Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
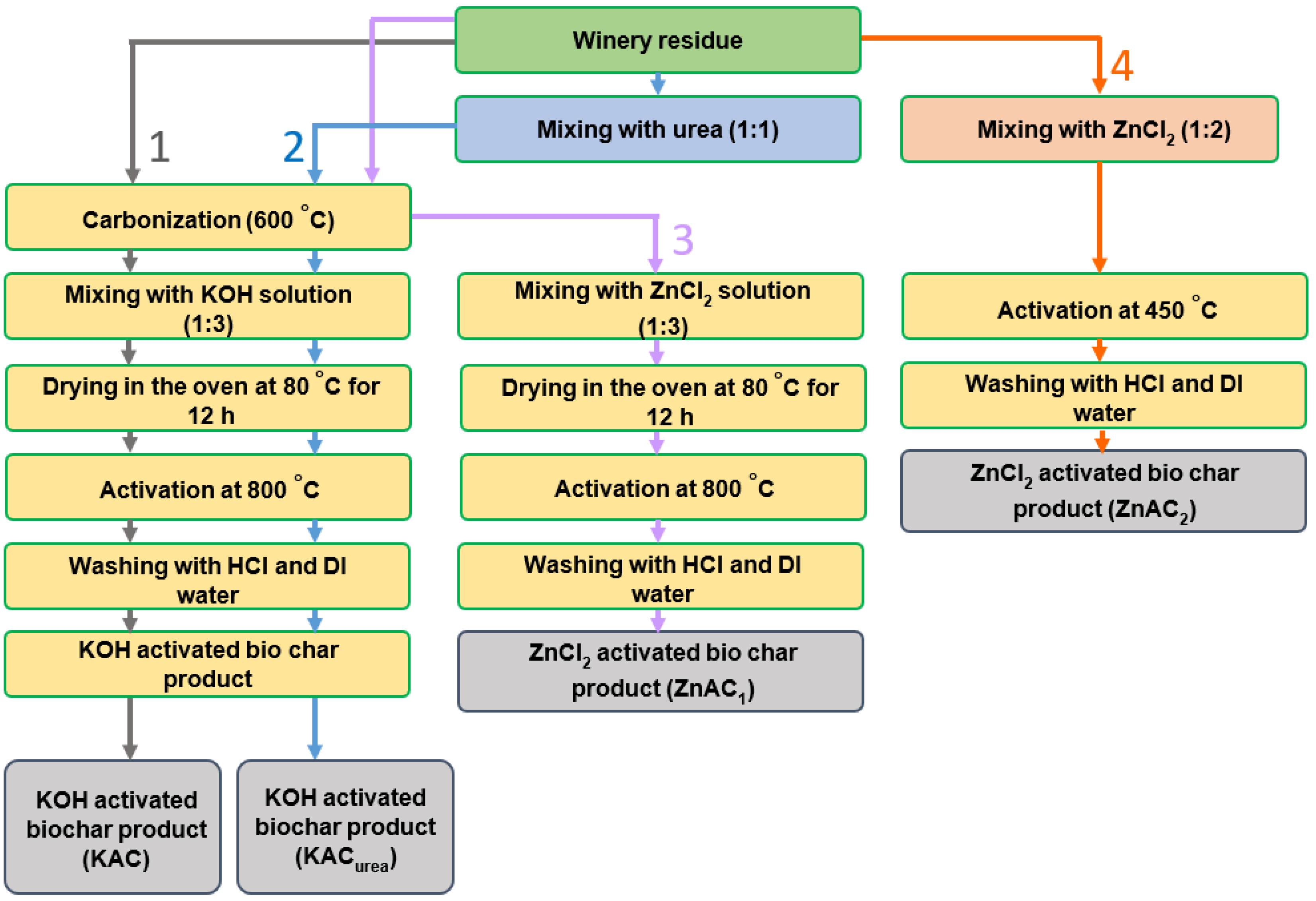
2.2. Synthesis of GM-Derived N-Doped AC
2.3. Characterization of Materials
2.4. Density Functional Theory (DFT) Calculations
2.5. Multilayer Perceptron (MLP) Model
3. Results and Discussion
3.1. Physicochemical Characterization of the GM-Derived N-Doped AC Materials
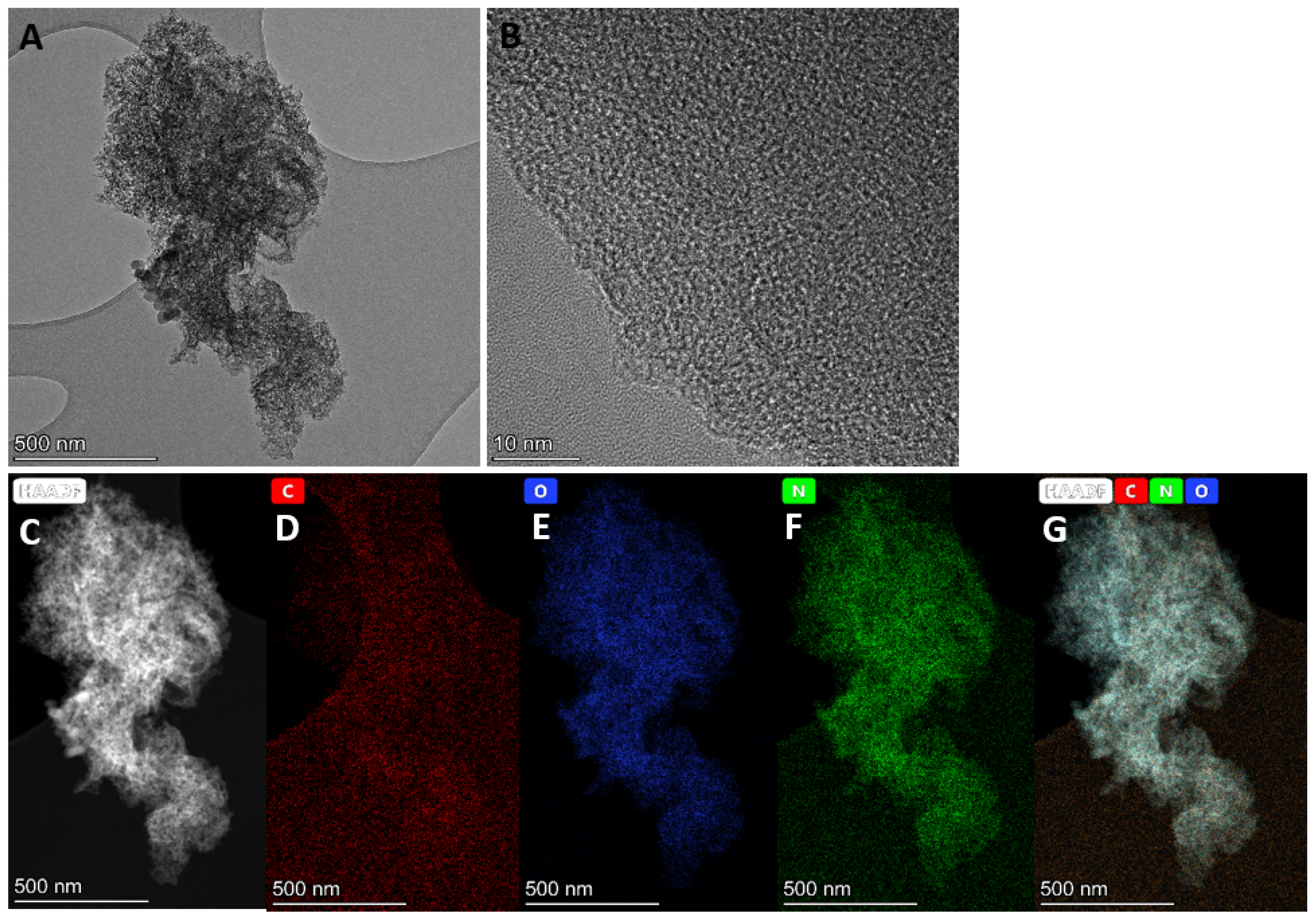
3.1.1. Morphology Analyses
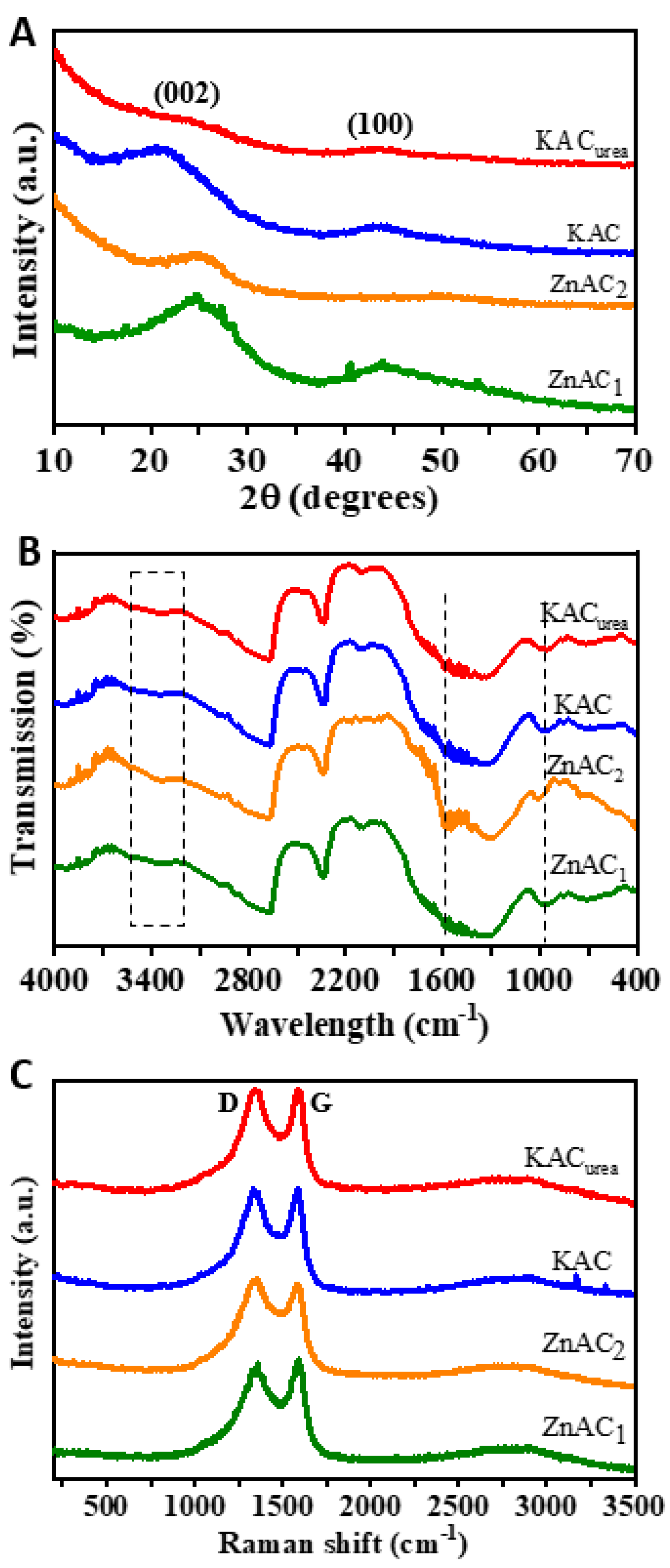
3.1.2. Structural and Spectral Analyses
3.1.3. Surface Area and Pore Structure
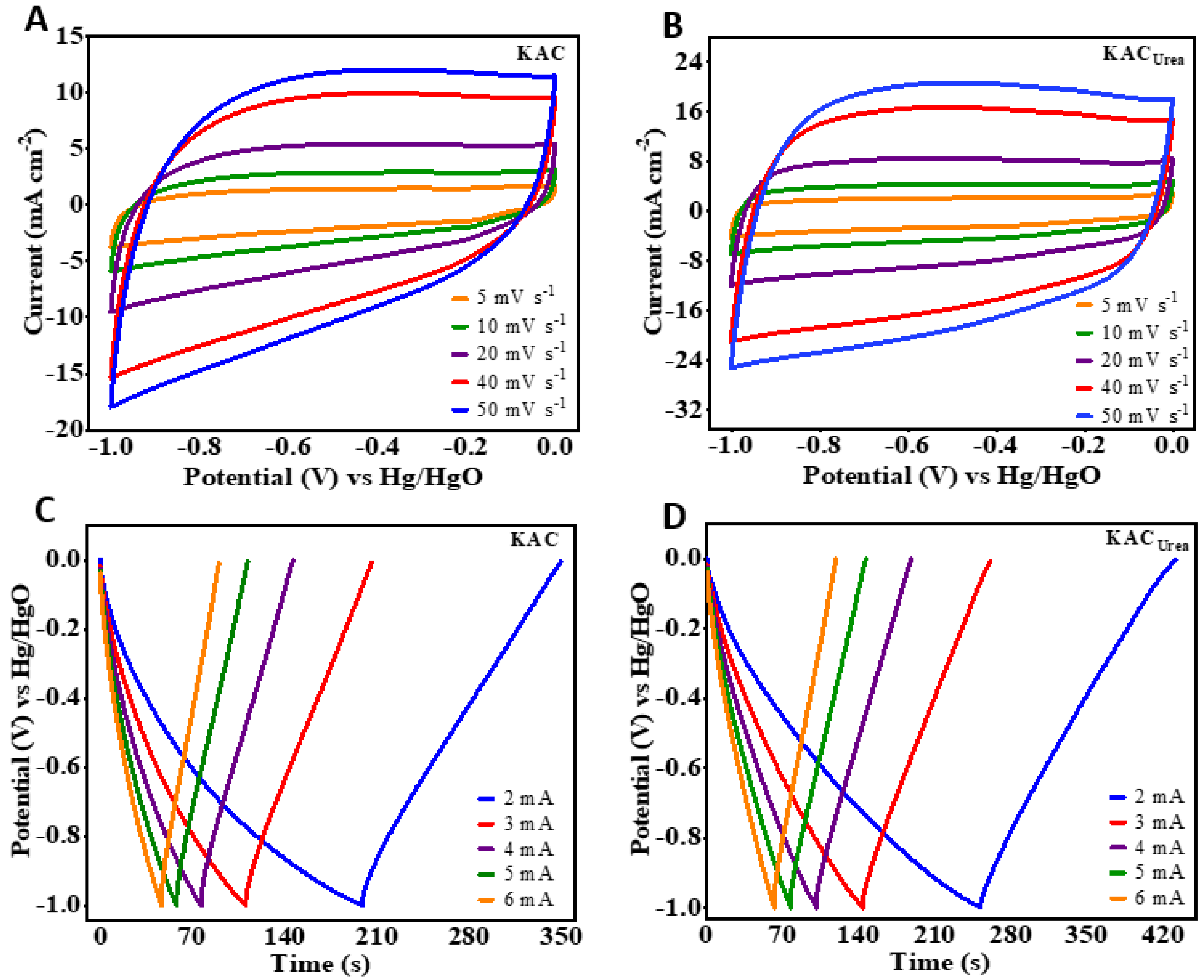
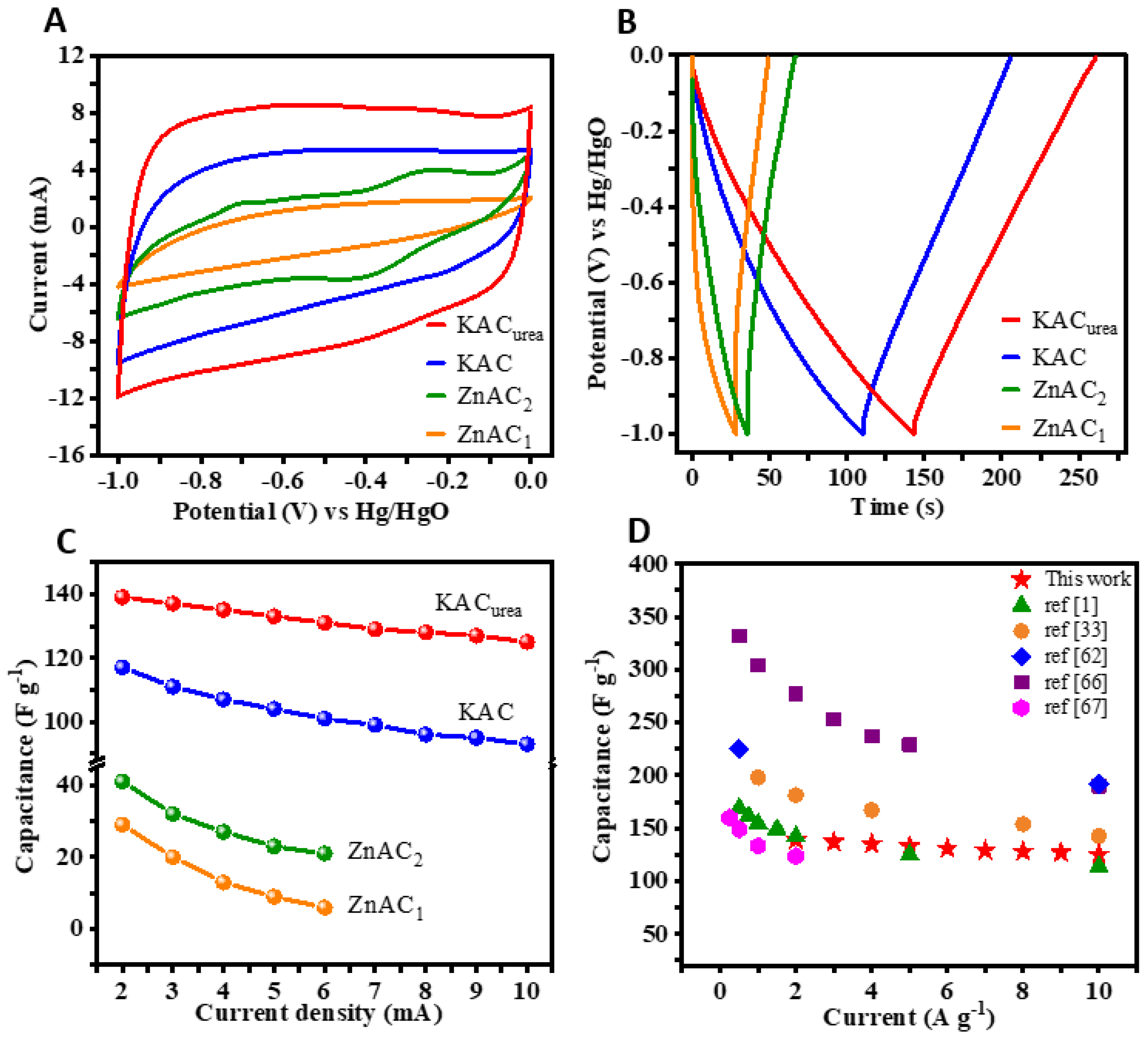
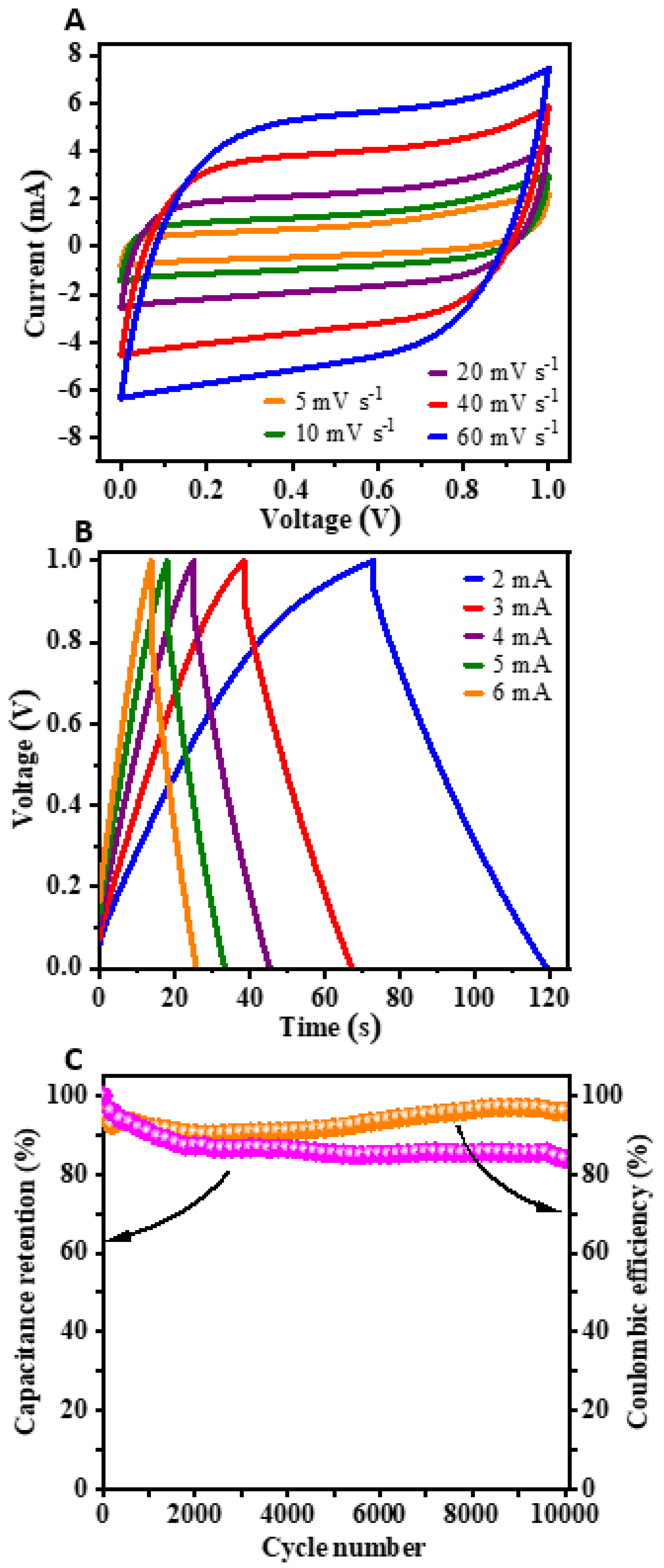
3.2. Supercapacitor Applications of GM-Derived N-Doped AC: Electrochemical Performance (CV, GCD) of the Single Electrode and Symmetric Capacitor Device
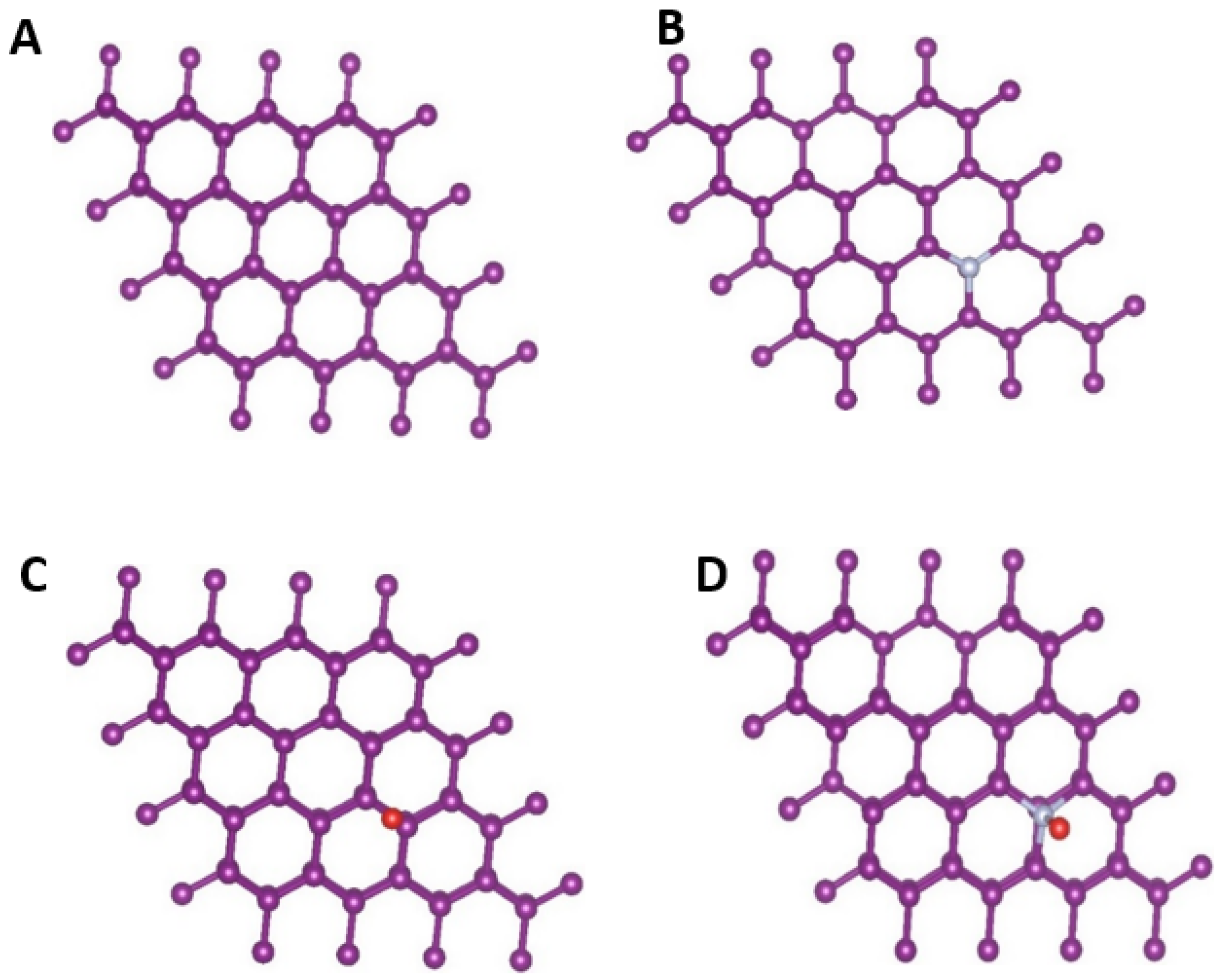
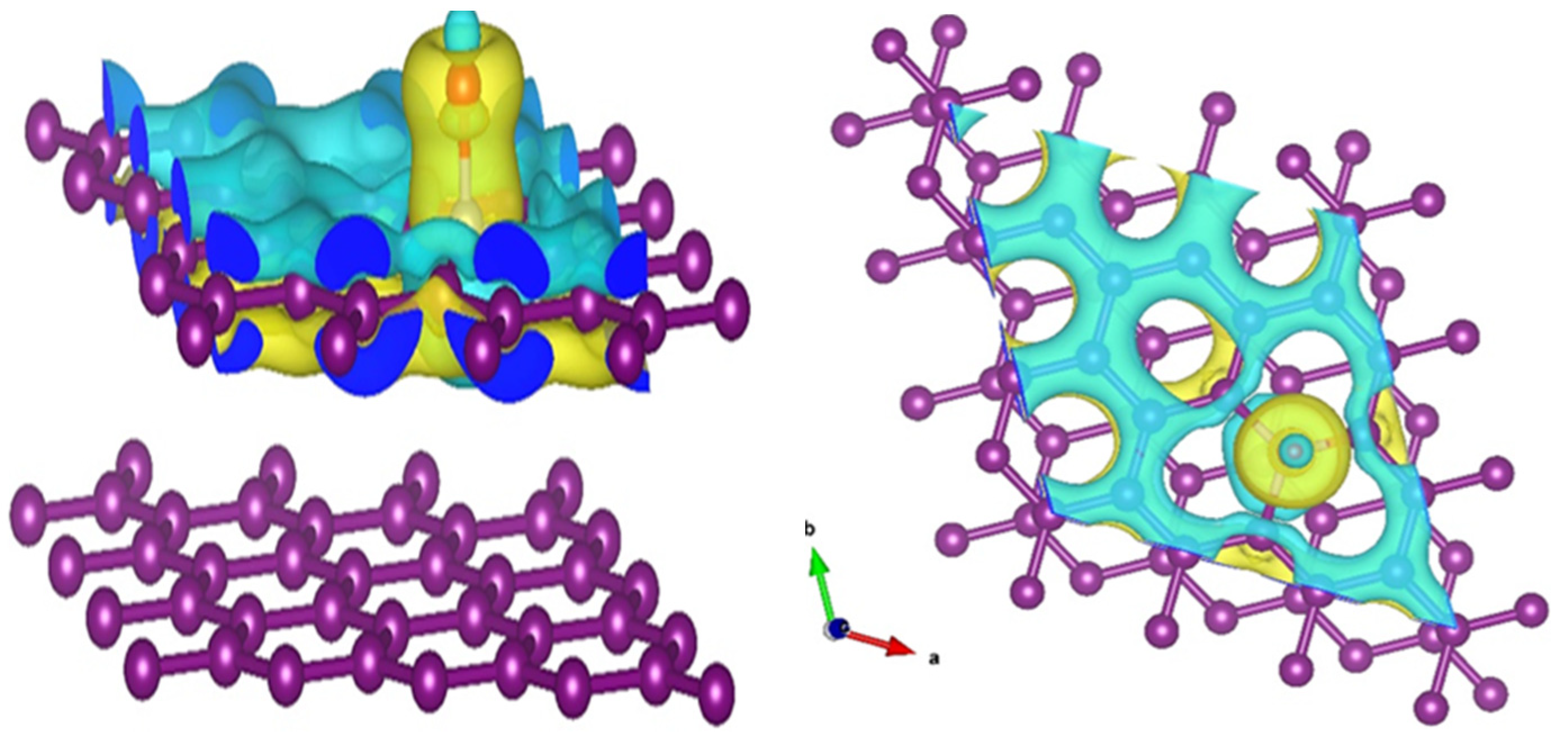
3.3. Theoretical Insights: Density Functional Theory (DFT) Study
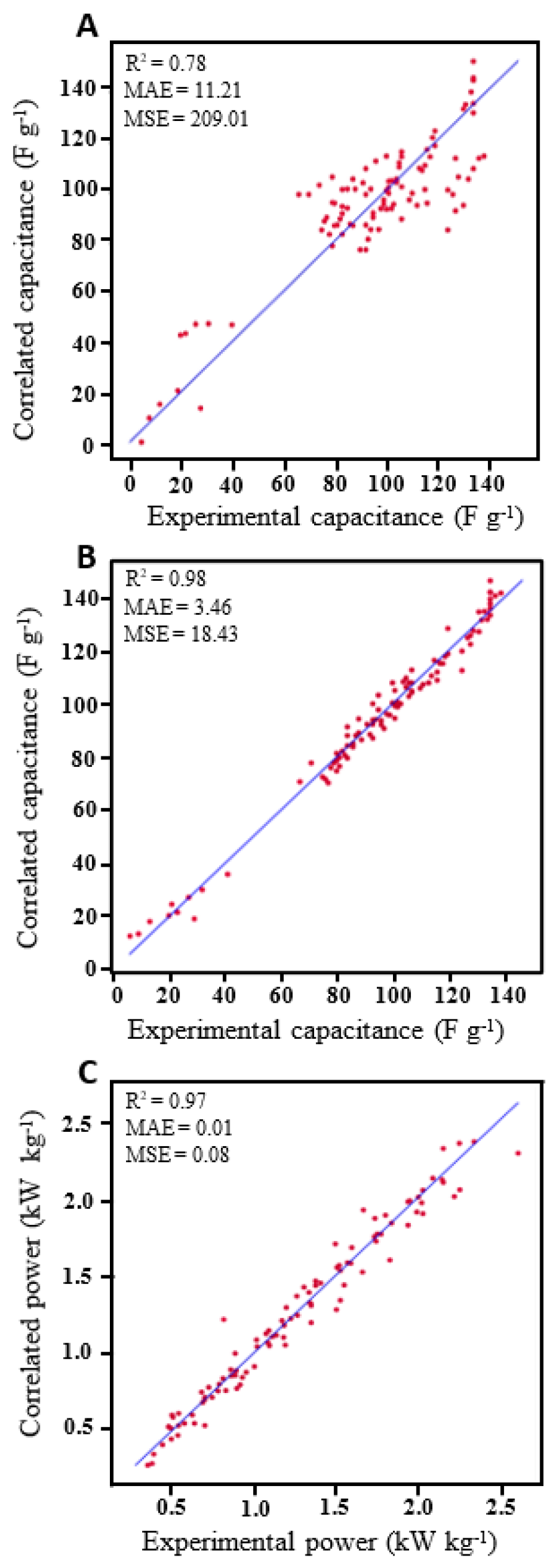
3.4. Machine Learning Insights: MLP Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wu, J.; Ma, H.; Han, G.; Yang, D.; Chen, Y.; Zhou, J. H3PO4-Assisted Synthesis of Apricot Shell Lignin-Based Activated Carbon for Capacitors: Understanding the Pore Structure/Electrochemical Performance Relationship. Energy Fuels 2021, 35, 8303–8312. [Google Scholar] [CrossRef]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, T.P.; Tetana, Z.N.; Ozoemena, K.I. Defective 3D Nitrogen-Doped Carbon Nanotube-Carbon Fibre Networks for High-Performance Supercapacitor: Transformative Role of Nitrogen-Doping from Surface-Confined to Diffusive Kinetics. Carbon N. Y. 2020, 169, 312–326. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Heimböckel, R.; Hoffmann, F.; Fröba, M. Insights into the Influence of the Pore Size and Surface Area of Activated Carbons on the Energy Storage of Electric Double Layer Capacitors with a New Potentially Universally Applicable Capacitor Model. Phys. Chem. Chem. Phys. 2019, 21, 3122–3133. [Google Scholar] [CrossRef] [Green Version]
- Bora, M.; Bhattacharjya, D.; Saikia, B.K. Coal-Derived Activated Carbon for Electrochemical Energy Storage: Status on Supercapacitor, Li-Ion Battery, and Li-S Battery Applications. Energy Fuels 2021, 35, 18285–18307. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Huang, J.; Xing, W.; Yan, Z. Functionalization of Petroleum Coke-Derived Carbon for Synergistically Enhanced Capacitive Performance. Nanoscale Res. Lett. 2016, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Zhang, H.; He, X.; Ma, H.; Dong, S.; Xie, X. Synthesis of Porous Carbons from Coal Tar Pitch for High-Performance Supercapacitors. New Carbon Mater. 2019, 34, 132–139. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Dou, G.; Salari, M.; Grinstaff, M.W. Biomass-Based Fuels and Activated Carbon Electrode Materials: An Integrated Approach to Green Energy Systems. ACS Sustain. Chem. Eng. 2017, 5, 3046–3054. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, K.; Tang, Q.; Wang, L.; Han, L.; Fang, H.; Zhang, Y.; Wang, S.; Wang, L. Large-Size Graphene-like Porous Carbon Nanosheets with Controllable N-Doped Surface Derived from Sugarcane Bagasse Pith/Chitosan for High Performance Supercapacitors. Carbon N. Y. 2017, 123, 290–298. [Google Scholar] [CrossRef]
- Yakaboylu, G.A.; Yumak, T.; Jiang, C.; Zondlo, J.W.; Wang, J.; Sabolsky, E.M. Preparation of Highly Porous Carbon through Slow Oxidative Torrefaction, Pyrolysis, and Chemical Activation of Lignocellulosic Biomass for High-Performance Supercapacitors. Energy Fuels 2019, 33, 9309–9329. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent Progress of Biomass-Derived Carbon Materials for Supercapacitors. J. Power Sources 2020, 451, 227794. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Yang, X.; Yu, Y.; Lin, H.; Sheng, K. Synthesis of Fe/N Co-Doped Porous Carbon Spheres Derived from Corncob for Supercapacitors with High Performances. Energy Fuels 2021, 35, 14157–14168. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated Bio-Chars Derived from Rice Husk via One- and Two-Step KOH-Catalyzed Pyrolysis for Phenol Adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Wong, S.I.; Li, Q.; Zhou, P.; Zhou, J.; Zhao, Y.; Sunarso, J.; Zhuo, S. Fishbone-Derived N-Doped Hierarchical Porous Carbon as an Electrode Material for Supercapacitor. J. Alloys Compd. 2020, 832, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of Garlic Skin-Derived 3D Hierarchical Porous Carbon for High-Performance Supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y. Comparison of Activated Carbons Prepared from Wheat Straw via ZnCl2 and KOH Activation. Waste Biomass Valorization 2017, 8, 549–559. [Google Scholar] [CrossRef]
- McNair, R.; Szekely, G.; Dryfe, R.A.W. Sustainable processing of electrodes for membrane capacitive deionization (MCDI). J. Clean. Prod. 2022, 342, 130922. [Google Scholar] [CrossRef]
- Wine Australia. Australian Wine: Production, Sales and Inventory Report 2019–20; Wine Australia: Kent Town, SA, Australia, 2021. [Google Scholar]
- Bacic, T. Recovery of Valuable Products from Lees and Integrated Approach to Minimise Waste and Add Value to Wine Production; Final report to Grape and Wine Research and Development Corporation; The University of Melbourne: Parkville, VIC, Australia, 2003. [Google Scholar]
- Guardia, L.; Suárez, L.; Querejeta, N.; Pevida, C.; Centeno, T.A. Winery Wastes as Precursors of Sustainable Porous Carbons for Environmental Applications. J. Clean. Prod. 2018, 193, 614–624. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Cui, L. Recent Progress of Mesoporous Materials for High Performance Supercapacitors. Microporous Mesoporous Mater. 2021, 314, 110870. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Kado, Y.; Soneda, Y.; Hatori, H.; Kodama, M. Advanced Carbon Electrode for Electrochemical Capacitors. J. Solid State Electrochem. 2019, 23, 1061–1081. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The preparation of active carbons from coal by chemical and physical activation. Carbon 1996, 34, 471–479. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical Porous Carbon from Mango Seed Husk for Electro-Chemical Energy Storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B—Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integration*. J. Mater. Chem. A 2019, 7, 2156–2164. [Google Scholar] [CrossRef]
- Su, H.; Lin, S.; Deng, S.; Lian, C.; Shang, Y.; Liu, H. Predicting the Capacitance of Carbon-Based Electric Double Layer Capacitors by Machine Learning. Nanoscale Adv. 2019, 1, 2162–2166. [Google Scholar] [CrossRef] [Green Version]
- Ran, F.; Yang, X.; Xu, X.; Li, S.; Liu, Y.; Shao, L. Green Activation of Sustainable Resources to Synthesize Nitrogen-Doped Oxygen-Riched Porous Carbon Nanosheets towards High-Performance Supercapacitor. Chem. Eng. J. 2021, 412, 128673. [Google Scholar] [CrossRef]
- Hong, K.L.; Qie, L.; Zeng, R.; Yi, Z.Q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.J.; Zhang, W.X.; et al. Biomass Derived Hard Carbon Used as a High Performance Anode Material for Sodium Ion Batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Ghosh, S.; Santhosh, R.; Jeniffer, S.; Raghavan, V.; Jacob, G.; Nanaji, K.; Kollu, P.; Jeong, S.K.; Grace, A.N. Natural Biomass Derived Hard Carbon and Activated Carbons as Electrochemical Supercapacitor Electrodes. Sci. Rep. 2019, 9, 16315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, E.; Liu, W.; Liu, S.; Zhang, Y.; Wang, H.; Chen, S.; Cheng, F.; Zhao, S.; Yang, H. Rich Sulfur Doped Porous Carbon Materials Derived from Ginkgo Leaves for Multiple Electrochemical Energy Storage Devices. J. Mater. Chem. A 2017, 5, 2204–2214. [Google Scholar] [CrossRef]
- Mopoung, S.; Moonsri, P.; Palas, W.; Khumpai, S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Sci. World J. 2015, 2015, 415961. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Li, T.; Zhu, L.; Wang, X.; Lin, Q. Preparation of Activated Carbons by Microwave Heating KOH Activation. Appl. Surf. Sci. 2007, 254, 506–512. [Google Scholar] [CrossRef]
- Cherifi, H.; Fatiha, B.; Salah, H. Kinetic Studies on the Adsorption of Methylene Blue onto Vegetal Fiber Activated Carbons. Appl. Surf. Sci. 2013, 282, 52–59. [Google Scholar] [CrossRef]
- Ding, Z.; Trouillet, V.; Dsoke, S. Are Functional Groups Beneficial or Harmful on the Electrochemical Performance of Activated Carbon Electrodes? J. Electrochem. Soc. 2019, 166, A1004–A1014. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying Disorder in Graphite-Based Systems by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Y.; Yuan, L. A Langmuir-like Desorption Model for Reflecting the Inhomogeneous Pore Structure of Coal and Its Experimental Verification. RSC Adv. 2015, 5, 2434–2440. [Google Scholar] [CrossRef]
- Yagmur, E.; Gokce, Y.; Tekin, S.; Semerci, N.I.; Aktas, Z. Characteristics and Comparison of Activated Carbons Prepared from Oleaster (Elaeagnus angustifolia L.) Fruit Using KOH and ZnCl2. Fuel 2020, 267, 117232. [Google Scholar] [CrossRef]
- Bouchemal, N.; Belhachemi, M.; Merzougui, Z.; Addoun, F. The Effect of Temperature and Impregnation Ratio on the Active Carbon Porosity. Desalin. Water Treat. 2009, 10, 115–120. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The Preparation of Activated Carbon from Macadamia Nutshell by Chemical Activation. Carbon N. Y. 1997, 35, 1723–1732. [Google Scholar] [CrossRef]
- Wabo, S.G.; Klepel, O. Nitrogen Release and Pore Formation through KOH Activation of Nitrogen-Doped Carbon Materials: An Evaluation of the Literature. Carbon Lett. 2021, 31, 581–592. [Google Scholar] [CrossRef]
- Li, Z.; Guo, K.; Chen, X. Controllable Synthesis of Nitrogen-Doped Mesoporous Carbons for Supercapacitor Applications. RSC Adv. 2017, 7, 30521–30532. [Google Scholar] [CrossRef] [Green Version]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent Advances in Biomass Derived Activated Carbon Electrodes for Hybrid Electrochemical Capacitor Applications: Challenges and Opportunities. Carbon N. Y. 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Fan, H.; Wang, G. Naturally Nitrogen Doped Porous Carbon Derived from Waste Shrimp Shells for High-Performance Lithium Ion Batteries and Supercapacitors. Microporous Mesoporous Mater. 2017, 246, 72–80. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical Properties of Carbon from Oil Palm Kernel Shell for High Performance Supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Li, M.; Wang, Y.; Sun, X.; Wang, F.; Yang, R. Soybean Root-Derived Hierarchical Porous Carbon as Electrode Material for High-Performance Supercapacitors in Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 33626–33634. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, L.; Liu, Y.; Zhao, G.; Chen, J.Y.; Yu, G. Biobased Nano Porous Active Carbon Fibers for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15205–15215. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Nonaqueous Electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Yin, H.; Lu, B.; Xu, Y.; Tang, D.; Mao, X.; Xiao, W.; Wang, D.; Alshawabkeh, A.N. Harvesting Capacitive Carbon by Carbonization of Waste Biomass in Molten Salts. Environ. Sci. Technol. 2014, 48, 8101–8108. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, S.; Wei, B. Supercapacitors from Activated Carbon Derived from Banana Fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Teixeira, J.G.; Almeida, I. Development of Easy Made Low Cost Bindless Monolithic Electrodes from Biomass with Controlled Properties to Be Used as Electrochemical Capacitors. Bioresour. Technol. 2011, 102, 2781–2787. [Google Scholar] [CrossRef] [Green Version]
- Gou, G.; Huang, F.; Jiang, M.; Li, J.; Zhou, Z. Hierarchical Porous Carbon Electrode Materials for Supercapacitor Developed from Wheat Straw Cellulosic Foam. Renew. Energy 2020, 149, 208–216. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Zhuang, L.; Zhang, L.; Wang, K.; Yao, X. Defective Carbons Derived from Macadamia Nut Shell Biomass for Efficient Oxygen Reduction and Supercapacitors. ChemElectroChem 2018, 5, 1874–1879. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhao, Y.; Zhang, B.; Xia, H.; Zhou, J.; Xie, W.; Li, H. Nano-Micro Carbon Spheres Anchored on Porous Carbon Derived from Dual-Biomass as High Rate Performance Supercapacitor Electrodes. J. Power Sources 2018, 381, 116–126. [Google Scholar] [CrossRef]
- Ramirez, N.; Sardella, F.; Deiana, C.; Schlosser, A.; Müller, D.; Kißling, P.A.; Klepzig, L.F.; Bigall, N.C. Capacitive Behavior of Activated Carbons Obtained from Coffee Husk. RSC Adv. 2020, 10, 38097–38106. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Liang, Y.; Lin, H.; Zhang, S.; Liu, J.; Jin, C.; Choe, U.; Sheng, K. Green Synthesis of Nitrogen-Doped Porous Carbon Derived from Rice Straw for High-Performance Supercapacitor Application. Energy Fuels 2020, 34, 8966–8976. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Kaur, A.; Maheshwari, P.H.; Sundriyal, S. Surface and Diffusion Charge Contribution Studies of Human Hair- Derived Heteroatom-Doped Porous Carbon Electrodes for Supercapacitors. Energy Fuels 2022, 36, 626–637. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, T.; Ju, W.; Shi, S. Materials discovery and design using machine learning. J. Mater. 2017, 3, 159–177. [Google Scholar] [CrossRef]
- Schmidt, J.; Marques, M.R.G.; Botti, S.; Marques, M.A.L. Recent advances and applications of machine learning in solid-state materials science. NPJ Comput. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]
- Isayev, O.; Oses, C.; Toher, C.; Gossett, E.; Curtarolo, S.; Tropsha, A. Universal fragment descriptors for predicting properties of inorganic crystals. Nat. Commun. 2017, 8, 15679. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Woo, T.K.; Wilmer, C.E.; Snurr, R.Q. Large-scale quantitative structure-property relationship (QSPR) analysis of methane storage in metal-organic frameworks. J. Phys. Chem. C 2013, 117, 7681–7689. [Google Scholar] [CrossRef]
- Van Der Linden, J.H.; Narsilio, G.A.; Tordesillas, A. Machine learning framework for analysis of transport through complex networks in porous, granular media: A focus on permeability. Phys. Rev. E 2016, 94, 022904. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Lee, L.; Kim, J. Inverse design of porous materials using artificial neural networks. Sci. Adv. 2020, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Diao, Y.; Yan, L.; Gao, K. A strategy assisted machine learning to process multi-objective optimization for improving mechanical properties of carbon steels. J. Mater. Sci. Technol. 2022, 109, 86–93. [Google Scholar] [CrossRef]
- Snyder, J.C.; Rupp, M.; Hansen, K.; Müller, K.R.; Burke, K. Finding density functionals with machine learning. Phys. Rev. Lett. 2012, 108, 253002. [Google Scholar] [CrossRef]
- Shi, S.; Gao, J.; Liu, Y.; Zhao, Y.; Wu, Q.; Ju, W.; Ouyang, C.; Xiao, R. Multi-scale computation methods: Their applications in lithium-ion battery research and development. Chin. Phys. B 2015, 25, 018212. [Google Scholar] [CrossRef]
- Chong, S.; Lee, S.; Kim, B.; Kim, J. Applications of Machine Learning in Metal-Organic Frameworks. Coord. Chem. Rev. 2020, 423, 213487. [Google Scholar] [CrossRef]
- Ge, M.; Su, F.; Zhao, Z.; Su, D. Deep Learning Analysis on Microscopic Imaging in Materials Science. Mater. Today Nano 2020, 11, 100087. [Google Scholar] [CrossRef]
- Krishnamurthy, D.; Weiland, H.; Barati Farimani, A.; Antono, E.; Green, J.; Viswanathan, V. Machine Learning Based Approaches to Accelerate Energy Materials Discovery and Optimization. ACS Energy Lett. 2019, 4, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, A.; Salehi, S. Dynamic Modeling of the Formation Damage and Mud Cake Deposition Using Filtration Theories Coupled with SEM Image Processing. J. Nat. Gas Sci. Eng. 2017, 42, 157–168. [Google Scholar] [CrossRef]
- Ezeakacha, C.P.; Rabbani, A.; Salehi, S.; Ghalambor, A. Integrated Image Processing and Computational Techniques to Characterize Formation Damage. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 7–9 February 2018. [Google Scholar] [CrossRef]
- Rogers, S.; Girolami, M. A First Course of Machine Learning, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhang, H.; Zhou, X.L.; Shao, L.M.; Lü, F.; He, P.J. Hierarchical Porous Carbon Spheres from Low-Density Polyethylene for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3801–3810. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte Selection for Supercapacitive Devices: A Critical Review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef] [Green Version]

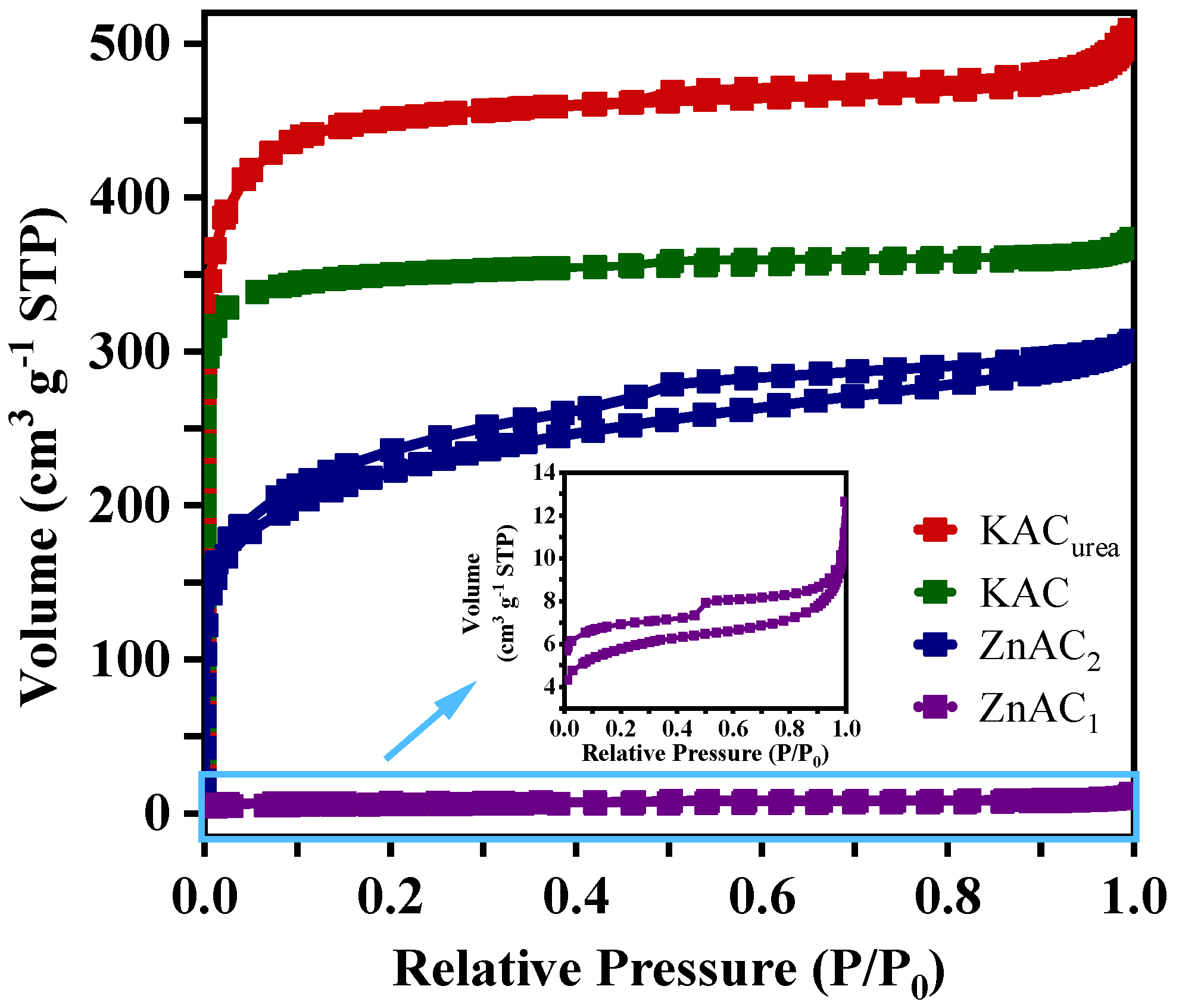
| Sample | BET Surface Area (m2 g−1) | t-Plot Micropore Area (m2 g−1) | Micropore Area (%) | Total Pore Volume (cm3 g−1) | t-Plot Micropore Volume (cm3 g−1) | Micropore Volume (%) |
|---|---|---|---|---|---|---|
| KACurea | 1356 | 1126 | 83.0 | 0.79 | 0.60 | 75.9 |
| KAC | 1128 | 998 | 88.5 | 0.58 | 0.53 | 91.4 |
| ZnAC1 | 29 | 26 | 89.7 | 0.02 | 0.01 | 50.0 |
| ZnAC2 | 711 | 275 | 38.7 | 0.46 | 0.15 | 32.6 |
| Ref. | Biomass Precursor | Activating Agent | Surface Area (m2 g−1) | Electrolyte | Current Density | Specific Capacitance (F g−1) |
|---|---|---|---|---|---|---|
| Wang et al. [1] | Apricot shell lignin | H3PO4 | 1474.82 | 6 M KOH | 0.5 A g−1 | 169.05 |
| Subramanian et al. [55] | Banana fibers | ZnCl2 KOH | 1097 686 | 1 M Na2SO4 | 0.5 A g−1 | 74 66 |
| Nabais et al. [56] | Coffee endocarp | CO2 KOH | 709 361 | 1 M H2SO4 | 10 mA (~0.2 A g−1) | 176 69 |
| Gou et al. [57] | Wheat straw | KOH | 772 | 6 M KOH | 0.5 A g−1 | 226.2 |
| Yan et al. [58] | Macadamia nutshell | KOH | 2202 | 1 M Na2SO4 | 1 A g−1 | 155 |
| Mondal et al. [49] | Shrimp shell | KOH | 1271 | 6 M KOH | 0.5 A g−1 | 239 |
| Liu et al. [59] | Rice straw | KOH | 1127 | 6 M KOH | 1 A g−1 | 337 |
| Huang et al. [52] | Wood sawdust | KOH | 2294 | 6 M KOH | 0.5 A g−1 | 225 |
| Ramirez et al. [60] | Coffee husk | Steam K2CO3 KOH | 1447 1156 2275 | 6 M KOH | 0.5 A g−1 | 138 129 106 |
| Xu et al. [61] | Rice straw | KHCO3 | 2786.5 | 6 M KOH | 1 A g−1 | 317 |
| Dubey et al. [62] | Human hair | KOH | 1992.4 | 1 M H2SO4 | 1 A g−1 | 274.5 |
| This work | GM | KOH | 1356 | 2M NaOH | 2 A g−1 | 139 |
| Configuration | Bond Length (Å) | Bader Charges | ||||
|---|---|---|---|---|---|---|
| C-O | C-N | N-O | O | N | C | |
| Pristine graphite + O | 2.27 | −0.35 | ||||
| N-doped graphite | 1.41 | −2.66 | 0.98 | |||
| 0.84 | ||||||
| 0.84 | ||||||
| N-doped graphite + O | 1.49 | 1.40 | −0.49 | −1.21 | 0.69 | |
| 0.39 | ||||||
| 0.39 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. https://doi.org/10.3390/nano12111847
Wickramaarachchi K, Minakshi M, Aravindh SA, Dabare R, Gao X, Jiang Z-T, Wong KW. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials. 2022; 12(11):1847. https://doi.org/10.3390/nano12111847
Chicago/Turabian StyleWickramaarachchi, Kethaki, Manickam Minakshi, S. Assa Aravindh, Rukshima Dabare, Xiangpeng Gao, Zhong-Tao Jiang, and Kok Wai Wong. 2022. "Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models" Nanomaterials 12, no. 11: 1847. https://doi.org/10.3390/nano12111847
APA StyleWickramaarachchi, K., Minakshi, M., Aravindh, S. A., Dabare, R., Gao, X., Jiang, Z.-T., & Wong, K. W. (2022). Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials, 12(11), 1847. https://doi.org/10.3390/nano12111847









