Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Date Palm Pits Extract
2.2.2. Biosynthesis of Nano Zinc Oxide and Composite Samples
2.3. Characterization of Date Palm Pits Extract and Prepared Nano Solid Samples
2.3.1. Characterization of Date Palm Pits Extract
2.3.2. Characterization of the Prepared Nano Solid Samples
2.4. Adsorption and Photocatalytic Degradation of Methylene Blue
2.4.1. Adsorption of MB onto the Prepared Solid Adsorbent
2.4.2. Photocatalytic Degradation of MB Using the Prepared Solid Nanocatalyst
3. Results and Discussion
3.1. Characterization of Date Palm Pits Extract and Prepared Solid Materials
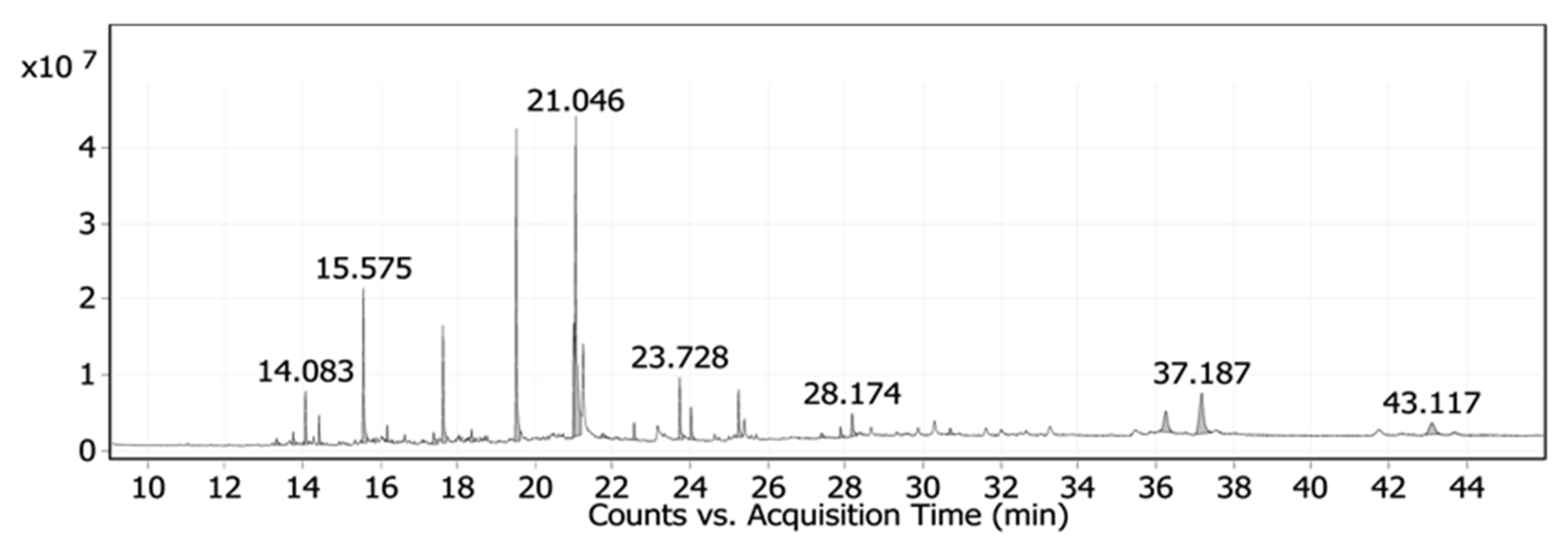
3.1.1. Chemical Characterization of Date Palm Pits Extract
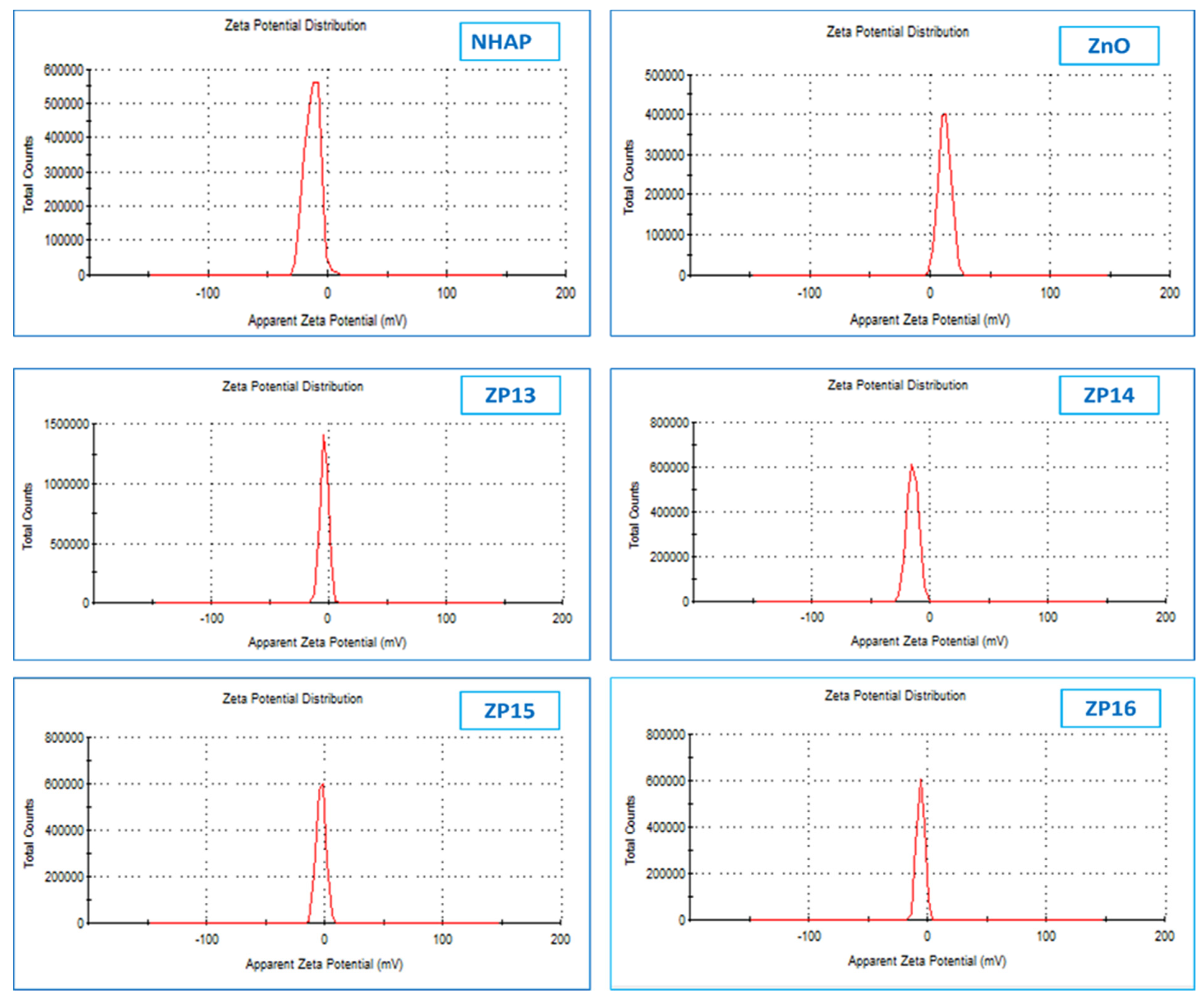
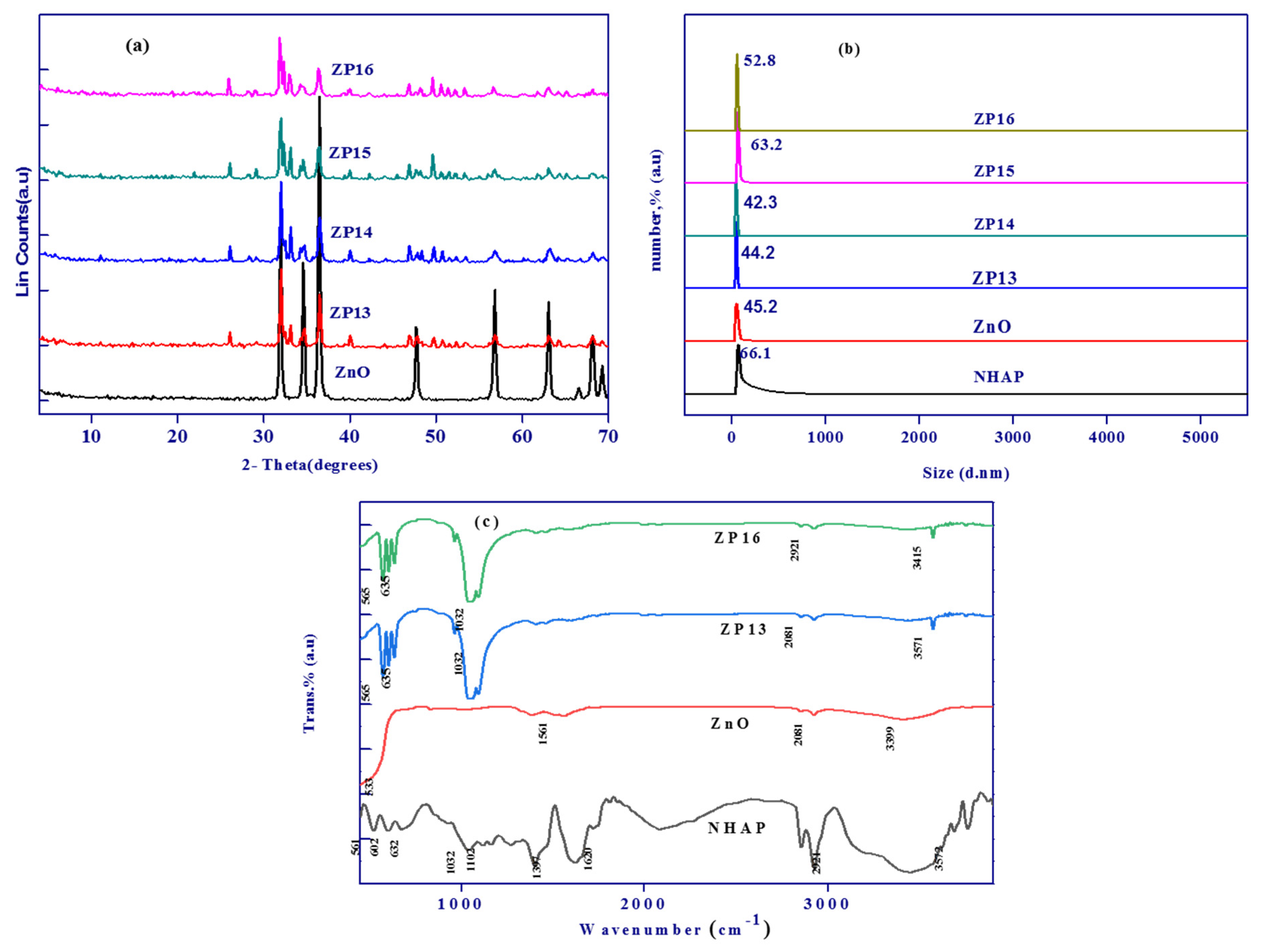
3.1.2. Characterization of the Prepared Solid Materials
3.2. Adsorption of Methylene Blue
3.2.1. Effect of Dosage
3.2.2. Effect of Initial pH of Methylene Blue Solution
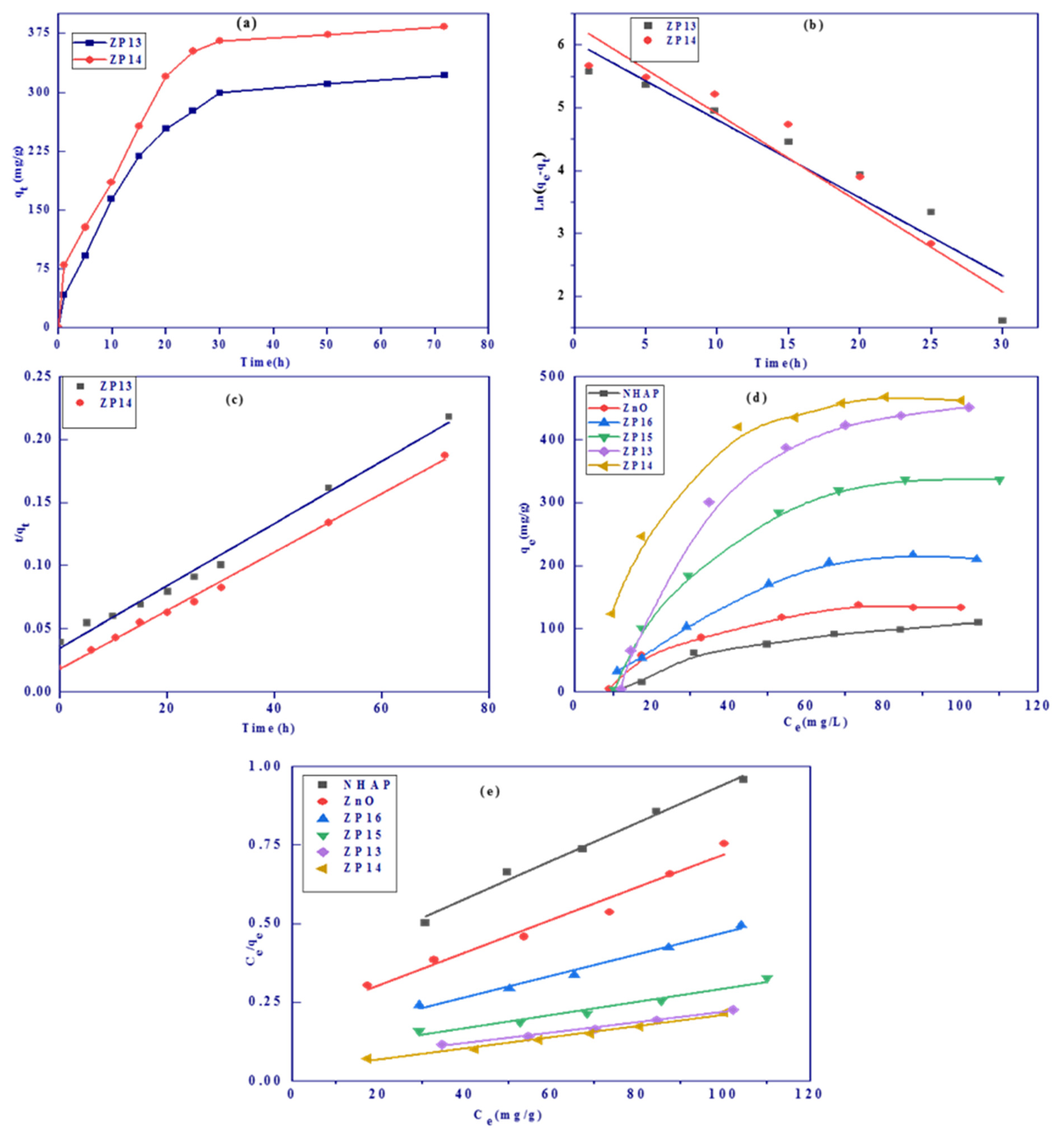
3.2.3. Effect of Contact Shaking Time and Kinetic Studies
3.2.4. Effect of Initial Methylene Blue Concentration
3.3. Photocatalytic Degradation of Methylene Blue
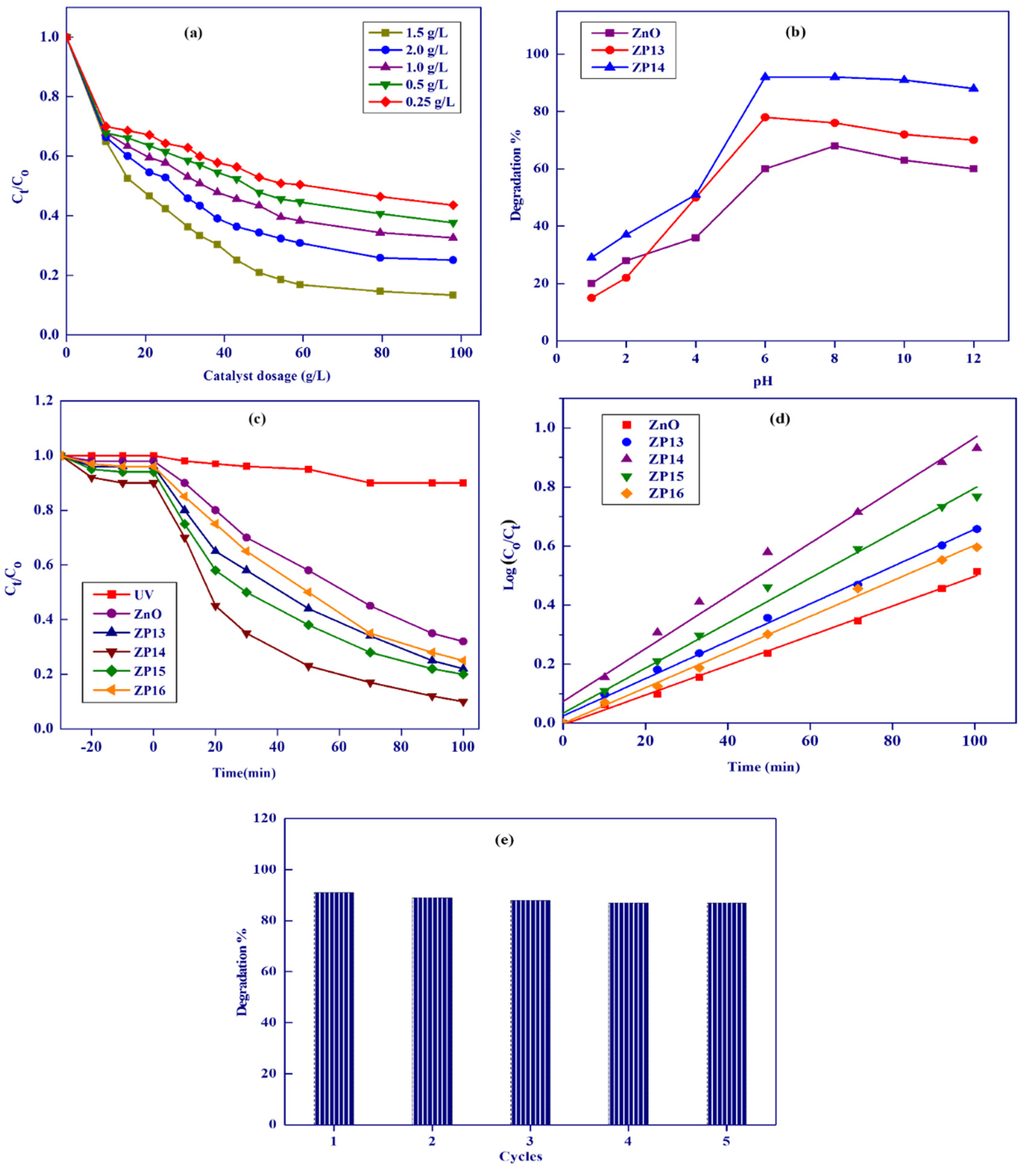
3.3.1. Effect of Photocatalyst Dosage
3.3.2. Effect of Initial Solution pH on Degradation of Dye
3.3.3. Comparison of Catalytic Activities of the Prepared Solid Photocatalysts
3.3.4. Kinetic Studies for Photocatalytic Degradation of Methylene Blue
3.3.5. Photocatalyst Reusability
3.4. Comparison between ZP14 and Other Solid Adsorbents and Photocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, A.; Jain, A.K. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Colloid Interface Sci. 2005, 281, 49–55. [Google Scholar] [CrossRef]
- Owamah, H.I.; Chukwujindu, I.S.; Asiagwu, A.K. Biosorptive capacity of yam peels waste for the removal of dye from aqueous solutions. Civ. Environ. Res. 2013, 3, 36–40. [Google Scholar]
- Franco, J.H.; da Silva, B.F.; Dias EF, G.; de Castro, A.A.; Ramalho, T.C.; Zanoni MV, B. Influence of auxochrome group in disperse dyes bearing azo groups as chromophore center in the biotransformation and molecular docking prediction by reductase enzyme: Implications and assessment for environmental toxicity of xenobiotics. Ecotoxicol. Environ. Saf. 2018, 160, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Han, L.; Ji, Y.; Wei, J.; Cai, G.; Gao, G.; Wu, J.; Yao, Z. Heavy metal investigation and risk assessment along the Le’An River from non-ferrous metal mining and smelting activities in Poyang, China. J. Environ. Biol. 2018, 39, 536–545. [Google Scholar] [CrossRef]
- Omer, S.; Hussein, M.A.; Hussein, B.H.M.; Magdi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- El-kousy, S.M.; El-shorbagy, H.G.; El-ghaffar, M.A.A. Chitosan/montmorillonite composites for fast removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2020, 254, 123236. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Shim, J.; Lee, Y.R. Degradation of organic pollutants by bio-inspired rectangular and hexagonal titanium dioxide nanostructures. J. Photochem. Photobiol. B Biol. 2017, 169, 90–95. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Nwanji, O.L.; Omorogie, M.O.; Olowoyo, J.; Babalola, J.O. Remediation of industrial dye by Fenton-activated biogenic waste. Surf. Interfaces 2020, 20, 100555. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye Pigment 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Cappelletti, G.; Pifferi, V.; Mostoni, S.; Falciola, L.; di Bari, C.; Spadavecchia, F.; Meroni, D.; Davoli, E.; Ardizzone, S. Hazardous o-toluidine mineralization by photocatalytic bismuth doped ZnO slurries. Chem. Commun. 2015, 51, 10459. [Google Scholar] [CrossRef] [Green Version]
- Kharissova, O.V.; Dias, H.R.; Kharisov, B.I.; Pérez, B.O.; Pérez VM, J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.A.; Doan, V.D.; Tran, A.V.; Nguyen, V.C.; Vasseghian, Y. Green synthesis of Nb-doped ZnO nanocomposite for photocatalytic degradation of tetracycline antibiotic under visible light. Mater. Lett. 2021, 308, 131129. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/hydroxyapatite/collagen scaffolds. J. Transl. Med. 2015, 13, 152. [Google Scholar] [CrossRef] [Green Version]
- El Bekkali, C.; Labrag, J.; Oulguidoum, A.; Chamkhi, I.; Laghzizil, A.; Nunzi, J.-M.; Robert, D.; Aurag, J. Porous ZnO/hydroxyapatite nanomaterials with effective photocatalytic and antibacterial activities for the degradation of antibiotics. Nanotechnol. Environ. Eng. 2022, 7, 1. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R.; Nair, A.S. Fabrication of chemically modified graphene oxide/nano hydroxyapatite composite for adsorption and subsequent photocatalytic degradation of aureomycine hydrochloride. J. Ind. Eng. Chem. 2017, 47, 415–430. [Google Scholar] [CrossRef]
- Hassan, A.; Hrdina, R. Chitosan/nanohydroxyapatite composite based scallop shells as an efficient adsorbent for mercuric ions: Static and dynamic adsorption studies. Int. J. Biol. Macromol. 2018, 109, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaabi, K.; Al-Khanbashi, A.; Hammami, A. Date palm fibers as polymeric matrix reinforcement: DPF/polyester composite properties. Polym. Compos. 2005, 26, 604–613. [Google Scholar] [CrossRef]
- Hassan, A.F.; El-Naggar, G.A.; Braish, A.G.; Amira, M.F.; Alshandoudi, L.M. Enhanced adsorption of chromium (VI) from aqueous medium by basic nanohydroxyapatite/chitosan composite based on egg shell. Desalination Water Treat. J. 2020, 206, 235–249. [Google Scholar] [CrossRef]
- Derkus, B.; Arslan, Y.E.; Emregul, K.C.; Emregul, E. Enhancement of aptamer immobilization using egg shell-derived nano-sized spherical hydroxyapatite for thrombin detection in neuroclinic. Talanta 2016, 158, 100–109. [Google Scholar] [CrossRef]
- Hassan, A.F.; Hrdina, R. Enhanced removal of arsenic from aqueous medium by modified silica nanospheres: Kinetic and thermodynamic studies. Arab. J. Sci. Eng. 2021, 1–13. [Google Scholar] [CrossRef]
- Hassan, A.F.; Elhadidy, H. Production of activated carbons from waste carpets and its application in methylene blue adsorption: Kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2017, 5, 955–963. [Google Scholar] [CrossRef]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res. Lett. 2020, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraynov, A.; Müller, T. Concepts for the stabilization of metal nanoparticles in ionic liquids. Appl. Ion. Liq. Sci. Technol. 2011, 9, 235–260. [Google Scholar]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Cyrille, G.; Thomas, B. Vibrational Circular Dichroism of Adsorbed Molecules: BINAS on Gold Nanoparticles. J. Phys. Chem. C 2010, 114, 15897–15902. [Google Scholar] [CrossRef]
- Bhattacharjee, C.R.; Purkayastha, D.D.; Bhattacharjee, S.; Nath, A. Homogeneous chemical precipitation route to ZnO nanosphericals. Assam Univ. J. Sci. Technol. 2011, 7, 122–127. [Google Scholar]
- Giri, P.K.; Bhattacharyya, S.; Chetia, B.; Kumari, S.; Singh, D.K.; Iyer, P.K. High-yield chemical synthesis of hexagonal ZnO nanoparticles and nanorods with excellent optical properties. J. Nanosci. Nanotechnol. 2011, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of nano-hydroxyapatite/chitosan composite for cadmium ions removal in wastewater treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Adsorptive removal of Pb2+, Co2+ and Ni2+ by hydroxyapatite/chitosan composite from aqueous solution. J. Taiwan Inst. Chem. Eng. 2012, 43, 125–131. [Google Scholar] [CrossRef]
- Anilreddy, B. Preparation and characterization of iron oxide nanoparticles on disaccharide templates. Asian J. Pharm. Res. Health Care 2009, 1, 172–183. [Google Scholar]
- Xu, Z.; Qian, G.; Feng, M. Using polyacrylamide to control particle size and synthesize porous nano hydroxyapatite. Results Phys. 2020, 16, 102991. [Google Scholar] [CrossRef]
- Skwarek, E.; Goncharuk, O.; Sternik, D.; Janusz, W.; Gdula, K.; Gun’ko, V.M. Structural, and adsorption properties and thermal stability of nanohydroxyapatite/polysaccharide composites. Nanoscale Res. Lett. 2017, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Edralin, E.J.M.; Garcia, J.L.; Rosa, F.M.D.; Punzalan, E.R. Sonochemical synthesis, characterization and photocatalytic properties of hydroxyapatite nano-rods derived from mussel shells. Mater. Lett. 2017, 196, 33–36. [Google Scholar] [CrossRef]
- Snehal, Y.; Chandra, M.; Prakash, M. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Khataee, A.; Kiranşan, M.; Karaca, S.; Arefi-Oskoui, S. Preparation and characterization of ZnO/MMT nanocomposite for photocatalytic ozonation of a disperse dye. Turk. J. Chem. 2016, 40, 546–564. [Google Scholar] [CrossRef]
- Hassan, A.F. Synthesis of carbon nano-onion embedded metal-organic frameworks as an efficient adsorbent for cadmium ions: Kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2019, 26, 24099–24111. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.F.; Alafid, F.; Hrdina, R. Preparation of melamine formaldehyde/nanozeolite Y composite based on nanosilica extracted from rice husks by sol-gel method: Adsorption of lead (II) ion. J. Sol-Gel Sci. Technol. 2020, 95, 11–22. [Google Scholar] [CrossRef]
- Hassan, A.; Abdel-Mohsen, A.-M.; Fouda, M.M. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef]
- Aroua, M.K.; Leong, S.P.P.; Teo, L.Y.; Yin, C.Y.; Daud, W.M.A.W. Real time determination of kinetics of adsorption of lead(II) onto palm shell-based activated carbon using ion selective electrode. Bioresour. Technol. 2008, 99, 5786–5792. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Cai, H.; Liu, T.; Huang, Q.; Zhang, X. Adsorptive removal of methylene blue from aqueous solution using coal fly ash-derivedmesoporous silica material. Adsorpt. Sci. Technol. 2019, 37, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Ren, D.; Xia, S.; Zhang, Y.; Zhao, J. Photocatalytic degradation of bisphenol A (BPA) using immobilized TiO2 and UV illumination in a horizontal circulating bed photocatalytic reactor (HCBPR). J. Hazard. Mater. 2009, 169, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Kazeminezhad, I.; Sadollahkhani, A. Influence of pH on the photocatalytic activity of ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 4206–4215. [Google Scholar] [CrossRef]
- Alkaim, A.; Aljeboree, A.; Alrazaq, N.; Baqir, S.; Hussein, F.; Lilo, A. Effect of pH on Adsorption and Photocatalytic Degradation Efficiency of Different Catalysts on Removal of Methylene Blue. Asian J. Chem. 2014, 26, 8445–8448. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Li, A.-D.; Li, X.-Y.; Zhai, H.-F.; Zhang, W.-Q.; Gong, Y.-P.; Li, H.; Wu, D. Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J. Solid State Chem. 2010, 183, 1359–1364. [Google Scholar] [CrossRef]
- Hassan, A.F.; Elhadidy, H.; Abdel-Mohsen, A. Adsorption and photocatalytic detoxification of diazinon using iron and nanotitania modified activated carbons. J. Taiwan Inst. Chem. Eng. 2017, 75, 299–903. [Google Scholar] [CrossRef]
- Kazeminezhad, I.; Sadollahkhani, A.; Farbod, M. Synthesis of ZnO nanoparticles and flower-like nanostructures using nonsono-and sono-electrooxidation methods. Mater. Lett. 2013, 92, 29–32. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Mbonu, J.I. Green synthesis, structural characterization and photocatalytic activities of Chitosan-ZnO Nano-composite. J. Inorg. Organomet. Polym. 2012, 31, 3356–3367. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-Molina, Á.; Morales-Torres, S.; Salemi, A.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Photocatalytic Performance of ZnO-Graphene Oxide Composites towards the Degradation of Vanillic Acid under Solar Radiation and Visible-LED. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef]
- Kutláková, K.M.; Tokarský, J.; Peikertová, P. Functional and eco-friendly nanocomposite kaolinite/ZnO with high photocatalytic activity. Appl. Catal. B Environ. 2015, 162, 392–400. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalination Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Vignesh, K.; Suganthi, A.; Rajarajan, M.; Sara, S.A. Photocatalytic activity of AgI sensitized ZnO nanoparticles under visible light irradiation. Powder Technol. 2012, 224, 331–337. [Google Scholar] [CrossRef]
- Qiu, P.; Li, W.; Thokchom, B.; Park, B.; Cui, M.; Zhao, D.; Khim, J. Uniform core-shell structured magnetic mesoporous TiO2 nanospheres as a highly efficient and stable sonocatalyst for the degradation of bisphenol-A. J. Mater. Chem. A 2015, 3, 6492–6500. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z. Effect of low Fe3+ doping on characteristics, sonocatalytic activity and reusability of TiO2 nanotubes catalysts for removal of Rhodamine B from water. J. Hazard. Mater. 2012, 235, 326–335. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Effect of annealing temperature on the characteristics, sonocatalytic activity and reusability of nanotubes TiO2 in the degradation of Rhodamine B. Appl. Catal. B Environ. 2010, 100, 393–402. [Google Scholar] [CrossRef]
- Zatta, L.; da Costa Gardolinski, J.E.F.; Wypych, F. Raw halloysite as reusable heterogeneous catalyst for esterification of lauric acid. Appl. Clay Sci. 2011, 51, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Salimi, F.; Rahimi, H.; Karami, C. Removal of methylene blue from water solution by modified nanogoethite by Cu. J. Desalination Water Treat. 2019, 137, 334–344. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Baeissa, E.S. Photocatalytic degradation of methylene blue dye under visible light irradiation using In/ZnO nanocomposite. Front. Nanosci. Nanotechnol. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K.; Kadiyala, N.K. Photocatalytic Degradation of Methylene Blue Dye by Nonconventional synthesized SnO2 Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 10, 339–350. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Rasool, R.T.; Hassan, M.; Zhang, L. Enhanced photo Fenton-like activity by effective and stable Al-Sm M-hexaferrite heterogenous catalyst magnetically detachable for methylene blue degradation. J. Alloys Compd. 2020, 821, 153410. [Google Scholar] [CrossRef]


| Sample | qm (mg/g) | PFO Kinetic Model | PSO Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| qexp (mg/g) | k1 (h−1) | R2 | qexp (mg/g) | k2 (g/mg/h) | R2 | ||
| ZP13 | 504.9 | 414.1 | 0.1204 | 0.873683 | 494.8 | 1.78 × 10−4 | 0.988964 |
| ZP14 | 596.1 | 501.2 | 0.1421 | 0.863212 | 561.0 | 3.04 × 10−4 | 0.996050 |
| Parameter | NHAP | ZnO | ZP13 | ZP14 | ZP15 | ZP16 |
|---|---|---|---|---|---|---|
| qm (mg/g) | 134.5 | 202.3 | 504.9 | 596.1 | 428.5 | 312.4 |
| b (L/mg) | 0.01819 | 0.02610 | 0.05479 | 0.03061 | 0.02510 | 0.02651 |
| RL | 0.26466 | 0.16077 | 0.14045 | 0.10977 | 0.16611 | 0.15873 |
| R2 | 0.985361 | 0.996475 | 0.983010 | 0.989900 | 0.967441 | 0.983482 |
| Solid Nanomaterials | Maximum Capacity | Reference | |
|---|---|---|---|
| Adsorption | Photocatalysis | ||
| Cu/nanogoethite | 12.8 mg/g | NA | [57] |
| Chitosan/montmorillonite composite | 181 mg/g | NA | [6] |
| Fe3O4/activated montmorillonite nanocomposite | 106.4 mg/g | NA | [58] |
| Fe-AC | 191.0 mg/g | NA | [46] |
| In/ZnO nanoparticles | NA | 89% | [59] |
| SnO2 nanoparticles | NA | 90% | [60] |
| Barium monoferrite | NA | 90% | [61] |
| ZP14 | 596.1 mg/g | 91% | [This study] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, M.S.; Ahmed, I.A.; Bader, D.M.D.; Hassan, A.F. Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2022, 12, 49. https://doi.org/10.3390/nano12010049
Elsayed MS, Ahmed IA, Bader DMD, Hassan AF. Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue. Nanomaterials. 2022; 12(1):49. https://doi.org/10.3390/nano12010049
Chicago/Turabian StyleElsayed, Maha S., Inas A. Ahmed, Dina M. D. Bader, and Asaad F. Hassan. 2022. "Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue" Nanomaterials 12, no. 1: 49. https://doi.org/10.3390/nano12010049







