Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of ZnO@Mt and ZnO@HNT Hybrid Nanostructures
2.1.2. Preparation of CS/PVOH/xZnO@Mt and CS/PVOH/xZnO@HNT Active Films
2.2. XRD Analysis
2.3. FTIR Spectrometry
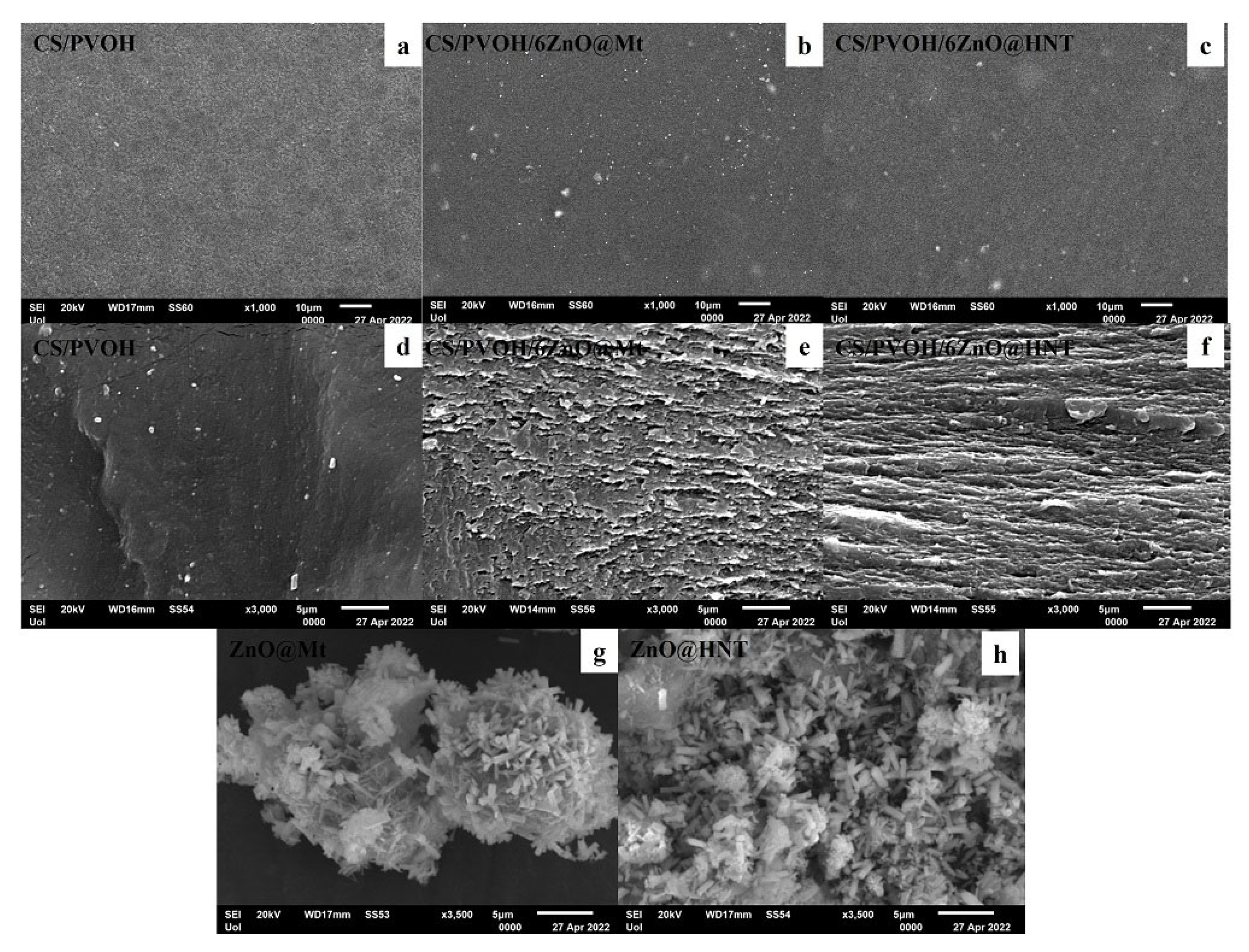
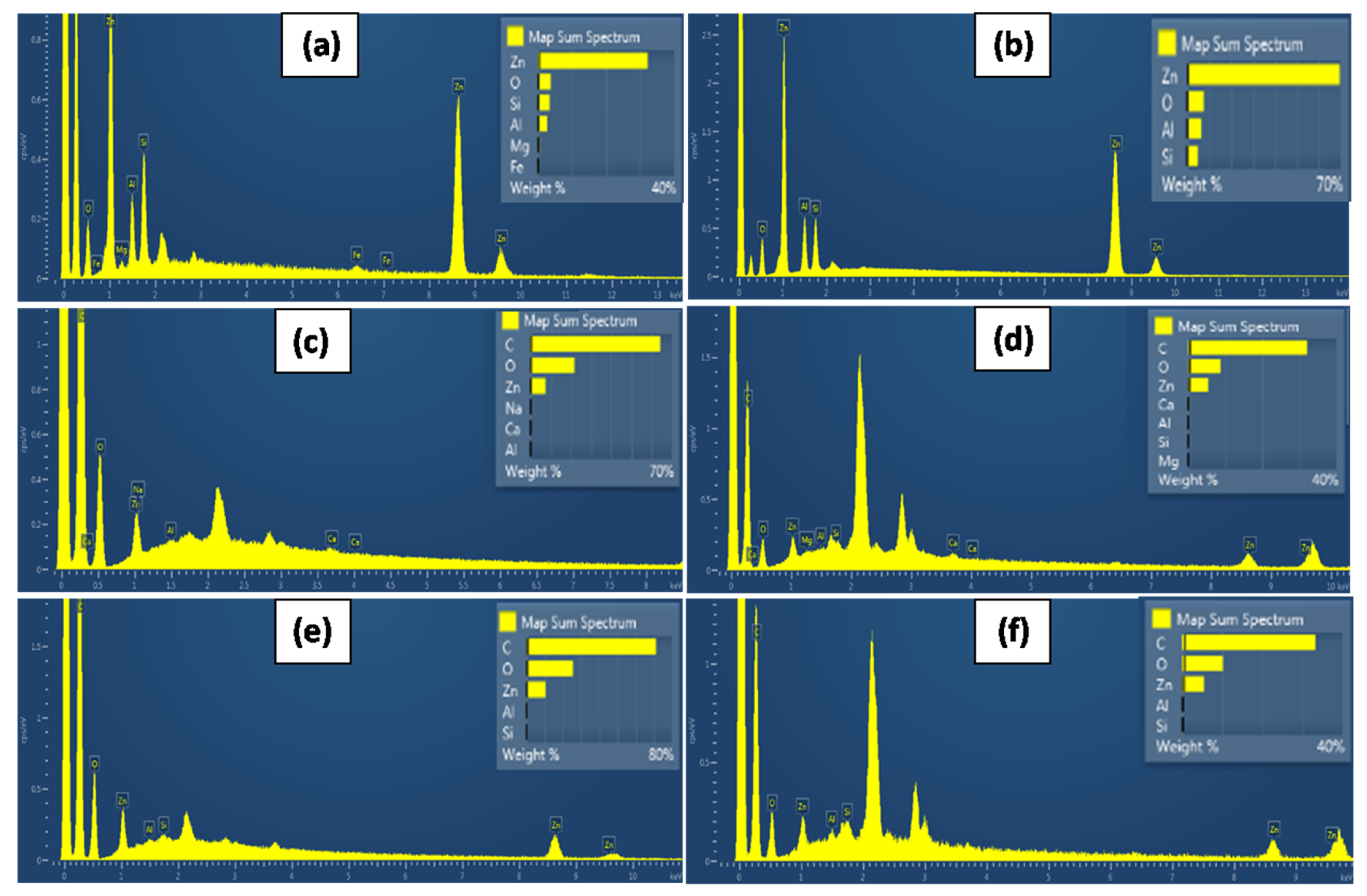
2.4. SEM Images
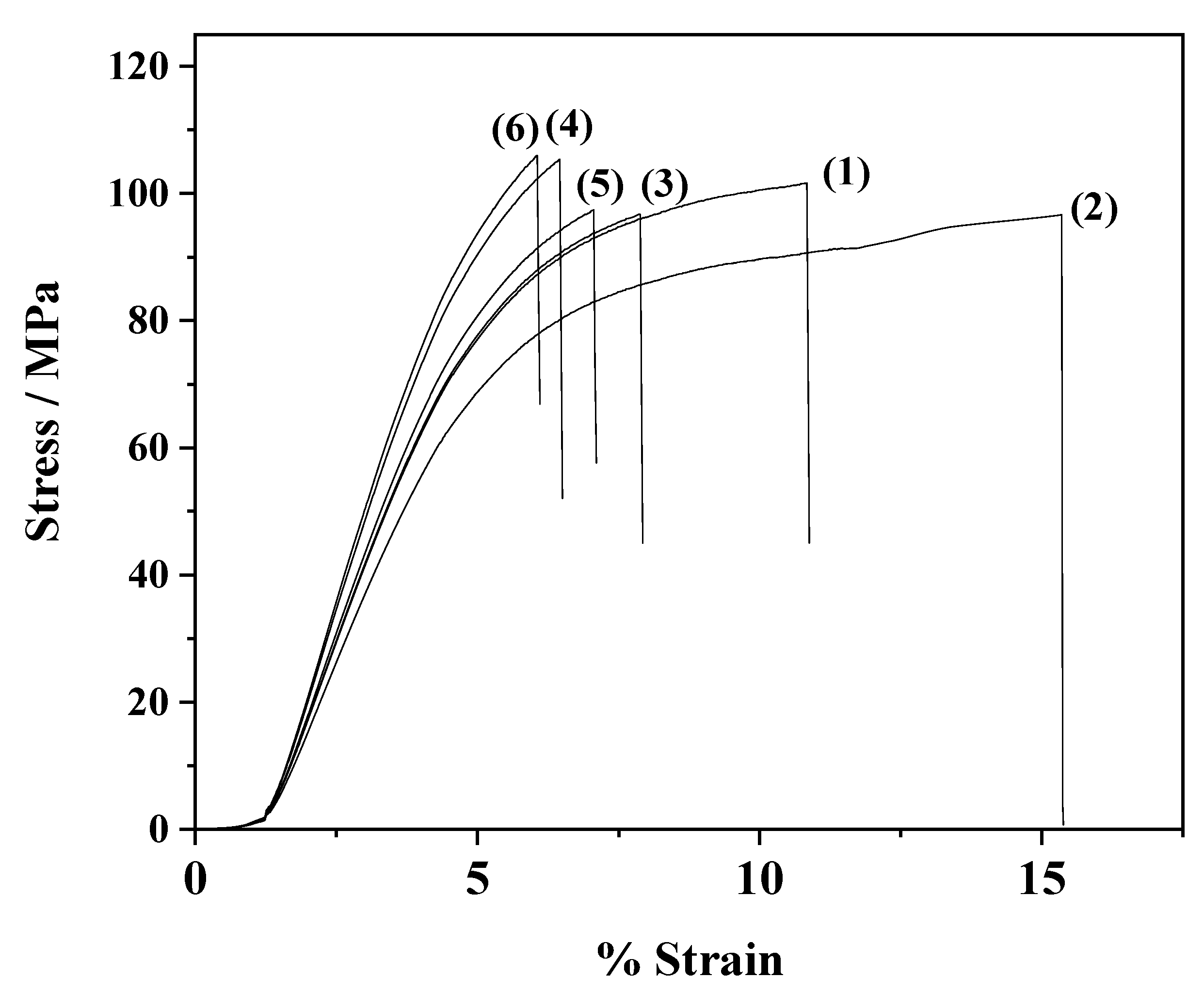
2.5. Tensile Properties
2.6. Water Sorption
2.7. Water Vapor Diffusivity
2.8. Oxygen Permeability
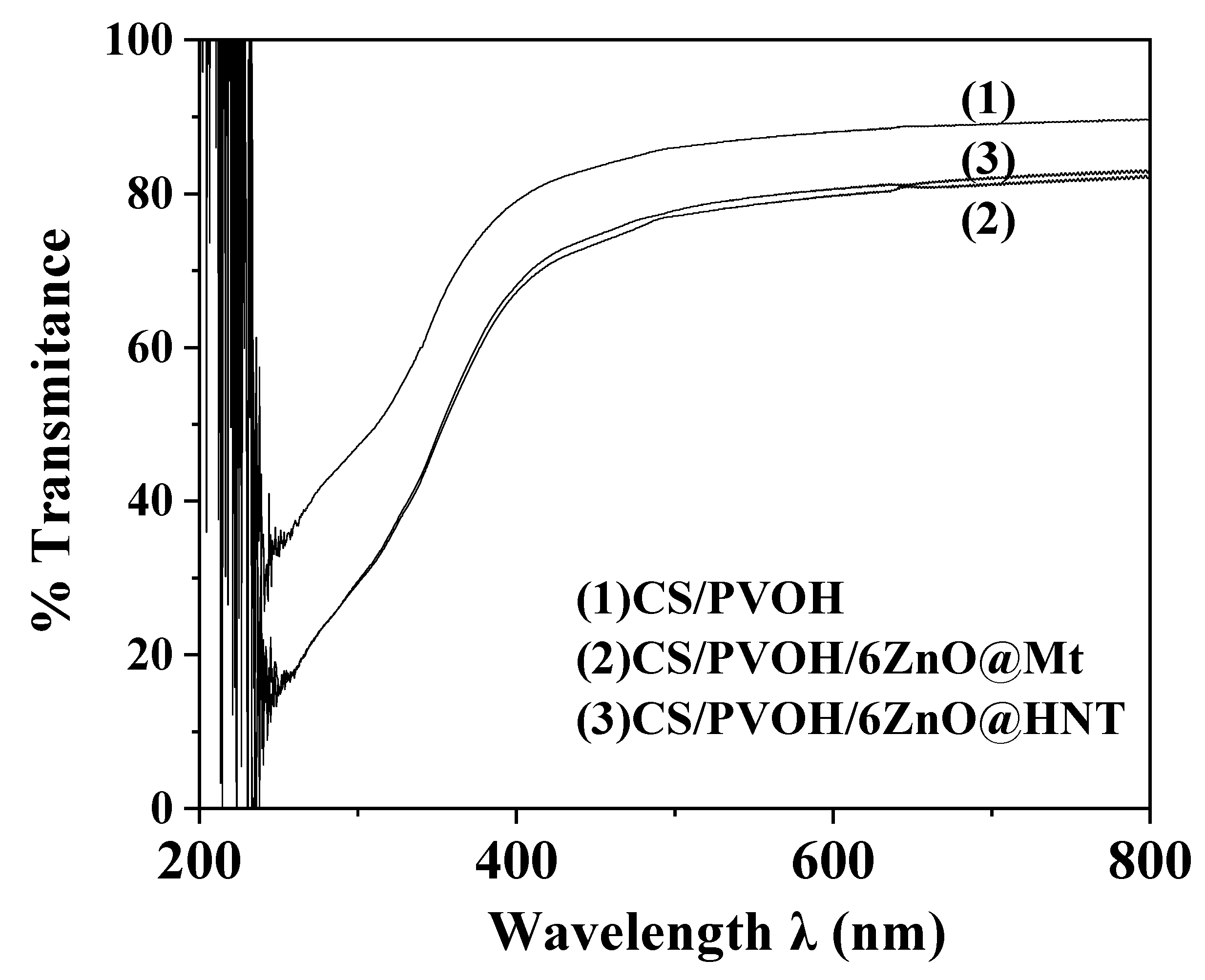
2.9. UV–Vis Transmittance Analysis of Films
2.10. Antimicrobial Activity Tests
3. Results
3.1. XRD
3.2. FTIR
3.3. SEM Images
3.4. Tensile Measurements
3.5. Water Sorption
3.6. Water and Oxygen Barrier
3.7. UV–Vis Film Transmittance
3.8. Antimicrobial Activity
3.9. Statistical Analysis of the Experimental Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Aider, M. Chitosan Application for Active Bio-Based Films Production and Potential in the Food Industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Tang, C.; Chen, N.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, X. Preparation and Properties of Chitosan Nanocomposites with Nanofillers of Different Dimensions. Polym. Degrad. Stab. 2009, 94, 124–131. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in Food and Beverage Packaging. J. Nanomater. 2019, 2019, 8927167. [Google Scholar] [CrossRef] [Green Version]
- Giannakas, E.A.; Leontion, A. Montmorillonite Composite Materials and Food Packaging. In Composites Materials for Food Packaging; John Wilwy and Sons Inc.; Scrivener Publishing LLC: Beverly, CA, USA, 2018; pp. 1–71. ISBN 978-1-119-16020-5. [Google Scholar]
- Barman, M.; Mahmood, S.; Augustine, R.; Hasan, A.; Thomas, S.; Ghosal, K. Natural Halloysite Nanotubes /Chitosan Based Bio-Nanocomposite for Delivering Norfloxacin, an Anti-Microbial Agent in Sustained Release Manner. Int. J. Biol. Macromol. 2020, 162, 1849–1861. [Google Scholar] [CrossRef]
- Applications of Halloysite Nanotubes in Food Packaging for Improving Film Performance and Food Preservation—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0956713521000141?via%3Dihub (accessed on 26 January 2022).
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Bionanocomposite Films for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-08-100596-5. [Google Scholar]
- Haerudin, H.; Pramono, A.W.; Kusuma, D.S.; Jenie, A.; Voelcker, N.H.; Gibson, C. Preparation and Characterization of Chitosan/Montmorillonite (MMT) Nanocomposite Systems. Int. J. Technol. 2010, 1, 65–73. [Google Scholar]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical and Barrier Properties Investigation of Chitosan–Clay Nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadi, K.; Giannakas, A.; Ladavos, A.K.; Barkoula, N.-M. Interplay between Processing and Performance in Chitosan-Based Clay Nanocomposite Films. Polym. Bull. 2015, 72, 1145–1161. [Google Scholar] [CrossRef]
- Dias, M.V.; Azevedo, V.M.; Borges, S.V.; Soares, N.D.F.F.; Fernandes, R.V.D.B.; Marques, J.J.; Medeiros, A.A. Development of Chitosan/Montmorillonite Nanocomposites with Encapsulated α-Tocopherol. Food Chem. 2014, 165, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Gomes, V.; Souza, L.; Pires, J.R.A.; Freitas, P.; Lopes, A.A.S.; Fernandes, F.M.B.; Paula, M.; Coelhoso, I.M.; Luisa, A. Bionanocomposites of Chitosan / Montmorillonite Incorporated with Rosmarinus o Ffi Cinalis Essential Oil: Development and Physical Characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/Clay Films’ Properties as Affected by Biopolymer and Clay Micro/Nanoparticles’ Concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic Effect of Chitosan and Halloysite Nanotubes on Improving Agar Film Properties. Food Hydrocoll. 2020, 101, 105471. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Lu, R.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Preparation, Characterization, and 3D Printing Verification of Chitosan/Halloysite Nanotubes/Tea Polyphenol Nanocomposite Films. Int. J. Biol. Macromol. 2021, 166, 32–44. [Google Scholar] [CrossRef]
- Jauković, V.; Krajišnik, D.; Daković, A.; Damjanović, A.; Krstić, J.; Stojanović, J.; Čalija, B. Influence of Selective Acid-Etching on Functionality of Halloysite-Chitosan Nanocontainers for Sustained Drug Release. Mater. Sci. Eng. C 2021, 123, 112029. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Silva, R.D.; Vahedi, V.; Churchman, G.J. Halloysite Nanotubes: Prospects and Challenges of Their Use as Additives and Carriers—A Focused Review. Clay Miner. 2016, 51, 479–487. [Google Scholar] [CrossRef]
- Saadat, S.; Pandey, G.; Tharmavaram, M.; Braganza, V.; Rawtani, D. Nano-Interfacial Decoration of Halloysite Nanotubes for the Development of Antimicrobial Nanocomposites. Adv. Colloid Interface Sci. 2020, 275, 102063. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical, Barrier and Antimicrobial Properties of Chitosan/PVOH/Clay Nanocomposites. Carbohydr. Polym. 2016, 140, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F. Effect of Plasticizers on the Barrier and Mechanical Properties of Biomimetic Composites of Chitosan and Clay. Carbohydr. Polym. 2015, 115, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Boura-Theodoridou, O.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.-M. Performance of ZnO/Chitosan Nanocomposite Films for Antimicrobial Packaging Applications as a Function of NaOH Treatment and Glycerol/PVOH Blending. Food Packag. Shelf Life 2020, 23, 100456. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial Properties of ZnO Nanomaterials: A Review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Boyer, R.F. A Review of: “Zinc and Its Role in Biology and Nutrition, Volume 15 of Metal Ions in Biological Systems, H. Sigel, Editor, Xxii + 493 Pages, Marcel Dekker, Inc., New York, 1983”. Synth. React. Inorg. Metal. Org. Chem. 1983, 13, 959–960. [Google Scholar] [CrossRef]
- Abdeen, Z.I.; El Farargy, A.F.; Negm, N.A. Nanocomposite Framework of Chitosan/Polyvinyl Alcohol/ZnO: Preparation, Characterization, Swelling and Antimicrobial Evaluation. J. Mol. Liq. 2018, 250, 335–343. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-Zinc Oxide Nanoparticle Composite Coating for Active Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A. Preparation, Characterization and Evaluation of Glycerol Plasticized Chitosan/PVA Blends for Burn Wounds. Int. J. Biol. Macromol. 2019, 124, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Kumar, S.; Koh, J.; Seena, S.; Singh, P.; Ramalho, A.; Sobral, A.J.F.N. Bio-Based Chitosan/Gelatin/Ag@ZnO Bionanocomposites: Synthesis and Mechanical and Antibacterial Properties. Cellulose 2019, 26, 5347–5361. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Ben Rhaiem, H.; Ben Haj Amara, A.; Ruiz-Hitzky, E. ZnO/Clay Nanoarchitectures: Synthesis, Characterization and Evaluation as Photocatalysts. Appl. Clay Sci. 2016, 131, 131–139. [Google Scholar] [CrossRef]
- Ding, J.; Hui, A.; Wang, W.; Yang, F.; Kang, Y.; Wang, A. Multifunctional Palygorskite@ZnO Nanorods Enhance Simultaneously Mechanical Strength and Antibacterial Properties of Chitosan-Based Film. Int. J. Biol. Macromol. 2021, 189, 668–677. [Google Scholar] [CrossRef]
- Salmas, C.; Giannakas, A.; Katapodis, P.; Leontiou, A.; Moschovas, D.; Karydis-Messinis, A. Development of ZnO/Na-Montmorillonite Hybrid Nanostructures Used for PVOH/ZnO/Na-Montmorillonite Active Packaging Films Preparation via a Melt-Extrusion Process. Nanomaterials 2020, 10, 1079. [Google Scholar] [CrossRef]
- Jia, W.; Dang, S.; Liu, H.; Zhang, Z.; Yu, C.; Liu, X.; Xu, B. Evidence of the Formation Mechanism of ZnO in Aqueous Solution. Mater. Lett. 2012, 82, 99–101. [Google Scholar] [CrossRef]
- Giannakas, A.; Patsaoura, A.; Barkoula, N.-M.; Ladavos, A. A Novel Solution Blending Method for Using Olive Oil and Corn Oil as Plasticizers in Chitosan Based Organoclay Nanocomposites. Carbohydr. Polym. 2017, 157, 550–557. [Google Scholar] [CrossRef]
- Vlacha, M.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.-M. On the Efficiency of Oleic Acid as Plasticizer of Chitosan/Clay Nanocomposites and Its Role on Thermo-Mechanical, Barrier and Antimicrobial Properties—Comparison with Glycerol. Food Hydrocoll. 2016, 57, 10–19. [Google Scholar] [CrossRef]
- Giannakas, A.; Giannakas, A.; Ladavos, A. Preparation and Characterization of Polystyrene/Organolaponite Nanocomposites. Polym. Plast. Technol. Eng. 2012, 51, 1411–1415. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef] [Green Version]
- Giannakas Na-Montmorillonite, V.S. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yuan, Z.-Y.; Du, G.-H.; Ren, T.-Z.; Bouvy, C.; Halasa, M.; Su, B.-L. Simple Approach to Highly Oriented ZnO Nanowire Arrays: Large-Scale Growth, Photoluminescence and Photocatalytic Properties. Nanotechnology 2006, 17, 588–594. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Cheng, C.-S.; Chang, C.-C.; Yang, S.; Chang, C.-S.; Hsieh, W.-F. Orientation-Enhanced Growth and Optical Properties of ZnO Nanowires Grown on Porous Silicon Substrates. Nanotechnology 2005, 16, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.K.; Hall, W.H. X-Ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Makarona, E.; Koutzagioti, C.; Salmas, C.; Ntalos, G.; Skoulikidou, M.-C.; Tsamis, C. Enhancing Wood Resistance to Humidity with Nanostructured ZnO Coatings. Nano Struct. Nano Objects 2017, 10, 57–68. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2020, 38, 537–565. [Google Scholar] [CrossRef] [Green Version]
- Gartner, C.; López, B.L.; Sierra, L.; Graf, R.; Spiess, H.W.; Gaborieau, M. Interplay between Structure and Dynamics in Chitosan Films Investigated with Solid-State NMR, Dynamic Mechanical Analysis, and X-ray Diffraction. Biomacromolecules 2011, 12, 1380–1386. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K.W. Preparation and Characterization of Chitosan-Based Nanocomposite Films with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Naveen Kumar, H.M.P.; Prabhakar, M.N.; Venkata Prasad, C.; Madhusudhan Rao, K.; Ashok Kumar Reddy, T.V.; Chowdoji Rao, K.; Subha, M.C.S. Compatibility Studies of Chitosan/PVA Blend in 2% Aqueous Acetic Acid Solution at 30 °C. Carbohydr. Polym. 2010, 82, 251–255. [Google Scholar] [CrossRef]
- Wu, L.M.; Tong, D.S.; Zhao, L.Z.; Yu, W.H.; Zhou, C.H.; Wang, H. Fourier Transform Infrared Spectroscopy Analysis for Hydrothermal Transformation of Microcrystalline Cellulose on Montmorillonite. Appl. Clay Sci. 2014, 95, 74–82. [Google Scholar] [CrossRef]
- Djomgoue, P.; Njopwouo, D. FT-IR Spectroscopy Applied for Surface Clays Characterization. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Al-Amiery, A.A.; Nassir, M.H.; Jaaz, A.H. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef] [Green Version]
- Anžlovar, A.; Crnjak Orel, Z.; Kogej, K.; Žigon, M. Polyol-Mediated Synthesis of Zinc Oxide Nanorods and Nanocomposites with Poly(Methyl Methacrylate). Available online: https://www.hindawi.com/journals/jnm/2012/760872/ (accessed on 6 January 2020).
- Soni, B.H.; Garg, N. Studies on ZnO Nanorods Synthesized by Hydrothermal Method and Their Characterization. J. Nano-Electron. Phys. 2013, 5, 04077-6. [Google Scholar]
- Mallakpour, S.; Madani, M. Transparent and Thermally Stable Improved Poly (Vinyl Alcohol)/Cloisite Na+/ZnO Hybrid Nanocomposite Films: Fabrication, Morphology and Surface Properties. Prog. Org. Coat. 2012, 74, 520–525. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The Effect of Different Preparation Methods on the Development of Chitosan/Thyme Oil/Montmorillonite Nanocomposite Active Packaging Films. J. Food Process. Preserv. 2019, 44, e14327. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the Combined Effect of Both Clay and Glycerol Plasticizer on the Properties of Chitosan Films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Hyder, M.N.; Chen, P. Pervaporation Dehydration of Ethylene Glycol with Chitosan-Poly(Vinyl Alcohol) Blend Membranes: Effect of CS-PVA Blending Ratios. J. Membr. Sci. 2009, 340, 171–180. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Kumar, K.R.; Tharanathan, R.N. Properties and Sorption Studies of Chitosan-Polyvinyl Alcohol Blend Films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Gas Barrier Properties Enhancement—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/B9780323358842000181?via%3Dihub (accessed on 17 May 2022).
- Influence of Packaging Material on Quality Characteristics of Minimally Processed Mridula Pomegranate (Punica Granatum) Arils during Cold Storage|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Influence-of-packaging-material-on-quality-of-arils-Bhatia-Asrey/aad1332359b35ef50b4c67b3e73395343d75373d (accessed on 17 May 2022).
- Huff, K. Active and Intelligent Packaging: Innovations for the Future. Ph.D. Thesis, Department of Food Science & Technology, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2008; pp. 1–13. [Google Scholar]
- Water Vapor Transmission Rate of Biomass Based Film Materials. Available online: https://www.jstage.jst.go.jp/article/eaef/4/2/4_2_37/_article/-char/en (accessed on 17 May 2022).
- Wiles, J.L.; Vergano, P.j.; Barron, F.h.; Bunn, J.m.; Testin, R.f. Water Vapor Transmission Rates and Sorption Behavior of Chitosan Films. J. Food Sci. 2000, 65, 1175–1179. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, Structural and Antimicrobial Properties of Poly Vinyl Alcohol–Chitosan Biodegradable Films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Gaume, J.; Rivaton, A.; Thérias, S.; Gardette, J.-L. A Promising Method for Measuring Oxygen Permeability of Polymers Using PEO Photooxidation as a Sensor. J. Membr. Sci. 2012, 411–412, 153–159. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-Solubility of Chitosan and Its Antimicrobial Activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhao, R.; Wang, C.; Hu, K.; Sun, Y.; Politis, C.; Shavandi, A.; Nie, L. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Des. Monomers Polym. 2020, 23, 118–133. [Google Scholar] [CrossRef]
- Suryawati, B. Zinc Homeostasis Mechanism and Its Role in Bacterial Virulence Capacity. AIP Conf. Proc. 2018, 2021, 070021. [Google Scholar] [CrossRef]
- Zinc as an Agent for the Prevention of Biofilm Formation by Pathogenic Bacteria—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23509865/ (accessed on 19 May 2022).
- Zinc-Dependent Mechanical Properties of Staphylococcus Aureus Biofilm-Forming Surface Protein SasG|PNAS. Available online: https://www.pnas.org/doi/10.1073/pnas.1519265113 (accessed on 19 May 2022).
- Cai, P.; Liu, X.; Ji, D.; Yang, S.; Walker, S.L.; Wu, Y.; Gao, C.; Huang, Q. Impact of Soil Clay Minerals on Growth, Biofilm Formation, and Virulence Gene Expression of Escherichia Coli O157:H7. Environ. Pollut. 2018, 243, 953–960. [Google Scholar] [CrossRef]
- Influence of Clay Minerals on Microorganisms: I. MONTMORILLONITE and Kaolinite on Bacteria. Available online: https://cdnsciencepub.com/doi/10.1139/m66-078 (accessed on 19 May 2022).
- Montmorillonite Mitigates the Toxic Effect of Heavy Oil on Hydrocarbon-Degrading Bacterial Growth: Implications for Marine Oil Spill Bioremediation|Clay Minerals|Cambridge Core. Available online: https://www.cambridge.org/core/journals/clay-minerals/article/abs/montmorillonite-mitigates-the-toxic-effect-of-heavy-oil-on-hydrocarbondegrading-bacterial-growth-implications-for-marine-oil-spill-bioremediation/016843E5C47A5619C14AC0674308B81D (accessed on 19 May 2022).
- Lv, G.; Pearce, C.W.; Gleason, A.; Liao, L.; MacWilliams, M.P.; Li, Z. Influence of Montmorillonite on Antimicrobial Activity of Tetracycline and Ciprofloxacin. J. Asian Earth Sci. 2013, 77, 281–286. [Google Scholar] [CrossRef]
- Parolo, M.E.; Avena, M.J.; Pettinari, G.; Zajonkovsky, I.; Valles, J.M.; Baschini, M.T. Antimicrobial Properties of Tetracycline and Minocycline-Montmorillonites. Appl. Clay Sci. 2010, 49, 194–199. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial Activity of Chitin, Chitosan and Its Oligomers Prepared from Shrimp Shell Waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Butnaru, E.; Cheaburu, C.N.; Yilmaz, O.; Pricope, G.M.; Vasile, C. Poly(Vinyl Alcohol)/Chitosan/Montmorillonite Nanocomposites for Food Packaging Applications: Influence of Montmorillonite Content. High Perform. Polym. 2016, 28, 1124–1138. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia Coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Wei, J.-C.; Yen, Y.-T.; Su, H.-L.; Lin, J.-J. Inhibition of Bacterial Growth by the Exfoliated Clays and Observation of Physical Capturing Mechanism. J. Phys. Chem. C 2011, 115, 18770–18775. [Google Scholar] [CrossRef]
- Clay: New Opportunities for Tissue Regeneration and Biomaterial Design—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23722321/ (accessed on 27 January 2022).
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and Antifungal Activities of Silver, Copper and Zinc Montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Bagchi, B.; Kar, S.; Dey, S.K.; Bhandary, S.; Roy, D.; Mukhopadhyay, T.K.; Das, S.; Nandy, P. In Situ Synthesis and Antibacterial Activity of Copper Nanoparticle Loaded Natural Montmorillonite Clay Based on Contact Inhibition and Ion Release. Colloids Surf B Biointerfaces 2013, 108, 358–365. [Google Scholar] [CrossRef]




| Code Name | CS gr-wt% | PVOH gr-wt% | ZnO@Mt gr-wt% | ZnO@HNT gr-wt% |
|---|---|---|---|---|
| CS | 2.0–100 | - | - | - |
| CS/PVOH | 2.0–100 | 0.25–20 | - | - |
| CS/PVOH/3ZnO@Mt | 2.0–100 | 0.5–20 | 0.08–3 | - |
| CS/PVOH/6ZnO@Mt | 2.0–100 | 0.59 | 0.17–6 | - |
| CS/PVOH/3ZnO@HNT | 2.0–100 | 0.5–20 | - | 0.08–3 |
| CS/PVOH/6ZnO@HNT | 2.0–100 | 0.59–20 | - | 0.17–6 |
| Code Name | E Modulus (MPa) | σuts (MPa) | εb% |
|---|---|---|---|
| CS | 3274 ± 62 | 92.2 ± 3.5 | 10.8 ± 1.4 |
| CS/PVOH | 2920 ± 105 | 86.4 ± 3.4 | 14.5 ± 0.8 |
| CS/PVOH/3ZnO@Mt | 3300 ± 83 | 93.8 ± 4.2 | 7.9 ± 1.7 |
| CS/PVOH/6ZnO@Mt | 3840 ± 51 | 106.4 ± 2.8 | 6.4 ± 10.2 |
| CS/PVOH/3ZnO@HNT | 3430 ± 89 | 95.8 ± 4.2 | 7.1 ± 1.8 |
| CS/PVOH/6ZnO@HNT | 3980 ± 51 | 109.1 ± 2.8 | 6.1 ± 1.2 |
| Sample Code | Mean Value ± Std. Err. Film Thick. (mm) | Mean Value ± Std. Err. % Water Sorption | Mean Value ± Std. Err. WVTR (g/cm2·Day) | DWV (10−4 cm2/s) | Mean Value ± Std. Err. OTR (mL/m2·Day) | PeO2 (10−10 cm2/s) |
|---|---|---|---|---|---|---|
| CS | 0.15 ± 0.002 | 175 ± 4 | 1.1764 ± 0.0102 | 89.313 | 40.1 ± 1.6 | 6.9618 |
| CS/PVOH | 0.17 ± 0.003 | 184 ± 5 | 0.1613 ± 0.0097 | 13.879 | 38.2 ± 1.9 | 7.5162 |
| CS/PVOH/3ZnO@Mt | 0.16 ± 0.002 | 191 ± 6 | 0.1521 ± 0.0091 | 12.317 | 26.9 ± 1.3 | 4.9815 |
| CS/PVOH/6ZnO@Mt | 0.13 ± 0.003 | 194 ± 5 | 0.1384 ± 0.0083 | 9.106 | 21.5 ± 1.1 | 3.2350 |
| CS/PVOH/3ZnO@HNT | 0.14 ± 0.001 | 190 ± 4 | 0.1452 ± 0.0087 | 10.289 | 19.5 ± 0.9 | 3.1597 |
| CS/PVOH/6ZnO@HNT | 0.15 ± 0.002 | 192 ± 7 | 0.1120 ± 0.0067 | 8.503 | 14.4 ± 0.4 | 2.5000 |
| Sample Code | Mean Value Film Thick. (mm) | Mean Value WVTR (g·m−2·day−1) | DWV (cm2·s−1) | Mean Value OTR (cm3·m−2·day−1) | PeO2 (cm2·s−1) |
|---|---|---|---|---|---|
| PP [65] | 0.0254 | 3.9 | 2.76 × 10−7 | - | - |
| PP [66] | 0.04 | 1.4 | 1.12 × 10−5 | 2702 | 1.25 × 10−8 |
| HDPE [65] | 0.0254 | 4.7–7.8 | (3.32–5.52) 10−7 | - | - |
| LDPE [65] | 0.0254 | 16–23 | (1.13–1.63) 10−6 | - | - |
| LDPE [66] | 0.05 | 5.6 | 5.61 × 10−5 | 6314 | 3.65 × 10−8 |
| LDPE [67] | 0.03175 | 2.66–16.29 | (5.65–11.73) × 10−7 | - | - |
| PLA [68] | 0.15–0.35 | 195.1–103 | (1.94–2.39) × 10−4 | 1400–377 | (4.21–5.76) × 10−8 |
| CS [69] | 0.02 | 1200 | 6.77 × 10−5 | - | - |
| Film Material | E. coli | S. aureus | S. enterica | L. monocytogenes | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition 1 (Mean Diameter of Clear Zone) | Contact 2 (Under the Film Disc) | Inhibition 1 (Mean Diameter of Clear Zone) | Contact 2 (Under the Film Disc) | Inhibition 1 (Mean Diameter of Clear Zone) | Contact 2 (Under the Film Disc) | Inhibition 1 (Mean Diameter of Clear Zone) | Contact 2 (Under the Film Disc) | |
| CS | 0.00 a | - | 0.00 a | - | 0.00 a | - | 0.00 a | + |
| CS/PVOH | 3.90 ± 0.14 b | - | 4.10 ± 0.14 b,c | - | 3.00 ± 0.00 b | - | 0.00 a | + |
| CS/PVOH/3ZnO@Mt | 4.04 ± 1.41 b | - | 5.00± 0.00 d,e | - | 4.00 ± 0.00 b,c | - | 2.00 ± 0.00 b | - |
| CS/PVOH/6ZnO@Mt | 5.00 ± 0.00 b,c | - | 3.50 ± 0.71 b | - | 4.50 ± 0.71 c,d | - | 0.00 a | - |
| CS/PVOH/3ZnO@HNT | 6.00 ± 0.72 c | - | 4.25 ± 0.35 b,c | - | 4.00 ± 0.01 b,c | - | 3.50 ± 0.71 c | - |
| CS/PVOH/6ZnO@HNT | 8.50 ± 0.71 d | - | 5.50 ± 0.71 e | - | 4.50 ± 0.71 c,d | - | 4.51 ± 0.71 c | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. https://doi.org/10.3390/nano12111843
Giannakas AE, Salmas CE, Moschovas D, Baikousi M, Kollia E, Tsigkou V, Karakassides A, Leontiou A, Kehayias G, Avgeropoulos A, et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials. 2022; 12(11):1843. https://doi.org/10.3390/nano12111843
Chicago/Turabian StyleGiannakas, Aris E., Constantinos E. Salmas, Dimitrios Moschovas, Maria Baikousi, Eleni Kollia, Vasiliki Tsigkou, Anastasios Karakassides, Areti Leontiou, George Kehayias, Apostolos Avgeropoulos, and et al. 2022. "Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications" Nanomaterials 12, no. 11: 1843. https://doi.org/10.3390/nano12111843












