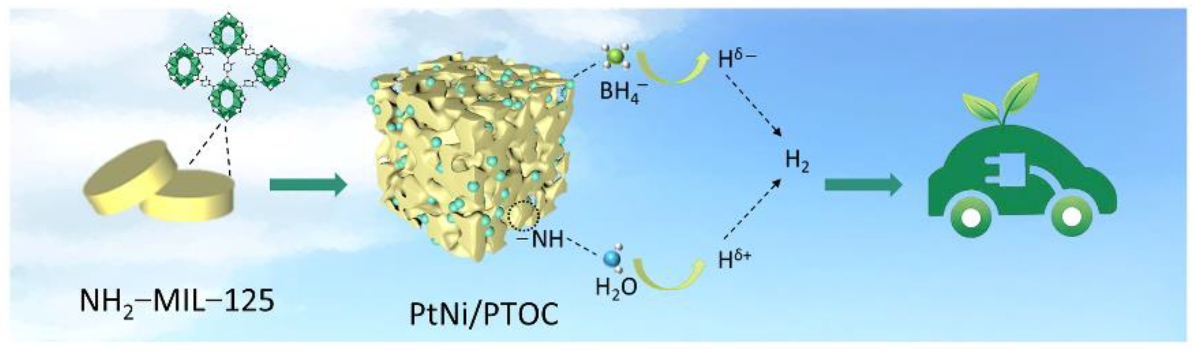
Bimetallic Pt-Ni Nanoparticles Confined in Porous Titanium Oxide Cage for Hydrogen Generation from NaBH4 Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NH2-MIL-125
2.3. Synthesis of PTOC
2.4. Preparation of PtNi/PTOC
2.5. Characterization
2.6. Hydrogen Production Testing
3. Results and Discussion
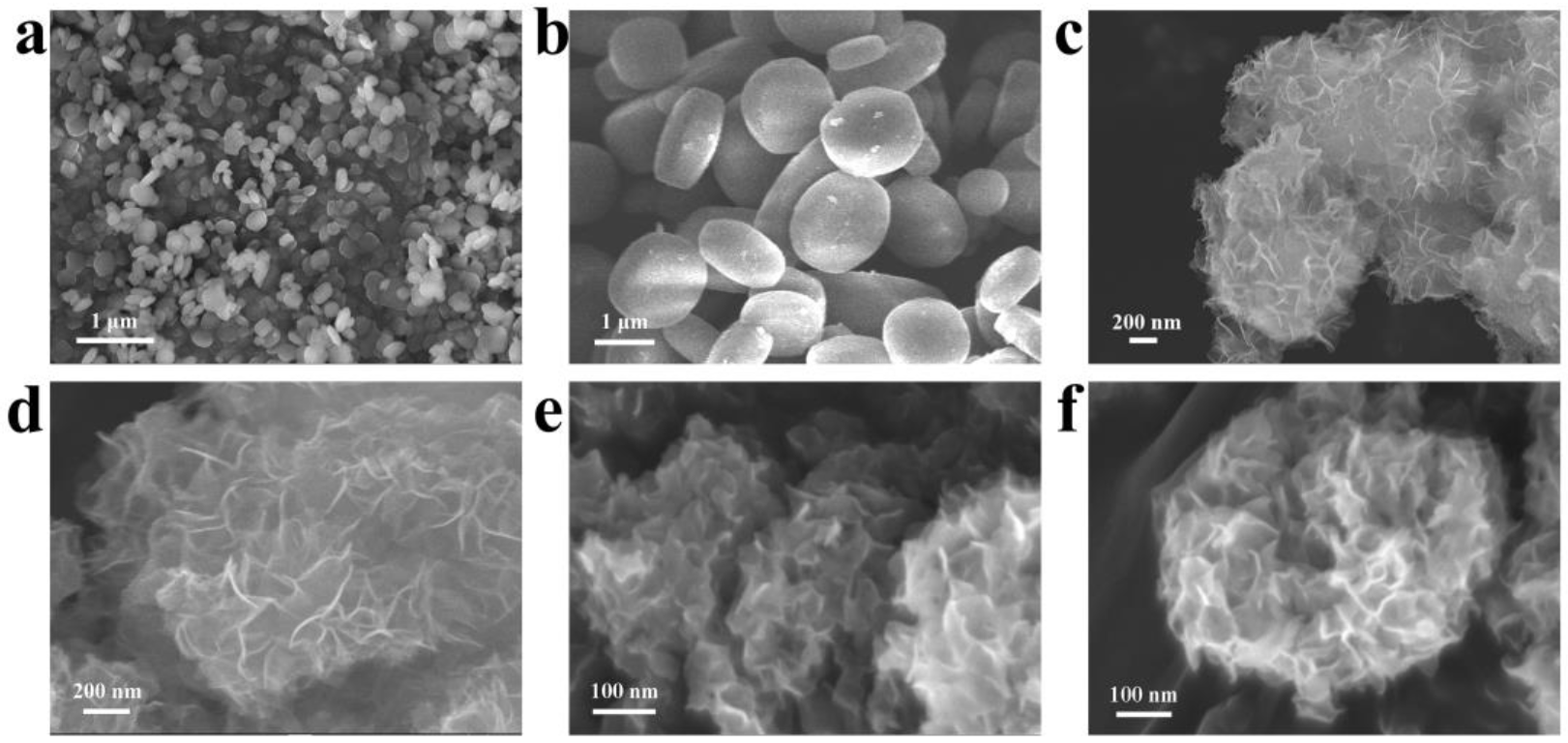
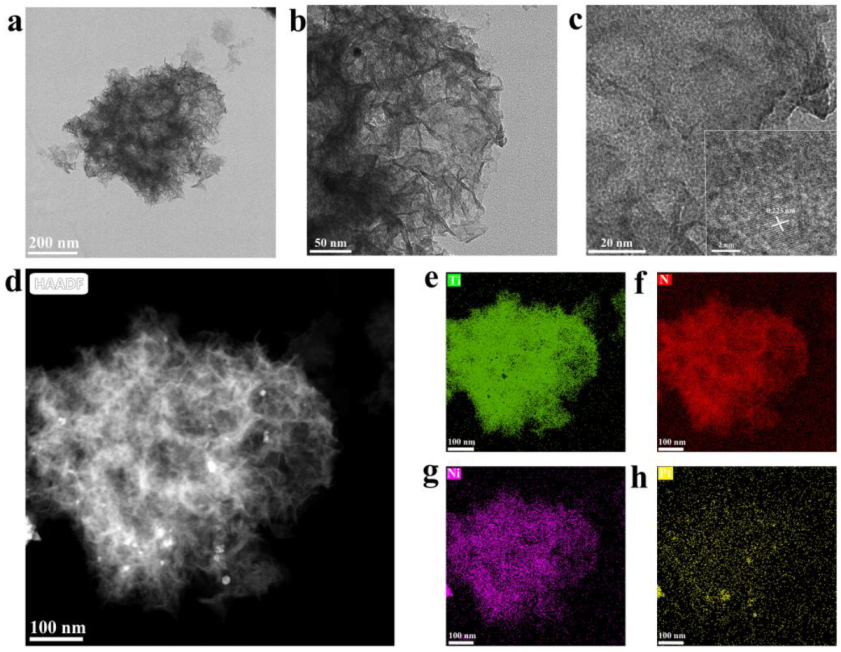
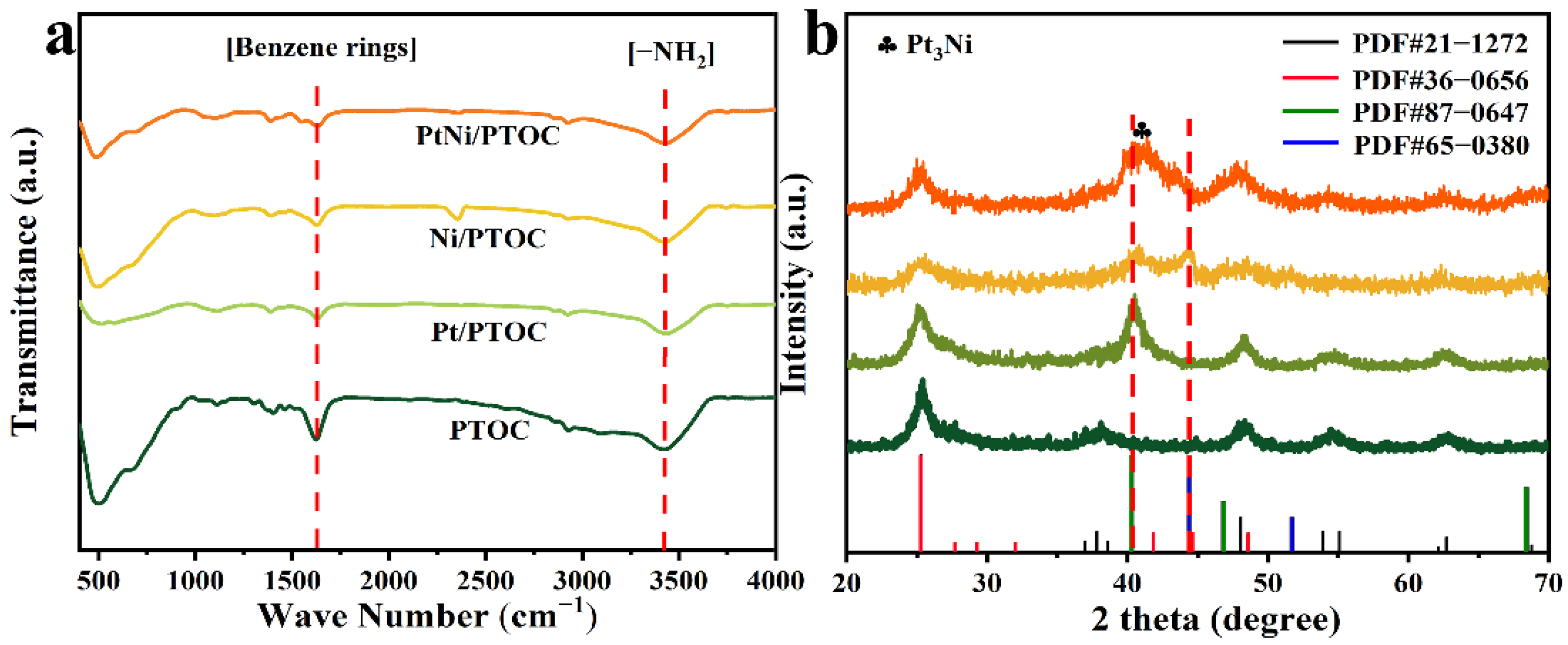
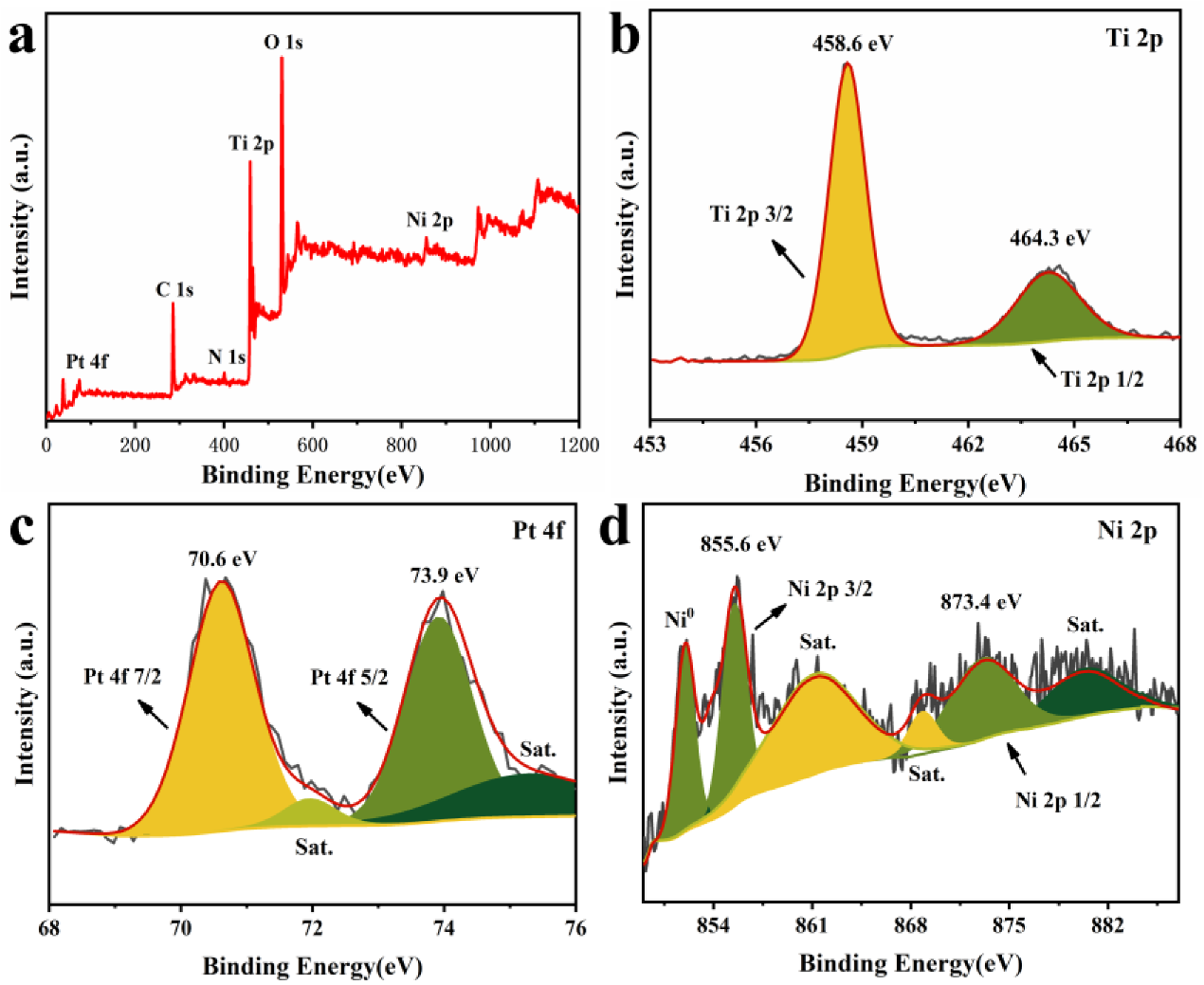
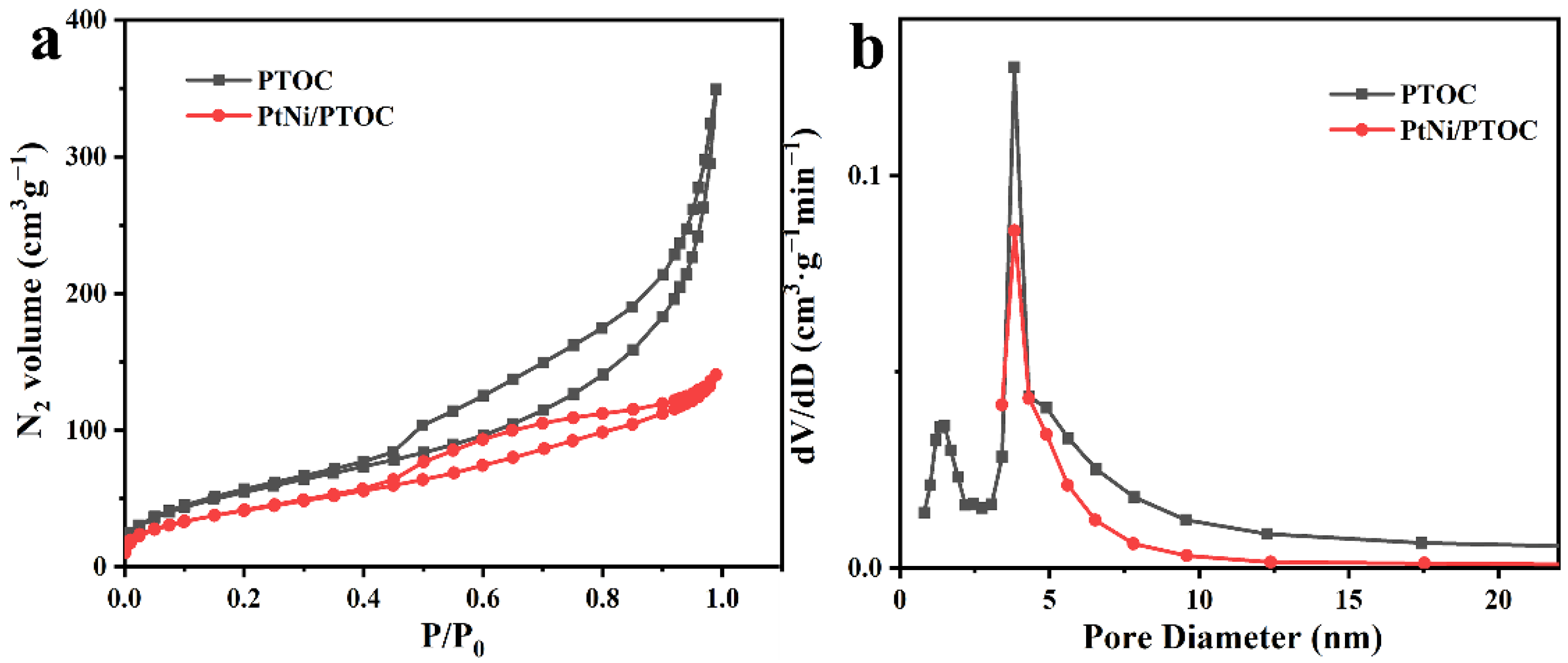
3.1. Catalyst Characterization
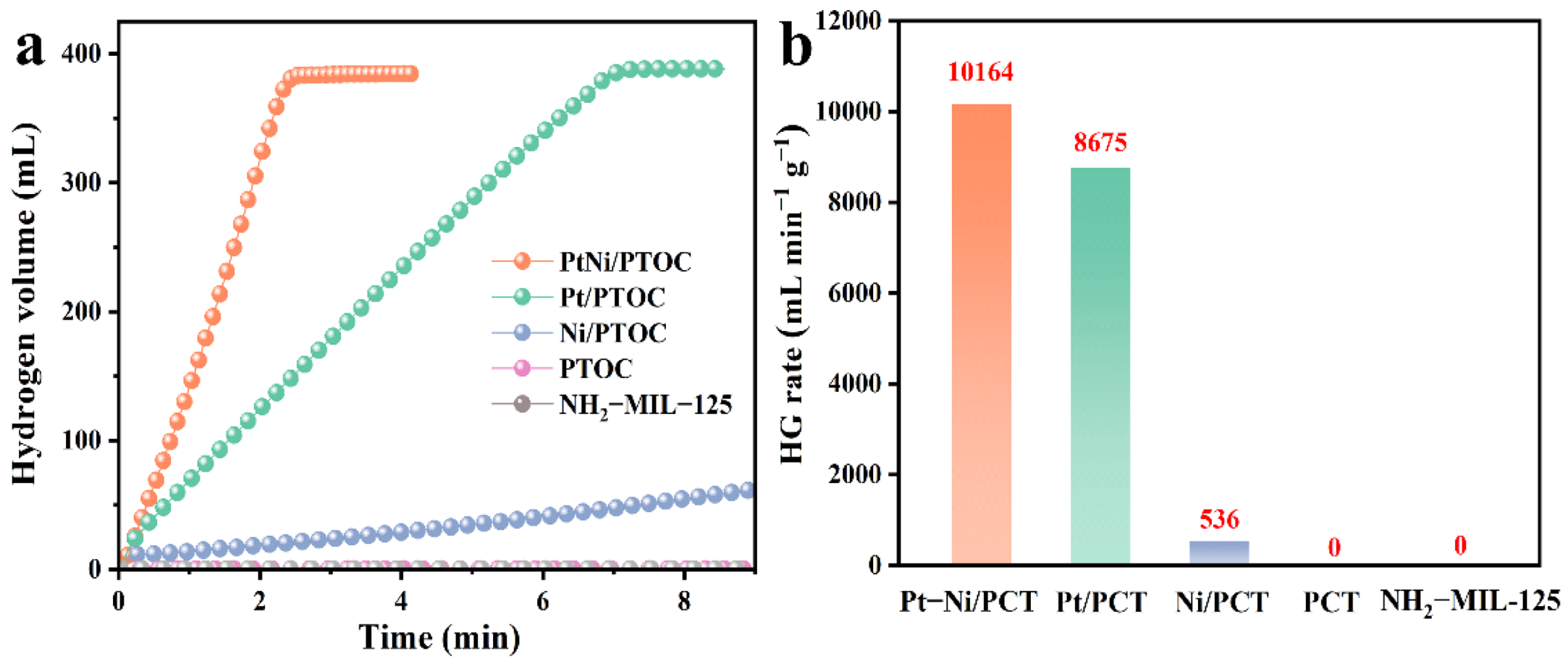
3.2. Effect of Different Types of Catalysts
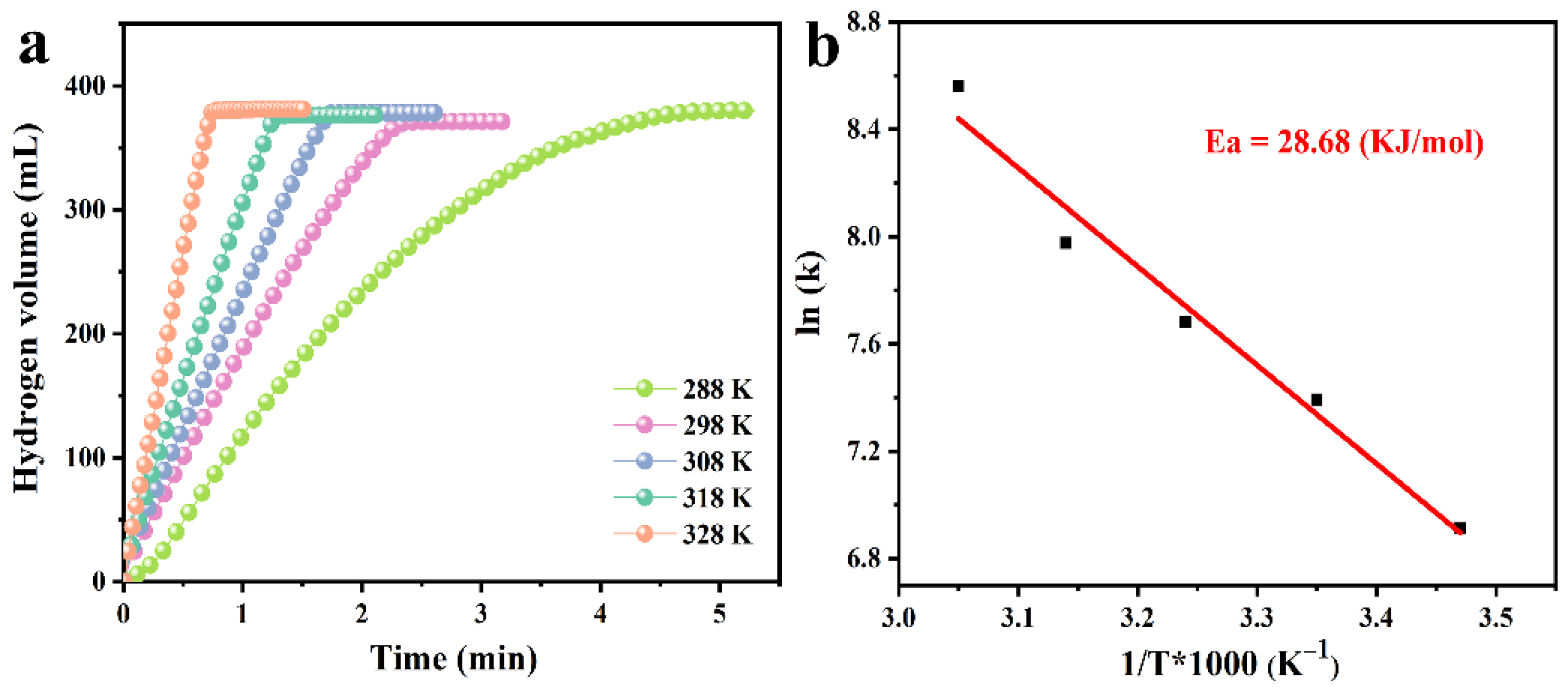
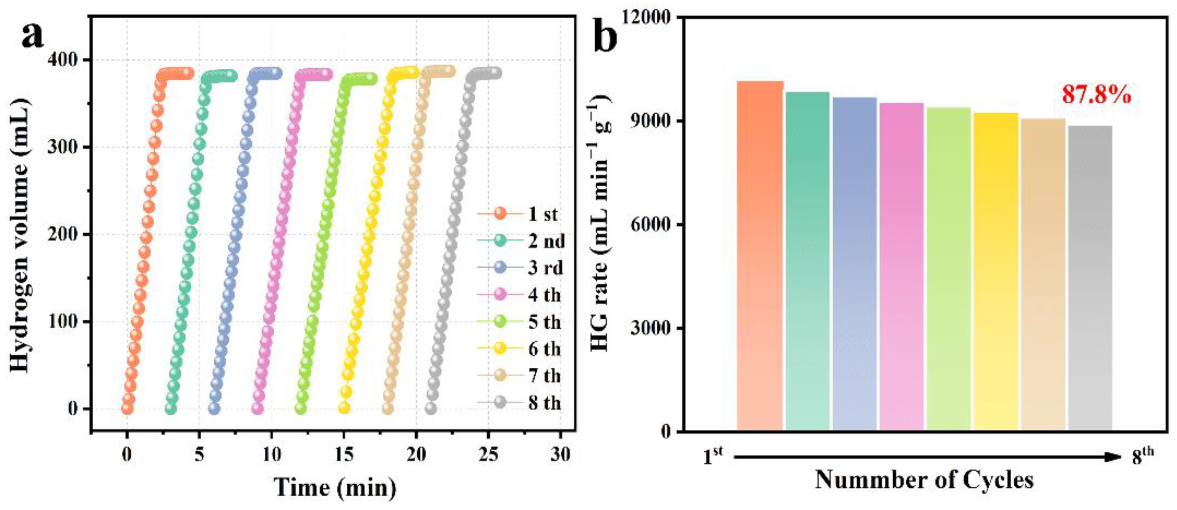
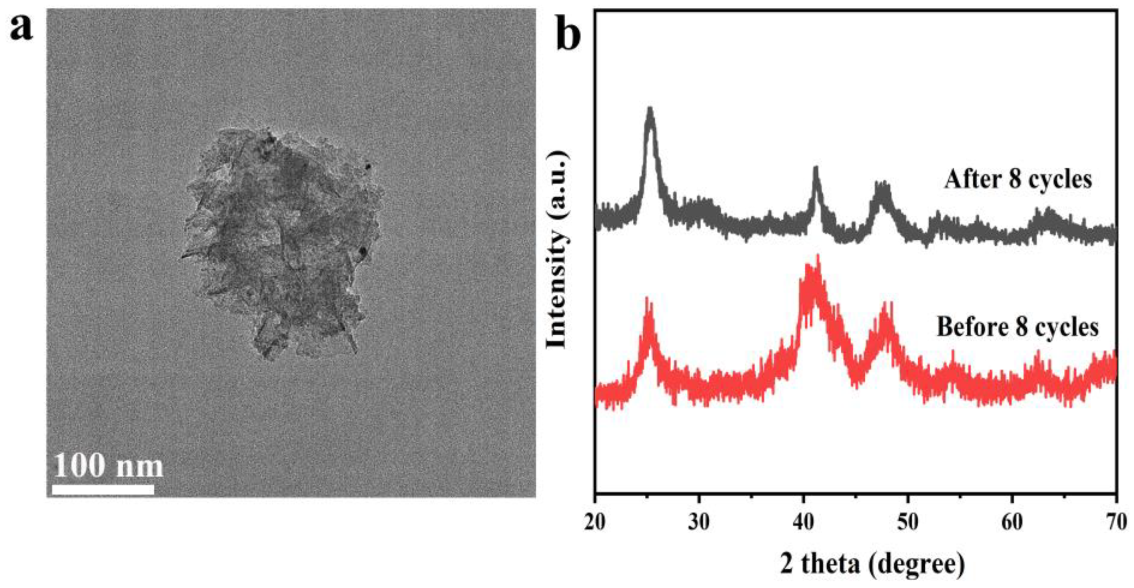
3.3. Stability of PtNi/PTOC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Xiao, X.; Wang, X.; Lu, Y.; Zhang, M.; Zheng, J.; Chen, L. Self-templated carbon enhancing catalytic effect of ZrO2 nanoparticles on the excellent dehydrogenation kinetics of MgH2. Carbon 2020, 166, 46–55. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, X.; He, J.; Dong, Z.; Wang, X.; Chen, M.; Chen, L. A dandelion-like amorphous composite catalyst with outstanding performance for sodium borohydride hydrogen generation. Int. J. Hydrogen Energy 2021, 46, 10809–10818. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Huang, Z.; Zhu, M. Closing the Loop for Hydrogen Storage: Facile Regeneration of NaBH4 from its Hydrolytic Product. Angew. Chem. Int. Ed. 2020, 59, 8623–8629. [Google Scholar] [CrossRef]
- Huang, Y.; An, C.; Zhang, Q.; Zang, L.; Shao, H.; Liu, Y.; Yuan, H.; Wang, C.; Wang, Y. Cos-effective mechanochemical synthesis of highly dispersed supported transition metal catalysts for hydrogen storage. Nano Energy 2021, 80, 105535. [Google Scholar] [CrossRef]
- Min, J.; Jeffery, A.; Kim, Y.; Jung, N. Electrochemical Analysis for Demonstrating CO Tolerance of Catalysts in Polymer Electrolyte Membrane Fuel Cells. Nanomaterials 2019, 9, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, Y.; Liu, J.; Chu, H.; Wei, S.; Yin, Q.; Kang, L.; Luo, X.; Sun, L.; Xu, F.; Huang, P.; et al. Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon. Nanomaterials 2021, 11, 3259. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Hu, W.; Wen, H.; Qiu, Y.; Tang, P.; Chen, M.; Wang, P. Hierarchical Nanostructured Co-Mo-B/CoMoO4−x Amorphous Composite for the Alkaline Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 42605–42612. [Google Scholar] [CrossRef]
- Shao, H.; Huang, G.; Liu, Y.; Guo, Y.; Wang, Y.N. Thermally stable Ni MOF catalyzed MgH2 for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 37977–37985. [Google Scholar] [CrossRef]
- Gao, H.; Shao, Y.; Shi, R.; Liu, Y.; Zhu, J.; Liu, J.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Effect of Few-Layer Ti3C2Tx Supported Nano-Ni via Self-Assembly Reduction on Hydrogen Storage Performance of MgH2. ACS Appl. Mater. Interfaces 2020, 12, 47684–47694. [Google Scholar] [CrossRef]
- Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Larichev, Y.V.; Netskina, O.V.; Komova, O.V.; Simagina, V.I. Comparative XPS study of Rh/Al2O3 and Rh/TiO2 as catalysts for NaBH4 hydrolysis. Int. J. Hydrogen Energy 2010, 35, 6501–6507. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Wu, Z.J.; Zheng, X.C.; Peng, Z.K.; Liu, P. Chitosan-reduced graphene oxide hybrids encapsulated Pd (0) nanocatalysts for H2 generation from ammonia borane. Int. J. Hydrogen Energy 2019, 44, 23610–23619. [Google Scholar] [CrossRef]
- Konuş, N.; Karataş, Y.; Gulcan, M. In situ formed ruthenium (0) nanoparticles supported on TiO2 catalyzed hydrogen generation from aqueous ammonia-borane solution at room temperature under air. Synth. React. Inorg. Met. Org. Chem. 2016, 46, 534–542. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, F.; Yang, L.; He, Z.; Huang, X.; Zhang, D.; Dong, H. Ultrasmall Ru nanoparticles supported on chitin nanofibers for hydrogen production from NaBH4 hydrolysis. Chin. Chem. Lett. 2020, 31, 2019–2022. [Google Scholar] [CrossRef]
- Dai, P.; Zhao, X.; Xu, D.; Wang, C.; Tao, X.; Liu, X.; Gao, J. Preparation, characterization, and properties of Pt/Al2O3/cordierite monolith catalyst for hydrogen generation from hydrolysis of sodium borohydride in a flow reactor. Int. J. Hydrogen Energy 2019, 44, 28463–28470. [Google Scholar] [CrossRef]
- Ro, G.; Kim, Y. H2 generation using Pt nanoparticles encapsulated in Fe3O4@SiO2@TiO2 multishell particles. Colloids Surf. A 2019, 577, 48–52. [Google Scholar] [CrossRef]
- Chen, J.; Gu, Y.; Kong, D.; Xiang, S.; Wang, P.; Zhang, H.; Zhang, S. Preparation of Colloidal Pt/Co Bimetallic Nanoparticle Catalysts and Their Catalytic Activity for Hydrogen Generation from Hydrolysis Reaction of NaBH4. Rare Met. Mater. Eng. 2014, 43, 2209–2214. [Google Scholar]
- Kotkondawar, A.V.; Rayalu, S. Enhanced H2 production from dehydrogenation of sodium borohydride over the ternary Co0.97Pt0.03/CeOx nanocomposite grown on CGO catalytic support. RSC Adv. 2020, 10, 38184–38195. [Google Scholar] [CrossRef]
- Ro, G.; Hwang, D.K.; Kim, Y. Hydrogen generation using Pt/Ni bimetallic nanoparticles supported on Fe3O4@SiO2@TiO2 multi-shell microspheres. J. Ind. Eng. Chem. 2019, 79, 364–369. [Google Scholar] [CrossRef]
- Guo, H.; Chen, C.; Chen, K.; Cai, H.; Chang, X.; Liu, S.; Wang, C. High performance carbon-coated hollow Ni12P5 nanocrystals decorated on GNS as advanced anodes for lithium and sodium storage. J. Mater. Chem. A 2017, 5, 22316–22324. [Google Scholar] [CrossRef]
- Aranishi, K.; Singh, A.K.; Xu, Q. Dendrimer-Encapsulated Bimetallic Pt-Ni Nanoparticles as Highly Efficient Catalysts for Hydrogen Generation from Chemical Hydrogen Storage Materials. Chem. Cat. Chem. 2013, 5, 2248–2252. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Zhou, W.; Zhou, X.; Sun, L.; Zhang, H.; Lu, L.; Zhang, S. Fabrication and catalytic activity of Pt/Ni/Fe trimetallic nanoparticles for hydrogen generation from NaBH4. Chem. J. Chin. Univ. Chin. 2014, 35, 2164–2169. [Google Scholar]
- Yang, D.S.; Kim, M.S.; Song, M.Y.; Yu, J.S. Highly efficient supported PtFe cathode electrocatalysts prepared by homogeneous deposition for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 13681–13688. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Rodríguez-Pérez, I.A.; Miao, W.; Chen, K.; Wang, W.; Han, S. Carbon nanospheres supported bimetallic Pt-Co as an efficient catalyst for NaBH4 hydrolysis. Appl. Surf. Sci. 2021, 540, 14829. [Google Scholar] [CrossRef]
- Qi, X.; Li, X.; Chen, B.; Lu, H.; Wang, L.; He, G. Highly active nanoreactors: Patchlike or thick Ni coating on Pt nanoparticles based on confined catalysis. ACS Appl. Mat. Interfaces 2016, 8, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Mandal, M.; Kundu, S.; Nath, S.; Pal, T. Bimetallic Pt-Ni nanoparticles can catalyze reduction of aromatic nitro compounds by sodium borohydride in aqueous solution. Appl. Catal. A Gen. 2004, 268, 61–66. [Google Scholar] [CrossRef]
- Hannauer, J.; Demirci, U.B.; Geantet, C.; Herrmann, J.M.; Miele, P. Transition metal-catalyzed dehydrogenation of hydrazine borane N2H4BH3 via the hydrolysis of BH3 and the decomposition of N2H4. Int. J. Hydrogen Energy 2012, 37, 10758–10767. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Cheng, L.; Hou, X.; Li, Y.; Han, S. Non-noble Co anchored on nanoporous graphene oxide, as an efficient and long-life catalyst for hydrogen generation from sodium borohydride. Colloids Surf. A 2019, 563, 112–119. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, L.; Fang, F.; Sun, D.; Guo, Z.; Liu, H.; Yu, X. General synthesis of transition metal oxide ultrafine nanoparticles embedded in hierarchically porous carbon nanofibers as advanced electrodes for lithium storage. Adv. Funct. Mater. 2016, 26, 6188–6196. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Wang, W.D.; Hu, X.; Dong, Z. Ru nanoclusters confined in porous organic cages for catalytic hydrolysis of ammonia borane and tandem hydrogenation reaction. Nanoscale 2019, 11, 21513–21521. [Google Scholar] [CrossRef] [PubMed]
- Kakanakova-Georgieva, A.; Gueorguiev, G.; Sangiovanni, D.; Suwannaharn, N.; Ivanov, I.; Cora, I.; Pécz, B.; Nicotra, G.; Giannazzo, F. Nanoscale phenomena ruling deposition and intercalation of AlN at the graphene/SiC interface. Nanoscale 2020, 12, 19470–19476. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Brito Mota, F.; Castilho, C.; Kakanakova-Georgieva, A.; Gueorguiev, G. Spin-orbit-induced gap modification in buckled honeycomb XBi and XBi3 (X = B, Al, Ga, and In) sheets. J. Phys.-Condens. Matter 2015, 27, 485306. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shi, J. Chemistry of mesoporous organosilica in nanotechnology: Molecularly organic-inorganic hybridization into frameworks. Adv. Mater. 2016, 28, 3235–3272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, L.; Li, Y.; Zheng, J.; Li, X. Metal-Organic-Framework-Based Catalyst for Highly Efficient H2 Generation from Aqueous NH3BH3 Solution. Chem. Eur. J. 2009, 15, 8951–8954. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Wu, Y.; Gao, J.; Zhang, Q. From isolated Ti-oxo clusters to infinite Ti-oxo chains and sheets: Recent advances in photoactive Ti-based MOFs. J. Mater. Chem. A 2020, 8, 15245–15270. [Google Scholar] [CrossRef]
- Karthik, P.; Shaheer, A.M.; Vinu, A.; Neppolian, B. Amine Functionalized Metal-Organic Framework Coordinated with Transition Metal Ions: D-d Transition Enhanced Optical Absorption and Role of Transition Metal Sites on Solar Light Driven H2 Production. Small 2020, 16, 1902990. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chen, L.; Wang, H.; Guo, Y.; Xu, M.; Zhang, Y.; Duan, C. Light control of charge transfer in metal/semiconductor heterostructures for efficient hydrogen evolution: Optical transition versus SPR. Int. J. Hydrogen Energy 2017, 42, 26713–26722. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, X.; Sun, L.; Yu, F.; Li, P.; Chen, J.; Wu, Y.; Cao, L.; Xu, C.; Yang, X.; et al. Hydrogen generation of a novel Al-NaMgH3 composite reaction with water. Int. J. Hydrogen Energy 2017, 42, 30535–30542. [Google Scholar] [CrossRef]
- Yang, F.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Synthesis of “needle-cluster” NiCo2O4 carbon nanofibers and loading of Co-B nanoparticles for hydrogen production through the hydrolysis of NaBH4. J. Alloys Compd. 2022, 911, 165069. [Google Scholar] [CrossRef]
- Zhou, H.; Kang, M.; Xie, B.; Wen, P.; Zhao, N. L-Alanine mediated controllable synthesis: Ultrathin Co3O4 nanosheets@ Ni foam for high performance supercapacitors. J. Alloys Compd. 2021, 874, 160030. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, C.; Chen, B.; Chen, J.; Fang, Q.; Wang, J. Novel photocatalytic performance of nanocage-like MIL-125-NH2 induced by adsorption of phenolic pollutants. Environ. Sci. Nano 2020, 7, 1525–1538. [Google Scholar] [CrossRef]
- Wang, W.; Hong, X.; Yao, Q.; Lu, Z.H. Bimetallic Ni-Pt nanoparticles immobilized on mesoporous N-doped carbon as a highly efficient catalyst for complete hydrogen evolution from hydrazine borane. J. Mater. Chem. A 2020, 8, 13694–13701. [Google Scholar] [CrossRef]
- Georgieva, J.; Valova, E.; Mintsouli, I.; Sotiropoulos, S.; Tatchev, D.; Armyanov, S.; Malet, L. Pt (Ni) electrocatalysts for methanol oxidation prepared by galvanic replacement on TiO2 and TiO2-C powder supports. J. Electroanal. Chem. 2015, 754, 65–74. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tian, C.; Geng, D.; Wang, D.; Bai, S. One-Pot Synthesis of Highly Efficient Carbon-Supported Polyhedral Pt3Ni Alloy Nanoparticles for Oxygen Reduction Reaction. Electrocatalysis 2019, 10, 613–620. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Song, N.; Wang, C.; Lu, X. Fabrication of Pt nanoparticles on nitrogen-doped carbon/Ni nanofibers for improved hydrogen evolution activity. J. Colloid Interface Sci. 2018, 514, 199–207. [Google Scholar] [CrossRef]
- Huff, C.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Nanocomposite catalyst derived from ultrafine platinum nanoparticles and carbon nanotubes for hydrogen generation. ECS. J. Solid State Technol. 2020, 9, 101008. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Guo, J.; Sun, L.; Ming, J.; Dong, H.; Yang, X. Ceria-induced strategy to tailor Pt atomic clusters on cobalt–nickel oxide and the synergetic effect for superior hydrogen generation. ACS. Sustain. Chem. Eng. 2018, 6, 7451–7457. [Google Scholar] [CrossRef]
- Lu, L.; Shu, H.; Ruan, Z.; Ni, J.; Zhang, H. Preparation of Graphene-supported Pt-Pd Catalyst and Its Catalytic Activity and Mechanism for Hydrogen Generation Reaction. Chem. J. Chin. Univ. Chi. 2018, 39, 949–955. [Google Scholar]
- Lale, A.; Wasan, A.; Kumar, R.; Miele, P.; Demirci, U.B.; Bernard, S. Organosilicon polymer-derived mesoporous 3D silicon carbide, carbonitride and nitride structures as platinum supports for hydrogen generation by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2016, 41, 15477–15488. [Google Scholar] [CrossRef]
- Li, K.; Ma, M.; Xie, L.; Yao, Y.; Kong, R.; Du, G.; Sun, X. Monolithically integrated NiCoP nanosheet array on Ti mesh: An efficient and reusable catalyst in NaBH4 alkaline media toward on-demand hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 19028–19034. [Google Scholar] [CrossRef]
- Li, X.; Zeng, C.; Fan, G. Ultrafast hydrogen generation from the hydrolysis of ammonia borane catalyzed by highly efficient bimetallic RuNi nanoparticles stabilized on Ti3C2X2 (X = OH and/or F). Int. J. Hydrogen Energy 2015, 40, 3883–3891. [Google Scholar] [CrossRef]
- Pornea, A.M.; Abebe, M.W.; Kim, H. Ternary NiCoP urchin like 3D nanostructure supported on nickel foam as a catalyst for hydrogen generation of alkaline NaBH4. Chem. Phys. 2019, 516, 152–159. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wu, S.; Wei, Y.; Meng, W.; Xie, Y.; Zhang, X. Hydrogen generation from alkaline NaBH4 solution using nanostructured Co-Ni-P catalysts. Int. J. Hydrogen Energy 2017, 42, 16529–16537. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, C.; Fang, Y.; Zhu, F.; Liu, H.; Ge, H. Hydrogen generation mechanism of BH4− spontaneous hydrolysis: A sight from ab initio calculation. Int. J. Hydrogen Energy 2016, 41, 22668–22676. [Google Scholar] [CrossRef]
| Sample | Tempera-ture (°C) | HG Rate (mL·min−1·gM−1) | Ea (kJ·mol–1) | Number of Cycles | Cyclic Stability | Ref. |
|---|---|---|---|---|---|---|
| CNSs@Pt0.1Co0.9 | 30 | 8943.0 | 38.0 | 5 | 85.2% | [25] |
| Pt/MWCNTs | 30 | 16.9 | 46.2 | 5 | 80.0% | [47] |
| Pt/CeO2-Co7Ni2Ox | 25 | 7834.8 | 47.4 | 5 | 85.0% | [48] |
| PtPd/GO | 25 | 3940.0 | 29.4 | 4 | 60.0% | [49] |
| Pt/Si3N4 | 80 | 13,000.0 | 35.2 | 5 | 82.5% | [50] |
| NiCoP NA/Ti | 30 | 3016.8 | 52.7 | 8 | 70.0% | [51] |
| RuNi/Ti3C2×2 | 30 | 1649.0 | 34.7 | 4 | 50% | [52] |
| PtNi/PTOC | 29 | 10,164.3 | 28.7 | 8 | 87.8% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Kang, L.; Sun, L.; Xu, F.; Pan, H.; Sang, Z.; Zhang, C.; Jia, X.; Sui, Q.; Bu, Y.; et al. Bimetallic Pt-Ni Nanoparticles Confined in Porous Titanium Oxide Cage for Hydrogen Generation from NaBH4 Hydrolysis. Nanomaterials 2022, 12, 2550. https://doi.org/10.3390/nano12152550
Yu Y, Kang L, Sun L, Xu F, Pan H, Sang Z, Zhang C, Jia X, Sui Q, Bu Y, et al. Bimetallic Pt-Ni Nanoparticles Confined in Porous Titanium Oxide Cage for Hydrogen Generation from NaBH4 Hydrolysis. Nanomaterials. 2022; 12(15):2550. https://doi.org/10.3390/nano12152550
Chicago/Turabian StyleYu, Yuqian, Li Kang, Lixian Sun, Fen Xu, Hongge Pan, Zhen Sang, Chenchen Zhang, Xinlei Jia, Qingli Sui, Yiting Bu, and et al. 2022. "Bimetallic Pt-Ni Nanoparticles Confined in Porous Titanium Oxide Cage for Hydrogen Generation from NaBH4 Hydrolysis" Nanomaterials 12, no. 15: 2550. https://doi.org/10.3390/nano12152550
APA StyleYu, Y., Kang, L., Sun, L., Xu, F., Pan, H., Sang, Z., Zhang, C., Jia, X., Sui, Q., Bu, Y., Cai, D., Xia, Y., Zhang, K., & Li, B. (2022). Bimetallic Pt-Ni Nanoparticles Confined in Porous Titanium Oxide Cage for Hydrogen Generation from NaBH4 Hydrolysis. Nanomaterials, 12(15), 2550. https://doi.org/10.3390/nano12152550






