathic pain in rodents. Int. Immunopharmacol. 2022, 107, 108693. [Google Scholar] [CrossRef]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M.; et al. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef]
- Deseure, K.R.; Adriaensen, H.; Colpaert, F. Effects of the combined continuous administration of morphine and the high-efficacy 5-HT1A agonist, F 13640 in a rat model of trigeminal neuropathic pain. Eur. J. Pain 2004, 8, 547–554. [Google Scholar] [CrossRef]
- Maione, S.; Piscitelli, F.; Gatta, L.; Vita, D.; De Petrocellis, L.; Palazzo, E.; de Novellis, V.; Di Marzo, V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 2011, 162, 584–596. [Google Scholar] [CrossRef]
- Patel, N.; Kommineni, N.; Surapaneni, S.K.; Kalvala, A.; Yaun, X.; Gebeyehu, A.; Arthur, P.; Duke, L.C.; York, S.B.; Bagde, A.; et al. Cannabidiol Loaded Extracellular Vesicles Sensitize Triple-Negative Breast Cancer to Doxorubicin in both in-vitro and in vivo Models Running Title: Formulation and anti-cancer potential of CBD loaded hUCMSC-derived Extracellular Vesicles in TNBC. Int. J. Pharm. 2021, 607, 120943. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lötvall, J.; Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef]
- Gulati, M.; Grover, M.; Singh, M.; Singh, S. Study of azathioprine encapsulation into liposomes. J. Microencapsul. 1998, 15, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Kommineni, N.; Nottingham, E.; Bagde, A.; Patel, N.; Rishi, A.K.; Dev, S.R.S.; Singh, M. Role of nano-lipid formulation of CARP-1 mimetic, CFM-4.17 to improve systemic exposure and response in osimertinib resistant non-small cell lung cancer. Eur. J. Pharm. Biopharm. 2021, 158, 172–184. [Google Scholar] [CrossRef]
- Haynes, A.; Shaik, M.S.; Krarup, H.; Singh, M. Evaluation of the Malvern Spraytec® with inhalation cell for the measurement of particle size distribution from metered dose inhalers. J. Pharm. Sci. 2004, 93, 349–363. [Google Scholar] [CrossRef]
- Babu, R.; Kanikkannan, N.; Kikwai, L.; Ortega, C.; Andega, S.; Ball, K.; Yim, S.; Singh, M. The influence of various methods of cold storage of skin on the permeation of melatonin and nimesulide. J. Control. Release 2003, 86, 49–57. [Google Scholar] [CrossRef]
- Marepally, S.; Boakye, C.H.; Patel, A.R.; Godugu, C.; Doddapaneni, R.; Desai, P.R.; Singh, M. Topical administration of dual siRNAs using fusogenic lipid nanoparticles for treating psoriatic-like plaques. Nanomedicine 2014, 9, 2157–2174. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; Ramirez, M.D.; Neelakantan, H.; Walker, E.A. Cannabidiol Prevents the Development of Cold and Mechanical Allodynia in Paclitaxel-Treated Female C57Bl6 Mice. Obstet. Anesth. Dig. 2011, 113, 947–950. [Google Scholar] [CrossRef]
- Saleh, A.; Schapansky, J.; Smith, D.R.; Young, N.; Odero, G.L.; Aulston, B.; Fernyhough, P.; Glazner, G.W. Normalization of NF-κB activity in dorsal root ganglia neurons cultured from diabetic rats reverses neuropathy-linked markers of cellular pathology. Exp. Neurol. 2013, 241, 169–178. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Yerra, V.G.; Sherkhane, B.; Gundu, C.; Arruri, V.; Kumar, R.; Kumar, A. Chronic hyperglycemia impairs mitochondrial unfolded protein response and precipitates proteotoxicity in experimental diabetic neuropathy: Focus on LonP1 mediated mitochondrial regulation. Pharmacol. Rep. 2020, 72, 1627–1644. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Lee, J.B.; Deshpande, K.; Garbuzenko, O.B.; Minko, T.; Kagan, L. Prevention of paclitaxel-induced neuropathy by formulation approach. J. Control. Release 2019, 303, 109–116. [Google Scholar] [CrossRef]
- Yerra, V.G.; Kalvala, A.K.; Kumar, A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J. Nutr. Biochem. 2017, 47, 41–52. [Google Scholar] [CrossRef]
- Martinov, T.; Mack, M.; Sykes, A.; Chatterjea, D. Measuring Changes in Tactile Sensitivity in the Hind Paw of Mice Using an Electronic von Frey Apparatus. J. Vis. Exp. 2013, 82, e51212. [Google Scholar]
- Singh, M.; Ghose, T.; Faulkner, G.; Kralovec, J.; Mezei, M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Cancer Res. 1989, 49, 3976–3984. [Google Scholar]
- Hedrick, E.; Lee, S.-O.; Doddapaneni, R.; Singh, M.; Safe, S. NR4A1 Antagonists Inhibit β1-Integrin-Dependent Breast Cancer Cell Migration. Mol. Cell. Biol. 2016, 36, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Bheereddy, P.; Yerra, V.G.; Kalvala, A.K.; Sherkhane, B.; Kumar, A. SIRT1 activation by polydatin alleviates oxidative damage and elevates mitochondrial biogenesis in experimental diabetic neuropathy. Cell. Mol. Neurobiol. 2020, 41, 1563–1577. [Google Scholar] [CrossRef]
- Demanèche, S.; Chapel, J.P.; Monrozier, L.J.; Quiquampoix, H. Dissimilar pH-dependent adsorption features of bovine serum albumin and α-chymotrypsin on mica probed by AFM. Colloids Surf. B Biointerfaces 2009, 70, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, A.I.; Eskelinen, V.M.; Haikala, H.M.; Tervonen, T.A.; Yan, Y.; Partanen, J.I.; Klefström, J. Myc-induced AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 2013, 110, E1839–E1848. [Google Scholar] [CrossRef]
- Huang, L.; Kondo, F.; Gosho, M.; Feng, G.G.; Harato, M.; Xia, Z.Y.; Ishikawa, N.; Fujiwara, Y.; Okada, S. Enhanced expression of WD repeat-containing protein 35 via CaMKK/AMPK activation in bupivacaine-treated Neuro2a cells. PLoS ONE 2014, 9, e98185. [Google Scholar] [CrossRef] [PubMed]
- Bojanic, C.; To, K.; Zhang, B.; Mak, C.; Khan, W.S. Human umbilical cord derived mesenchymal stem cells in peripheral nerve regeneration. World J. Stem Cells 2020, 12, 288–302. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, M.; He, S.; Li, B.; Liu, C.; Min, J.; Hong, L. Effects of RSC96 Schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7841. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Frias, J.; Marcilla, A.; Galiano, A.; Simões, N. Identification of exosomes in the infective stage of entomopathogenic nematodes. J. Extracell. Vesicles 2018, 7, 251. [Google Scholar]
- Gounou, C.; Tan, S.; Arraud, N.; Brisson, A.R. Isolation of microvesicles and exosomes by fluorescence-triggered FACS. J. Extracell. Vesicles 2018, 7, 20–21. [Google Scholar]
- Ridolfi, A.; Brucale, M.; Montis, C.; Caselli, L.; Paolini, L.; Borup, A.; Boysen, A.T.; Loria, F.; van Herwijnen, M.J.C.; Kleinjan, M.; et al. AFM-Based High-Throughput Nanomechanical Screening of Single Extracellular Vesicles. Anal. Chem. 2020, 92, 10274–10282. [Google Scholar] [CrossRef]
- Gazze, S.A.; Thomas, S.J.; Garcia-Parra, J.; James, D.W.; Rees, P.; Marsh-Durban, V.; Corteling, R.; Gonzalez, D.; Conlan, R.S.; Francis, L.W. High content, quantitative AFM analysis of the scalable biomechanical properties of extracellular vesicles. Nanoscale 2021, 13, 6129–6141. [Google Scholar] [CrossRef]
- Hardij, J.; Cecchet, F.; Berquand, A.; Gheldof, D.; Chatelain, C.; Mullier, F.; Chatelain, B.; Dogné, J.-M. Characterisation of tissue factor-bearing extracellular vesicles with AFM: Comparison of air-tapping-mode AFM and liquid Peak Force AFM. J. Extracell. Vesicles 2013, 2, 21045. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Zoellner, H.; Paknejad, N.; Cornwell, J.; Chami, B.; Romin, Y.; Boykov, V.; Fujisawa, S.; Kelly, E.; Lynch, G.W.; Rogers, G.; et al. Cell-projection pumping: A hydrodynamic cell-stiffness dependent mechanism for cytoplasmic transfer between mammalian cells. bioRxiv 2019, 14, 531798. [Google Scholar]
- Leiva-Sabadini, C.; Alvarez, S.; Barrera, N.P.; Schuh, C.M.; Aguayo, S. Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides Against Oral Streptococci. Int. J. Nanomed. 2021, 16, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Alsteens, D.; Trabelsi, H.; Soumillion, P.; Dufrêne, Y.F. Multiparametric atomic force microscopy imaging of single bacteriophages extruding from living bacteria. Nat. Commun. 2013, 4, 2926. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; Tam, A.; Mukhopadhyay, D.; Bhattacharya, S. AFM study: Cell cycle and probe geometry influences nanomechanical characterization of Panc1 cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, Y.; Yang, R.; Liu, C.; Hsu, J.-M.; Jiang, Z.; Sun, L.; Wei, Y.; Li, C.-W.; Yu, D.; et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene 2021, 40, 4992–5001. [Google Scholar] [CrossRef]
- Shiue, S.-J.; Rau, R.-H.; Shiue, H.-S.; Hung, Y.-W.; Li, Z.-X.; Yang, K.D.; Cheng, J.-K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Yoon, T.G.; Kang, M.; Kim, H.J.; Kang, K.S. Effect of subcutaneous treatment with human umbilical cord blood-derived multipotent stem cells on peripheral neuropathic pain in rats. Korean J. Physiol. Pharmacol. 2017, 21, 153–160. [Google Scholar] [CrossRef]
- North, R.Y.; Li, Y.; Ray, P.; Rhines, L.D.; Tatsui, C.E.; Rao, G.; Johansson, C.A.; Zhang, H.; Kim, Y.H.; Zhang, B.; et al. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 2019, 142, 1215–1226. [Google Scholar] [CrossRef]
- Livni, L.; Lees, J.G.; Barkl-Luke, M.E.; Goldstein, D.; Moalem-Taylor, G. Dorsal root ganglion explants derived from chemotherapy-treated mice have reduced neurite outgrowth in culture. Neurosci. Lett. 2019, 694, 14–19. [Google Scholar] [CrossRef]
- Sahenk, Z.; Barohn, R.; New, P.; Mendell, J.R. Taxol neuropathy: Electrodiagnostic and sural nerve biopsy findings. Arch. Neurol. 1994, 51, 726–729. [Google Scholar] [CrossRef]
- A Argyriou, A.; Park, S.B.; Islam, B.; Tamburin, S.; Velasco, R.; Alberti, P.; Bruna, J.; Psimaras, D.; Cavaletti, G.; Cornblath, D.R. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1361–1369. [Google Scholar] [CrossRef]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK Inhibitor Compound C Is a Potent AMPK-Independent Antiglioma AgentAntiglioma Actions of Compound C. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.; Flatters, S. Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain 2017, 158, 1499. [Google Scholar] [CrossRef]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.-S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef]
- Zhou, Q.; Xie, M.; Zhu, J.; Yi, Q.; Tan, B.; Li, Y.; Ye, L.; Zhang, X.; Zhang, Y.; Tian, J.; et al. PINK1 contained in huMSC-derived exosomes prevents cardiomyocyte mitochondrial calcium overload in sepsis via recovery of mitochondrial Ca2+ efflux. Stem Cell Res. Ther. 2021, 12, 269. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Ma, C.; Lyu, Y.; Ma, X.; Ma, X. Exosomes derived from human placental mesenchymal stem cells reduce human lung microvascular endothelial cell injury induced by lipopolysaccharide via enhancing autophagy. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2021, 37, 225–232. [Google Scholar]
- Gallily, R.; Yekhtin, Z.; Tarshis, M.; Sionov, R.V. Cannabidiol (CBD) Prevents Palmitic Acid-Induced Drop in Mitochondrial Membrane Potential. Pharmacol. Pharm. 2019, 10, 387–395. [Google Scholar] [CrossRef]
- Brenneman, D.E.; Kinney, W.A.; Ward, S.J. Knockdown siRNA Targeting the Mitochondrial Sodium-Calcium Exchanger-1 Inhibits the Protective Effects of Two Cannabinoids Against Acute Paclitaxel Toxicity. J. Mol. Neurosci. 2019, 68, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Inyang, K.E.; McDougal, T.A.; Ramirez, E.D.; Williams, M.; Laumet, G.; Kavelaars, A.; Heijnen, C.J.; Burton, M.; Dussor, G.; Price, T.J. Alleviation of paclitaxel-induced mechanical hypersensitivity and hyperalgesic priming with AMPK activators in male and female mice. Neurobiol. Pain 2019, 6, 100037. [Google Scholar] [CrossRef]
- Larsson, N.-G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef]
- Trecarichi, A.; Flatters, S. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int. Rev. Neurobiol. 2019, 145, 83–126. [Google Scholar]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Arbab, A.A.I.; Lu, X.; Abdalla, I.M.; Idris, A.A.; Chen, Z.; Li, M.; Mao, Y.; Xu, T.; Yang, Z. Metformin Inhibits Lipoteichoic Acid–Induced Oxidative Stress and Inflammation Through AMPK/NRF2/NF-κB Signaling Pathway in Bovine Mammary Epithelial Cells. Front. Vet. Sci. 2021, 8, 661380. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Q.; Yang, B.; Xu, H.; Nasif, O.; Muruganantham, S.; Chen, J. Phyllanthin Averts Oxidative Stress and Neuroinflammation in Cerebral Ischemic-Reperfusion Injury through Modulation of the NF-κB and AMPK/Nrf2 Pathways. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 85–97. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Thampi, P.; Rao, H.V.; Mitter, S.K.; Cai, J.; Mao, H.; Li, H.; Seo, S.; Qi, X.; Lewin, A.S.; Romano, C.; et al. The 5HT1a Receptor Agonist 8-Oh DPAT Induces Protection from Lipofuscin Accumulation and Oxidative Stress in the Retinal Pigment Epithelium. PLoS ONE 2012, 7, e34468. [Google Scholar] [CrossRef]
- Pascual, D.; Goicoechea, C.; Suardíaz, M.; Martín, M.I. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 2005, 118, 23–34. [Google Scholar] [CrossRef]
- Do Monte, F.H.; Souza, R.R.; Bitencourt, R.M.; Kroon, J.A.; Takahashi, R.N. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav. Brain Res. 2013, 250, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, O.; Ramos, J.A.; Decio, A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur. J. Neurosci. 2007, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; E Ponde, D.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Normal Control | PTX | PTX + EVs | PTX + CBD (5 mg/kg) | PTX + EVs + CBD | PTX + CBD-EVs (5 mg/kg) |
|---|---|---|---|---|---|---|
| Paw withdrawal latency to hot stimuli (s) | 10.86 ± 0.36 | 4.05 ± 0.31 *** | 6.41 ± 0.98 ^ | 7.3 ± 0.4 ^^ | 6.93 ± 0.82 ^^ | 9.8 ± 0.66 ^^^ |
| Paw withdrawal latency to cold stimuli (s) | 13.43 ± 0.43 | 5.78 ± 0.24 *** | 7.9 ± 0.61 ^ | 8.1 ± 0.31 ^^ | 8.23 ± 0.93 ^^ | 10.09 ± 0.8 ^^^ |
| Paw withdrawal latency (s) | 10.89 ± 0.40 | 3.85 ± 0.8 *** | 5.99 ± 0.91 ^ | 6.86 ± 0.9 ^ | 6.79 ± 0.79 ^ | 8.10 ± 0.45 ^^^ |
| Paw withdrawal threshold (g) | 4.63 ± 0.24 | 1.91 ± 0.36 *** | 2.97 ± 0.6 ^ | 3.13 ± 0.1 ^ | 3.11 ± 0.87 ^ | 4.19 ± 0.7 ^^^ |
| Average Body weight (g) | 23.18 ± 1.11 | 22.65 ± 1.23 | 23.85 ± 1.99 | 23.99 ± 1.20 | 23.03 ± 1.08 | 23.21 ± 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalvala, A.K.; Bagde, A.; Arthur, P.; Kulkarni, T.; Bhattacharya, S.; Surapaneni, S.; Patel, N.K.; Nimma, R.; Gebeyehu, A.; Kommineni, N.; et al. Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics 2023, 15, 554. https://doi.org/10.3390/pharmaceutics15020554
Kalvala AK, Bagde A, Arthur P, Kulkarni T, Bhattacharya S, Surapaneni S, Patel NK, Nimma R, Gebeyehu A, Kommineni N, et al. Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics. 2023; 15(2):554. https://doi.org/10.3390/pharmaceutics15020554
Chicago/Turabian StyleKalvala, Anil Kumar, Arvind Bagde, Peggy Arthur, Tanmay Kulkarni, Santanu Bhattacharya, Sunil Surapaneni, Nil Kumar Patel, Ramesh Nimma, Aragaw Gebeyehu, Nagavendra Kommineni, and et al. 2023. "Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy" Pharmaceutics 15, no. 2: 554. https://doi.org/10.3390/pharmaceutics15020554








