Surface-Enhanced Carboxyphenyl Diazonium Functionalized Screen-Printed Carbon Electrode for the Screening of Tuberculosis in Sputum Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
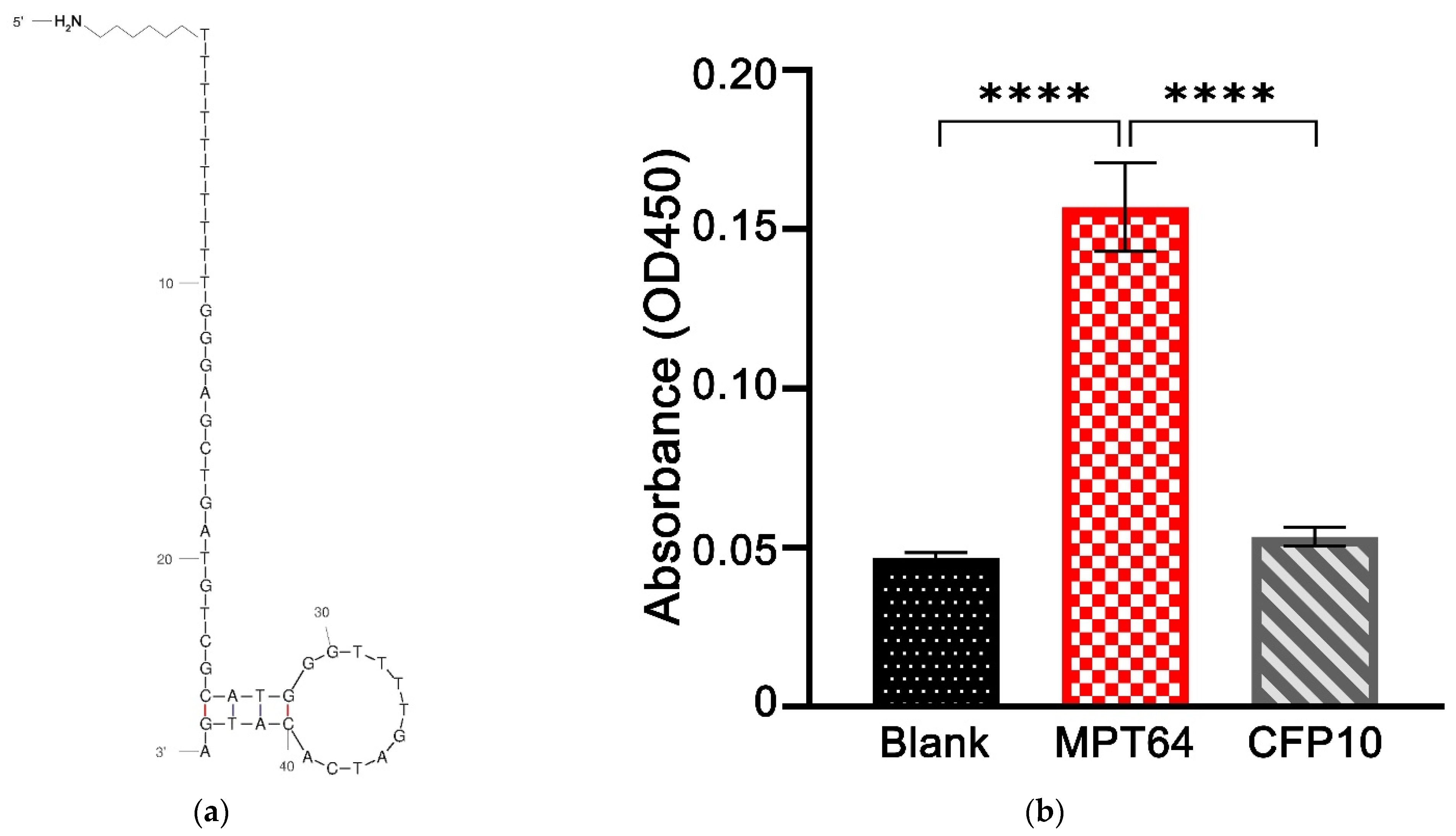
2.3. Binding Assay of MPT64 Aptamer
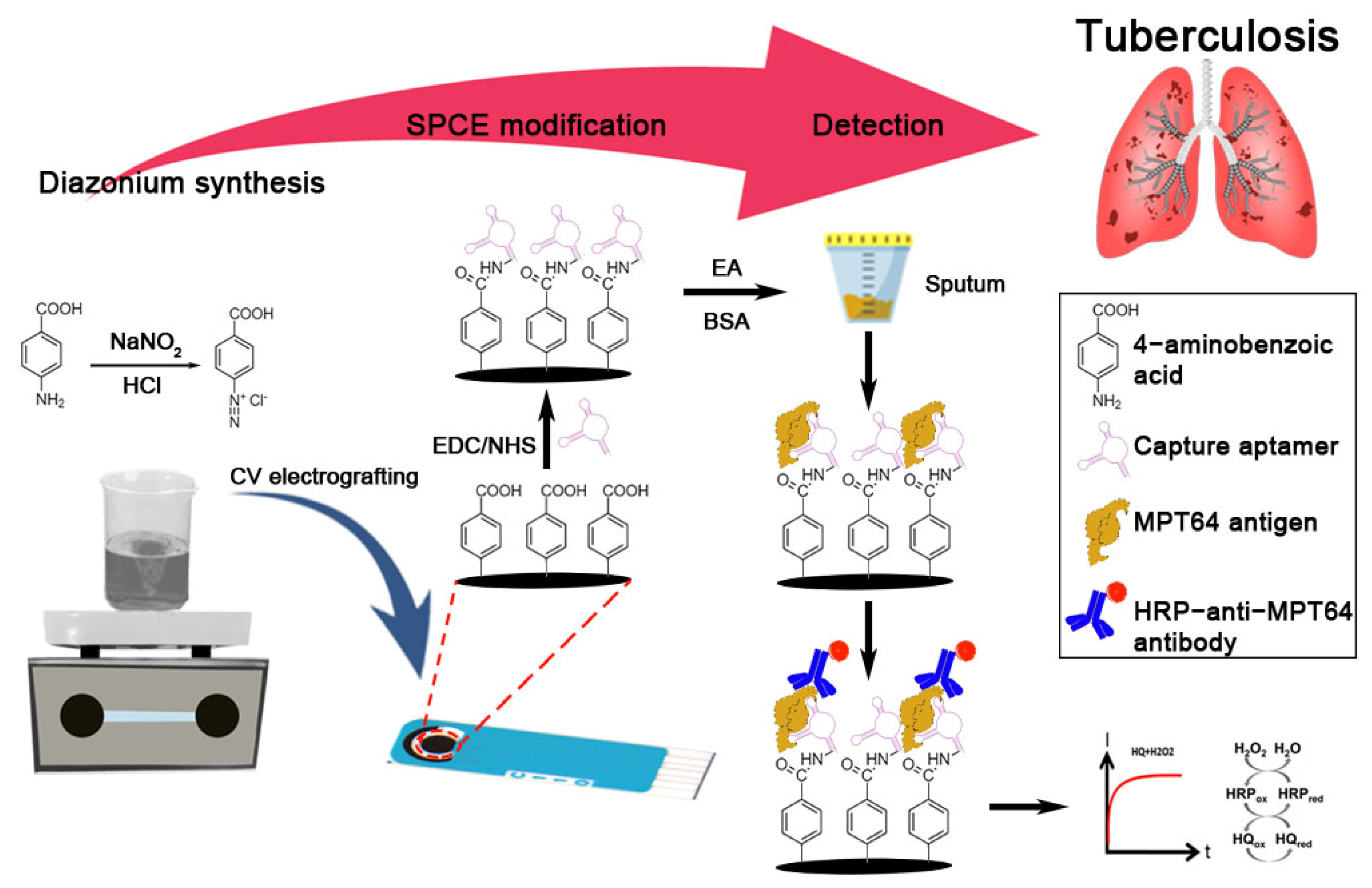
2.4. In Situ Electrografting of Carboxyphenyl Film on the Carbon Electrode and Immobilization of MPT64 Aptamer
2.5. Characterization of Carboxyphenyl Diazonium-Modified Electrode
2.6. Detection Studies
2.7. MPT64 Antigen Detection in Spiked Serum
2.8. Clinical Sample Analysis
2.9. Data Analysis
3. Results
3.1. Principle of Aptasensor
3.2. Binding Assay of the MPT64 Aptamer
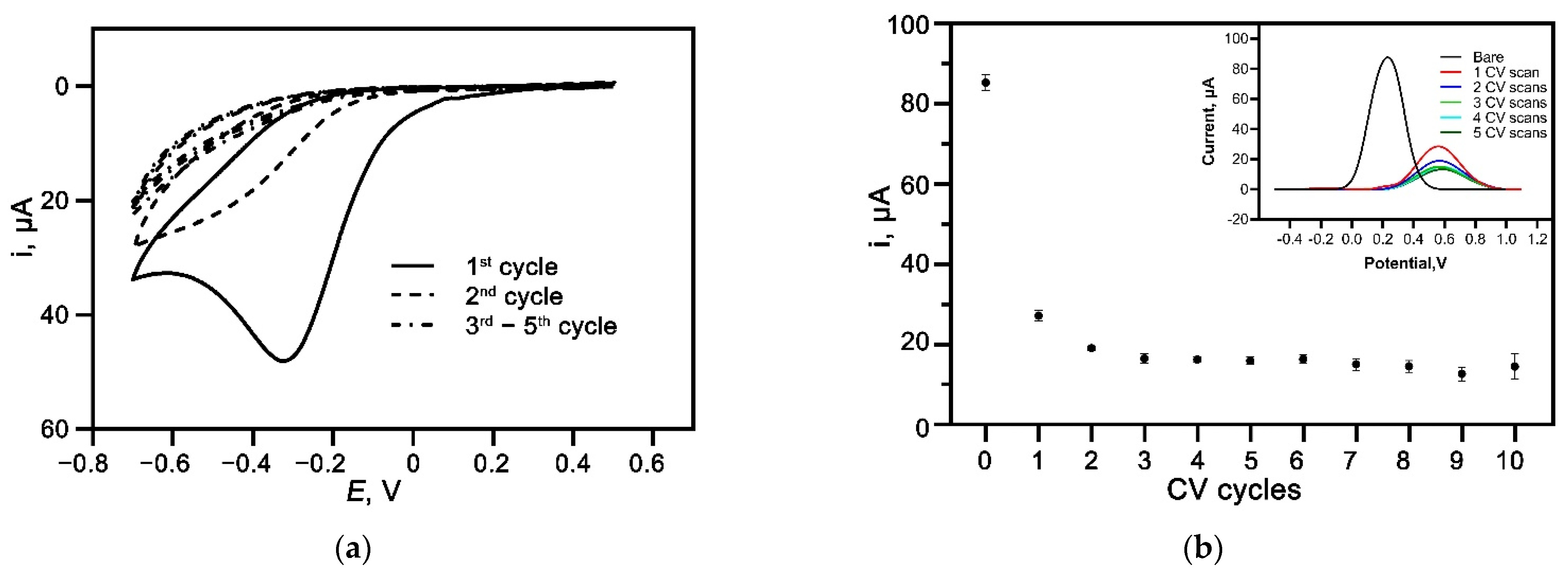
3.3. Electrografting of Diazonium Salt of 4-aminobenzoic Acid
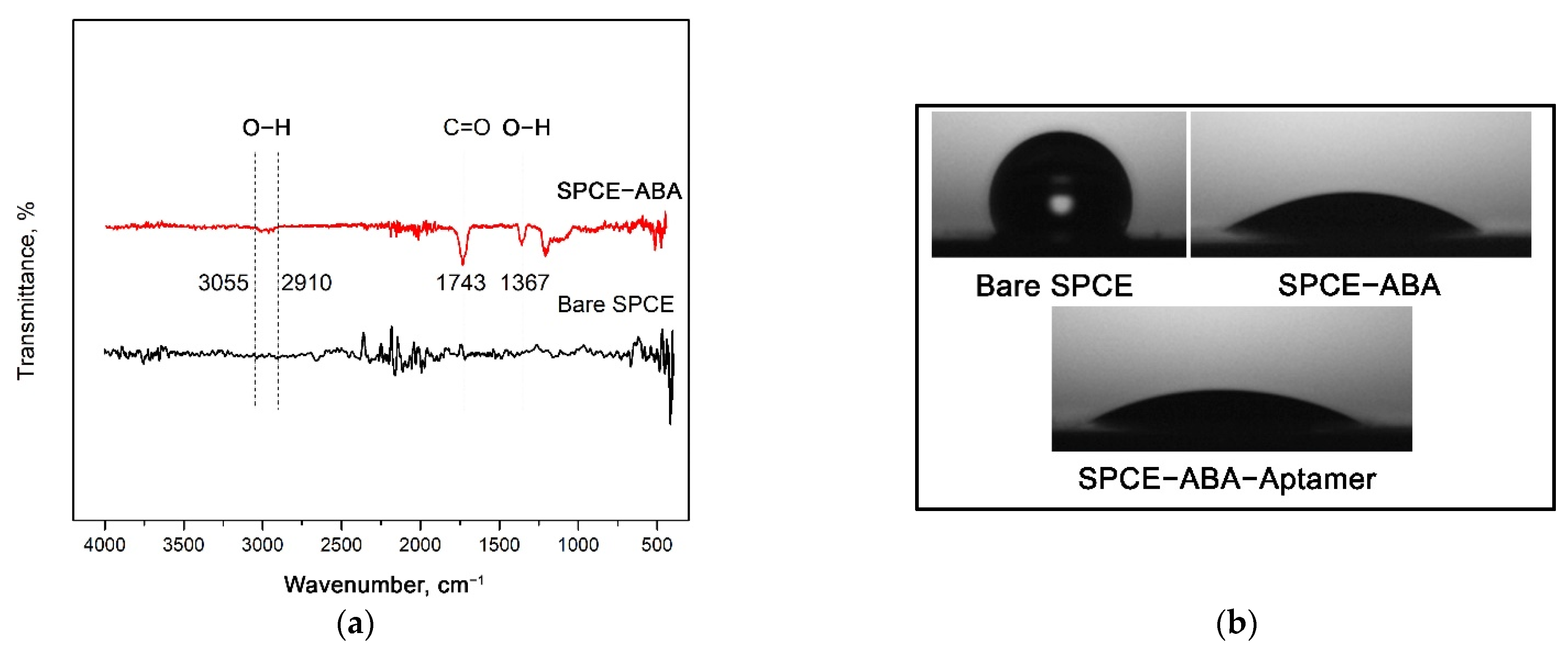
3.4. FTIR and Contact Angle Analyses of Carboxyphenyl Diazonium Film
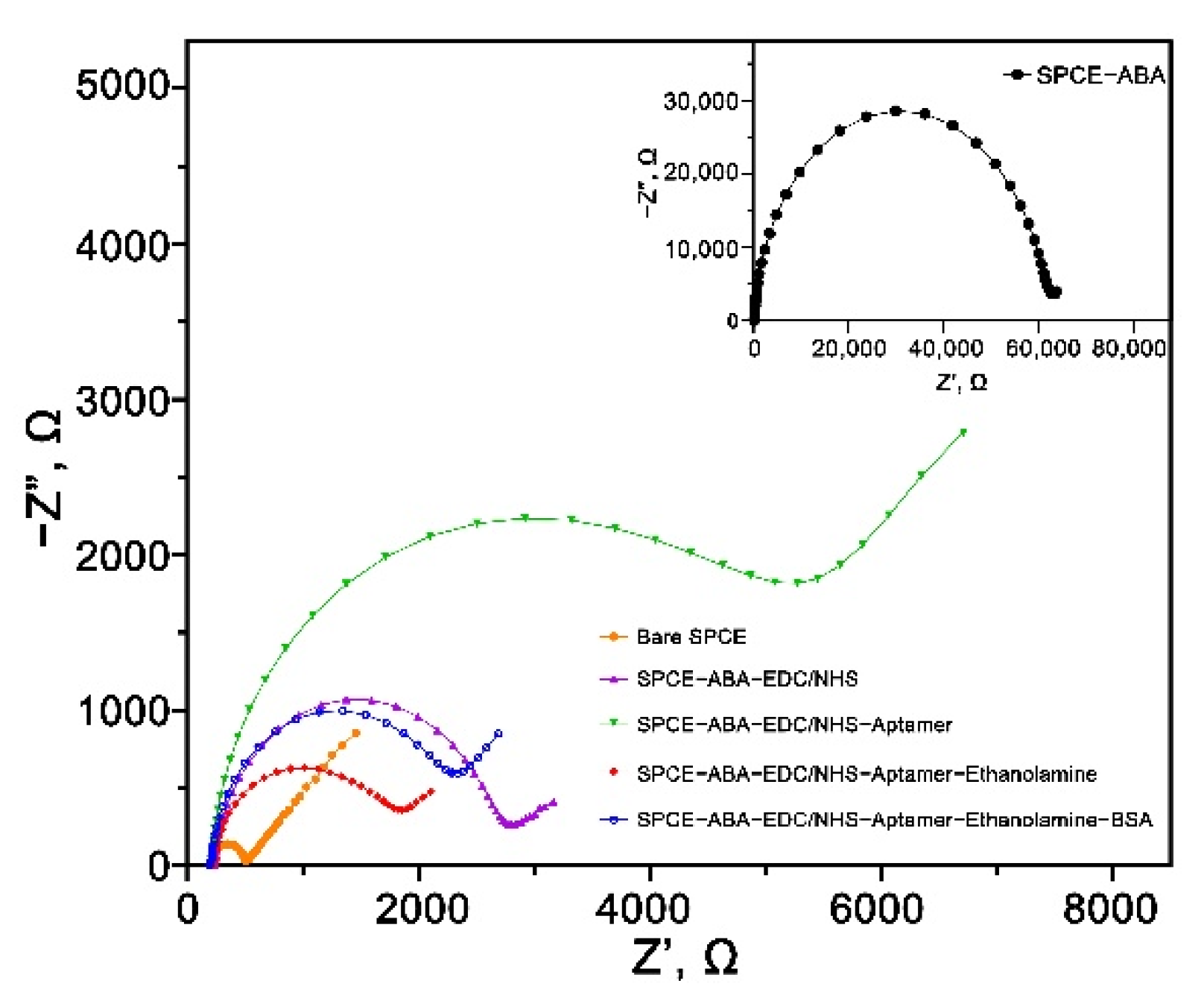
3.5. EIS Characterization of the Carboxyphenyl Diazonium Modified Aptasensor
3.6. Optimization of Aptasensor Conditions
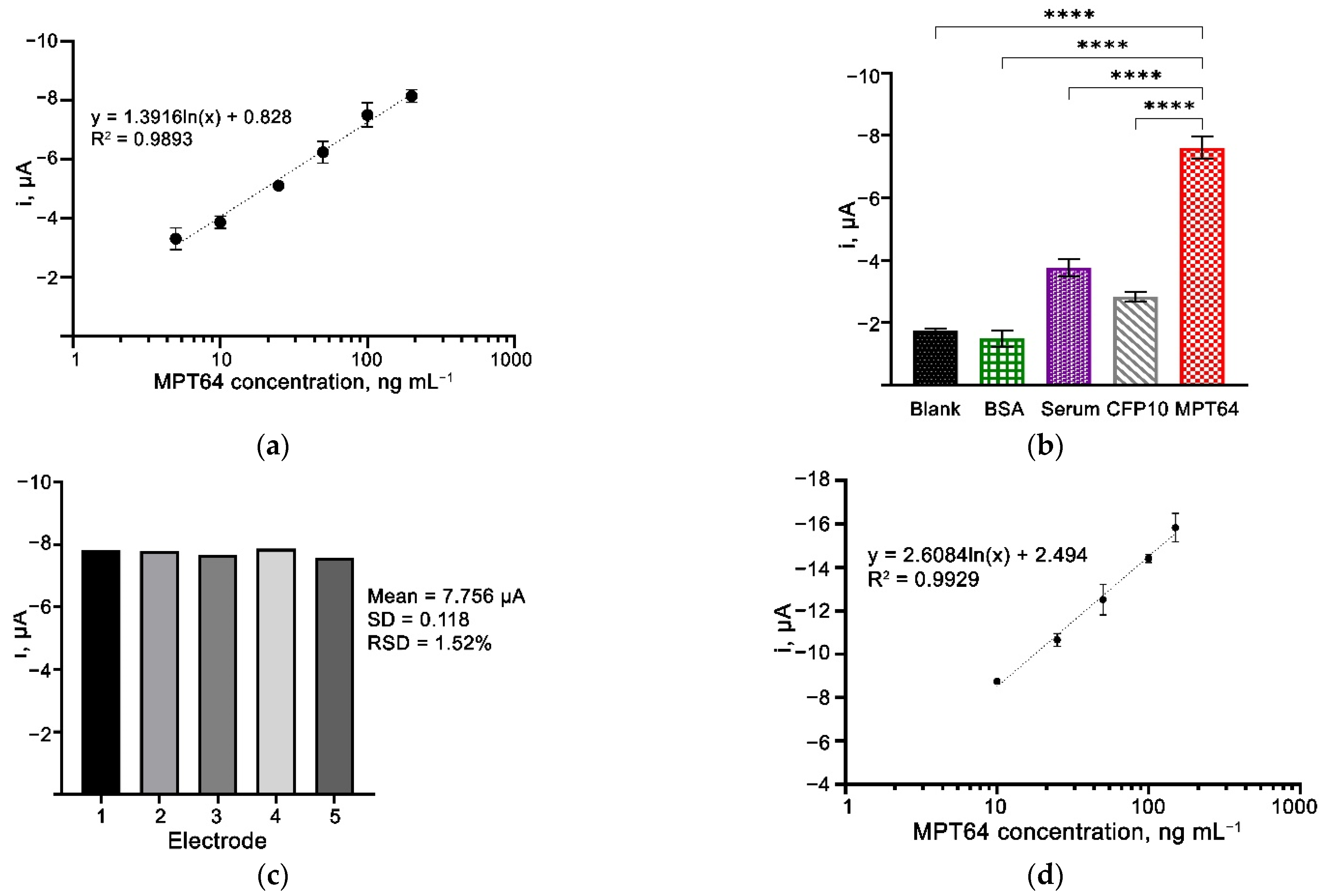
3.7. Analytical Performance of the Aptasensor
3.8. Selectivity and Reproducibility of the Aptasensor
3.9. Spiked-Sample Analysis
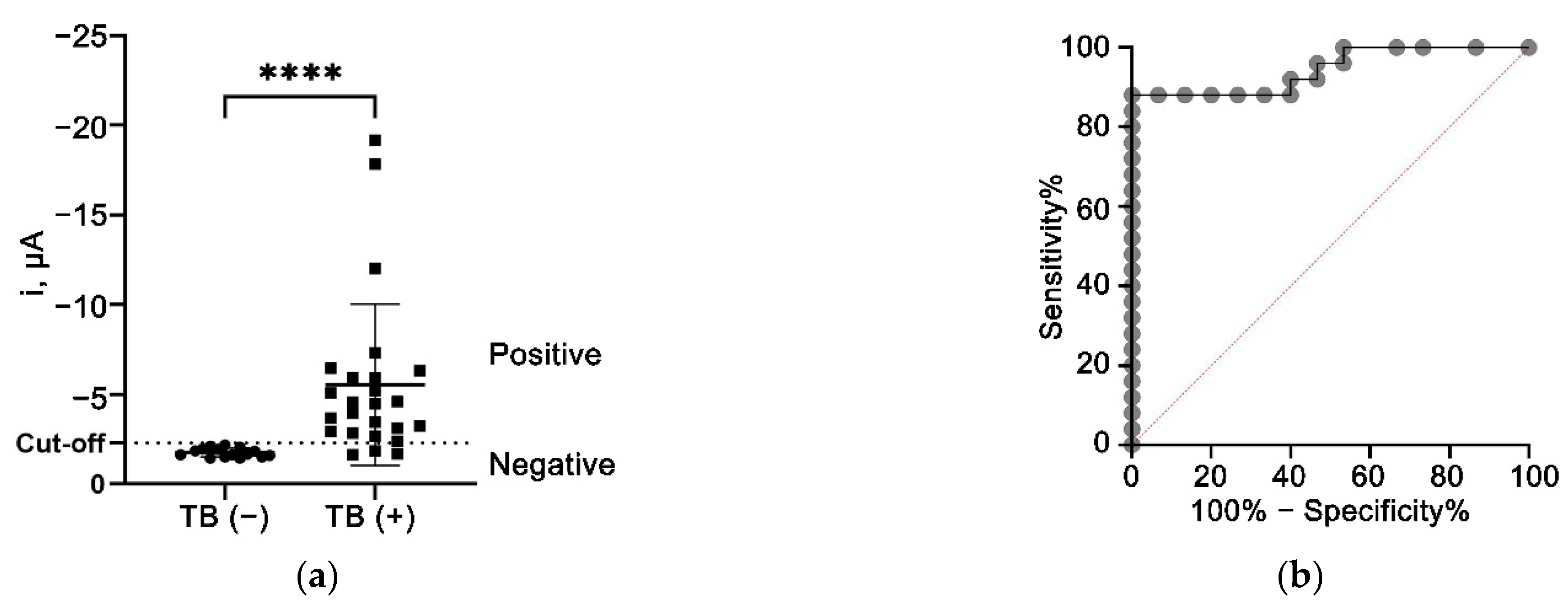
3.10. Clinical Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.K.; Mohan, A. Extrapulmonary Tuberculosis. In Mycobacterium Tuberculosis: Molecular Infection Biology, Pathogenesis, Diagnostics and New Interventions; Hasnain, S.E., Ehtesham, N.Z., Grover, S., Eds.; Springer: Singapore, 2019; pp. 37–53. ISBN 97898-13294134. [Google Scholar]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2021; Available online: http://apps.who.int/iris (accessed on 20 January 2022).
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor Nanoengineering: Design, Operation, and Implementation for Biomolecular Analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. A Graphene-Based Electrochemical Competitive Immunosensor for the Sensitive Detection of Okadaic Acid in Shellfish. Nanoscale 2012, 4, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.H.; Yusof, N.A.; Raston, N.H.A.; Noor, S.S.M.; Sulaiman, Y.; Abdullah, J. A Novel Amperometric Aptamer-Antibody Sandwich Assay for the Detection of Tuberculosis with Diazonium Electrografted Enhanced Modified Electrode. IEEE Sens. J. 2021, 21, 22442–22449. [Google Scholar] [CrossRef]
- Mousavisani, S.Z.; Raoof, J.B.; Turner, A.P.F.; Ojani, R.; Mak, W.C. Label-Free DNA Sensor Based on Diazonium Immobilisation for Detection of DNA Damage in Breast Cancer 1 Gene. Sens. Actuators B Chem. 2018, 264, 59–66. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Atasiei, R.; Hanganu, A.; Vasile, E.; Brezoiu, A.M.; Pilan, L. Organic Layers via Aryl Diazonium Electrochemistry: Towards Modifying Platinum Electrodes for Interference Free Glucose Biosensors. Electrochim. Acta 2016, 206, 226–237. [Google Scholar] [CrossRef]
- Zhou, W.; Jimmy Huang, P.J.; Ding, J.; Liu, J. Aptamer-Based Biosensors for Biomedical Diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [Green Version]
- Sypabekova, M.; Dukenbayev, K.; Tsepke, A.; Akisheva, A.; Oralbayev, N.; Kanayeva, D. An Aptasensor for the Detection of Mycobacterium Tuberculosis Secreted Immunogenic Protein MPT64 in Clinical Samples towards Tuberculosis Detection. Sci. Rep. 2019, 9, 16273. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yuan, Y.; Chen, Y.; Zhang, P.; Bai, Y.; Bai, L. Aptamer Based Voltammetric Biosensor for Mycobacterium Tuberculosis Antigen ESAT-6 Using a Nanohybrid Material Composed of Reduced Graphene Oxide and a Metal-Organic Framework. Mikrochim. Acta 2018, 185, 379. [Google Scholar] [CrossRef]
- Thakur, H.; Kaur, N.; Sabherwal, P.; Sareen, D.; Prabhakar, N. Aptamer Based Voltammetric Biosensor for the Detection of Mycobacterium Tuberculosis Antigen MPT64. Microchim. Acta 2017, 184, 1915–1922. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Guo, S.; Cao, J.; Zhou, J.; Zuo, J.; Bai, L. A Sandwich-Type Electrochemical Aptasensor for Mycobacterium Tuberculosis MPT64 Antigen Detection Using C60NPs Decorated N-CNTs/GO Nanocomposite Coupled with Conductive PEI-Functionalized Metal-Organic Framework. Biomaterials 2019, 216, 119253. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.; Sun, D.; Yu, W.; Tan, W.; Luo, Z.; Chen, Z. Dual-Aptamer-Based Voltammetric Biosensor for the Mycobacterium Tuberculosis Antigen MPT64 by Using a Gold Electrode Modified with a Peroxidase Loaded Composite Consisting of Gold Nanoparticles and a Zr(IV)/Terephthalate Metal-Organic Framework. Microchim. Acta 2018, 185, 543. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, J.; Liu, M.; Wu, Y.; Shen, Z.; Li, G. Sensitive Detection of Human Breast Cancer Cells Based on Aptamer–Cell–Aptamer Sandwich Architecture. Anal. Chim. Acta 2013, 764, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Ghibaudi, E.; Todaro, G.; Pia Ferrari, R. Enzymatic Degradation of 2,6-Dichlorophenol by Horseradish Peroxidase: UV–Visible and Mass Spectrophotometric Characterization of the Reaction Products. J. Inorg. Biochem. 2002, 92, 75–81. [Google Scholar] [CrossRef]
- Ferrari, R.P.; Laurenti, E.; Trotta, F.; Ferrari, R.P.; Laurenti, E.; Trotta, F. Oxidative 4-Dechlorination of 2,4,6-Trichlorophenol Catalyzed by Horseradish Peroxidase. JBIC J. Biol. Inorg. Chem. 1999, 4, 232–237. [Google Scholar] [CrossRef]
- Feyzizarnagh, H.; Park, B.W.; Sharma, L.; Patania, M.M.; Yoon, D.Y.; Kim, D.S. Amperometric Mediatorless Hydrogen Peroxide Sensor with Horseradish Peroxidase Encapsulated in Peptide Nanotubes. Sens. Biosens. Res. 2016, 7, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.-X.; Hu, S.-Q.; Gao, N.; Shen, G.-L.; Yu, R.-Q. An Amperometric Hydrogen Peroxide Biosensor Based on Immobilizing Horseradish Peroxidase to a Nano-Au Monolayer Supported by Sol–Gel Derived Carbon Ceramic Electrode. Bioelectrochemistry 2004, 65, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, T.; Li, J.; Chen, X. A Magnetic-Controlled Amperometric Biosensor Based on Composite Bio-Particulates Fe3O4 and Nano-Au with the Signal Enhancement by Increasing Loading of Horseradish Peroxidase. Int. J. Electrochem. Sci. 2011, 6, 4953–4966. [Google Scholar]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. Electrochemical Immunosensor for Cardiac Troponin I Detection Based on Covalent Organic Framework and Enzyme-Catalyzed Signal Amplification. Anal. Chem. 2021, 93, 13572–13579. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H. An Antifouling Interface Integrated with HRP-Based Amplification to Achieve a Highly Sensitive Electrochemical Aptasensor for Lysozyme Detection. Analyst 2019, 144, 5794–5801. [Google Scholar] [CrossRef]
- Ou, D.; Sun, D.; Lin, X.; Liang, Z.; Zhong, Y.; Chen, Z. A Dual-Aptamer-Based Biosensor for Specific Detection of Breast Cancer Biomarker HER2 via Flower-like Nanozymes and DNA Nanostructures. J. Mater. Chem. B 2019, 7, 3661–3669. [Google Scholar] [CrossRef]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Delamar, M.; Hitmi, R.; Pinson, J.; Saveant, J.M. Covalent Modification of Carbon Surfaces by Grafting of Functionalized Aryl Radicals Produced from Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1992, 114, 5883–5884. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A Powerful Method for Surface Modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Baranton, S.; Bélanger, D. Electrochemical Derivatization of Carbon Surface by Reduction of in Situ Generated Diazonium Cations. J. Phys. Chem. B 2005, 109, 24401–24410. [Google Scholar] [CrossRef] [PubMed]
- Combellas, C.; Jiang, D.E.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Steric Effects in the Reaction of Aryl Radicals on Surfaces. Langmuir 2009, 25, 286–293. [Google Scholar] [CrossRef]
- Laforgue, A.; Addou, T.; Bélanger, D. Characterization of the Deposition of Organic Molecules at the Surface of Gold by the Electrochemical Reduction of Aryldiazonium Cations. Langmuir 2005, 21, 6855–6865. [Google Scholar] [CrossRef]
- Ngoc Le, H.T.; Park, J.; Chinnadayyala, S.R.; Cho, S. Sensitive Electrochemical Detection of Amyloid Beta Peptide in Human Serum Using an Interdigitated Chain-Shaped Electrode. Biosens. Bioelectron. 2019, 144, 111694. [Google Scholar] [CrossRef]
- Sypabekova, M.; Jolly, P.; Estrela, P.; Kanayeva, D. Electrochemical Aptasensor Using Optimized Surface Chemistry for the Detection of Mycobacterium Tuberculosis Secreted Protein MPT64 in Human Serum. Biosens. Bioelectron. 2019, 123, 141–151. [Google Scholar] [CrossRef]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; del Valle, M.; Marty, J.L. A Novel Electrochemical Aptamer-Antibody Sandwich Assay for Lysozyme Detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Lavania, S.; Tyagi, S.; Dhiman, A.; Rath, D.; Anthwal, D.; Gupta, R.K.; Sharma, N.; Gadpayle, A.K.; Taneja, R.S.; et al. A Novel Aptamer-Based Test for the Rapid and Accurate Diagnosis of Pleural Tuberculosis. Anal. Biochem. 2019, 564–565, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azmi, U.Z.; Yusof, N.A.; Kusnin, N.; Abdullah, J.; Suraiya, S.; Ong, P.S.; Ahmad Raston, N.H.; Abd Rahman, S.F.; Mohamad Fathil, M.F. Sandwich Electrochemical Immunosensor for Early Detection of Tuberculosis Based on Graphene/Polyaniline-Modified Screen-Printed Gold Electrode. Sensors 2018, 18, 3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulaikha, U.; Azmi, M.; Yusof, N.A.; Abdullah, J.; Ainliah, S.; Ahmad, A.; Nabilah, F.; Faudzi, M.; Hanun, N.; Raston, A.; et al. Portable Electrochemical Immunosensor for Detection of Mycobacterium Tuberculosis Secreted Protein CFP10-ESAT6 in Clinical Sputum Samples. Microchim. Acta 2021, 188, 20. [Google Scholar] [CrossRef]
- Chutichetpong, P.; Cheeveewattanagul, N.; Srilohasin, P.; Rijiravanich, P.; Chaiprasert, A.; Surareungchai, W. Rapid Screening Drug Susceptibility Test in Tuberculosis Using Sandwich Electrochemical Immunosensor. Anal. Chim. Acta 2018, 1025, 108–117. [Google Scholar] [CrossRef]
- Díaz-González, M.; González-García, M.B.; Costa-García, A. Immunosensor for Mycobacterium Tuberculosis on Screen-Printed Carbon Electrodes. Biosens. Bioelectron. 2005, 20, 2035–2043. [Google Scholar] [CrossRef]
- Torati, S.R.; Reddy, V.; Yoon, S.S.; Kim, C. Electrochemical Biosensor for Mycobacterium Tuberculosis DNA Detection Based on Gold Nanotubes Array Electrode Platform. Biosens. Bioelectron. 2016, 78, 483–488. [Google Scholar] [CrossRef]
- Rotherham, L.S.; Maserumule, C.; Dheda, K.; Theron, J.; Khati, M. Selection and Application of SsDNA Aptamers to Detect Active TB from Sputum Samples. PLoS ONE 2012, 7, e46862. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Lee, J.; So, J.; Lee, J.B.; Lee, H.; Chang, Y.; Shin, S.; Jo, J.; Ban, C. Homogeneous Fluorescent Aptasensor for Active Tuberculosis Diagnosis by Direct Quantification of Circulating TB7.7 Based on Aptamer Beacon with Graphene Oxide. Sens. Actuators B Chem. 2020, 317, 128126. [Google Scholar] [CrossRef]
- Stamm, C.E.; Pasko, B.L.; Chaisavaneeyakorn, S.; Franco, L.H.; Nair, V.R.; Weigele, B.A.; Alto, N.M.; Shiloh, M.U. Screening Mycobacterium Tuberculosis Secreted Proteins Identifies Mpt64 as a Eukaryotic Membrane-Binding Bacterial Effector. mSphere 2019, 4, e00354-19. [Google Scholar] [CrossRef] [Green Version]
- Mehaffy, C.; Dobos, K.M.; Nahid, P.; Kruh-Garcia, N.A. Second Generation Multiple Reaction Monitoring Assays for Enhanced Detection of Ultra-Low Abundance Mycobacterium Tuberculosis Peptides in Human Serum. Clin. Proteom. 2017, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Feng, Y.; Duan, S.; Su, L.; Zhang, J.; He, F. Mycobacterium Tuberculosis Strain H37Rv Electrochemical Sensor Mediated by Aptamer and AuNPs–DNA. ACS Sens. 2019, 4, 849–855. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis: Rapid Diagnostics for Tuberculosis Detection; 2021 update; World Health Organization: Geneva, Switzerland, 2021; ISBN 97892-40029415. [Google Scholar]

| Technique | Target | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Amperometry | CFP10 | 5–500 ng mL−1 | 1.22 ng mL−1 | [5] |
| DPV | CFP10 | 20–100 ng mL−1 | 15 ng mL−1 | [34] |
| DPV | CFP10-ESAT6 | 10–500 ng mL−1 | 1.5 ng mL−1 | [35] |
| Amperometry | MPT64 | 0.3–50 ng mL−1 | 0.43 ng mL−1 | [36] |
| SWV | Ag231 | 5–100 ng mL−1 | 1.0 ng mL−1 | [37] |
| CV | M. tuberculosis DNA | 0.001–100 ng µL−1 | 0.05 ng µL−1 | [38] |
| Amperometry | MPT64 | 5–200 ng mL−1 | 1.11 ng mL−1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunus, M.H.; Yusof, N.A.; Ismail, S.; Md Noor, S.S.; Mohammad, F.; Sulaiman, Y.; Ahmad Raston, N.H.; Abdullah, J.; Soleiman, A.A. Surface-Enhanced Carboxyphenyl Diazonium Functionalized Screen-Printed Carbon Electrode for the Screening of Tuberculosis in Sputum Samples. Nanomaterials 2022, 12, 2551. https://doi.org/10.3390/nano12152551
Yunus MH, Yusof NA, Ismail S, Md Noor SS, Mohammad F, Sulaiman Y, Ahmad Raston NH, Abdullah J, Soleiman AA. Surface-Enhanced Carboxyphenyl Diazonium Functionalized Screen-Printed Carbon Electrode for the Screening of Tuberculosis in Sputum Samples. Nanomaterials. 2022; 12(15):2551. https://doi.org/10.3390/nano12152551
Chicago/Turabian StyleYunus, Muhammad Hafiznur, Nor Azah Yusof, Suhainie Ismail, Siti Suraiya Md Noor, Faruq Mohammad, Yusran Sulaiman, Nurul Hanun Ahmad Raston, Jaafar Abdullah, and Ahmed A. Soleiman. 2022. "Surface-Enhanced Carboxyphenyl Diazonium Functionalized Screen-Printed Carbon Electrode for the Screening of Tuberculosis in Sputum Samples" Nanomaterials 12, no. 15: 2551. https://doi.org/10.3390/nano12152551
APA StyleYunus, M. H., Yusof, N. A., Ismail, S., Md Noor, S. S., Mohammad, F., Sulaiman, Y., Ahmad Raston, N. H., Abdullah, J., & Soleiman, A. A. (2022). Surface-Enhanced Carboxyphenyl Diazonium Functionalized Screen-Printed Carbon Electrode for the Screening of Tuberculosis in Sputum Samples. Nanomaterials, 12(15), 2551. https://doi.org/10.3390/nano12152551






