Comparative Study between Traditional and Nano Calcium Phosphate Fertilizers on Growth and Production of Snap Bean (Phaseolus vulgaris L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.1.1. Nano Calcium Phosphate Preparation
2.1.2. Nano Calcium Phosphate (NCaP) Characterization
2.2. Experimental Procedure
2.2.1. Pot Experiment
2.2.2. Field Experiment
2.3. Data Recording
2.4. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Nano Calcium Phosphate (NCaP)
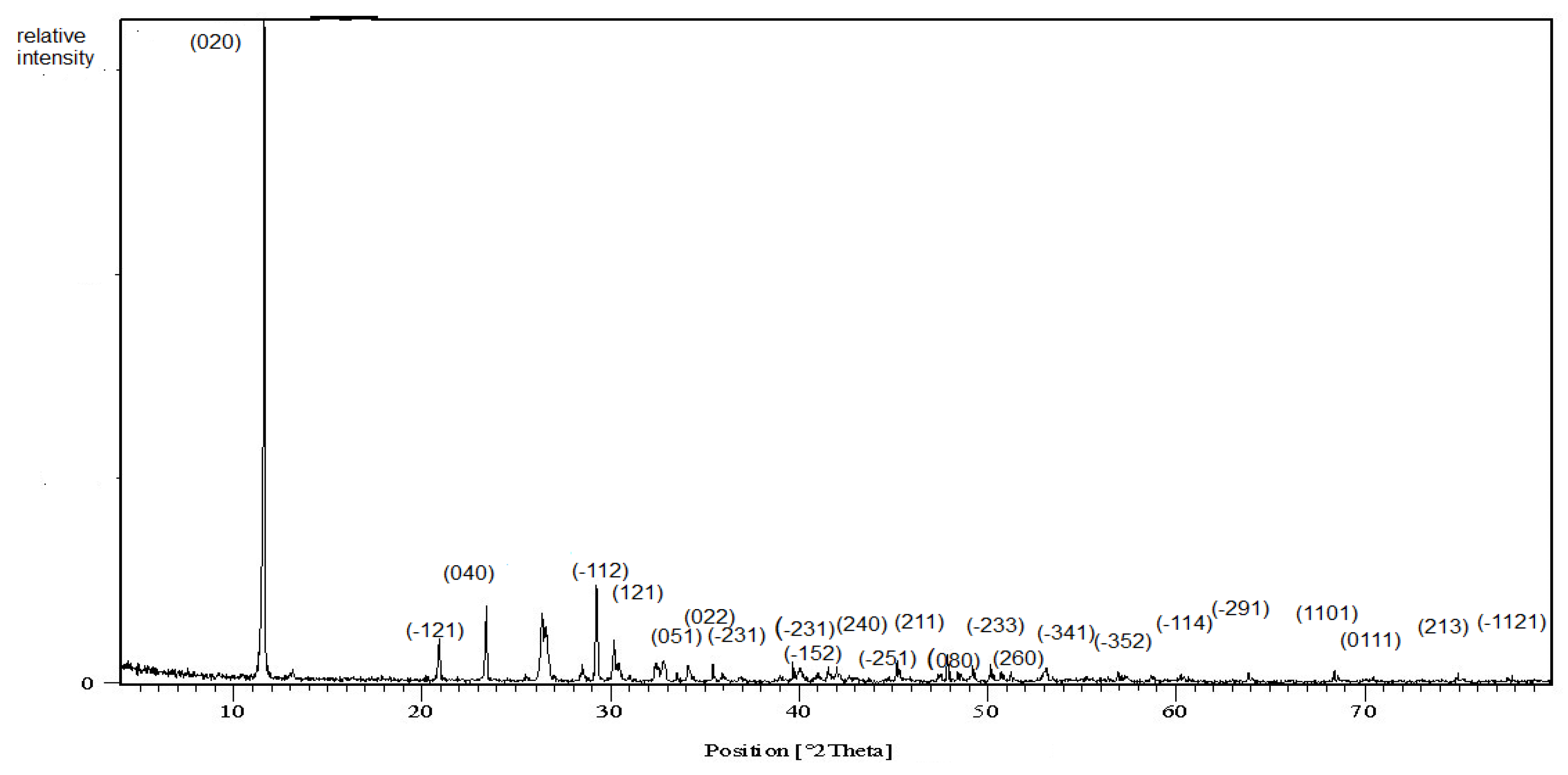
3.1.1. X-ray Diffraction Pattern
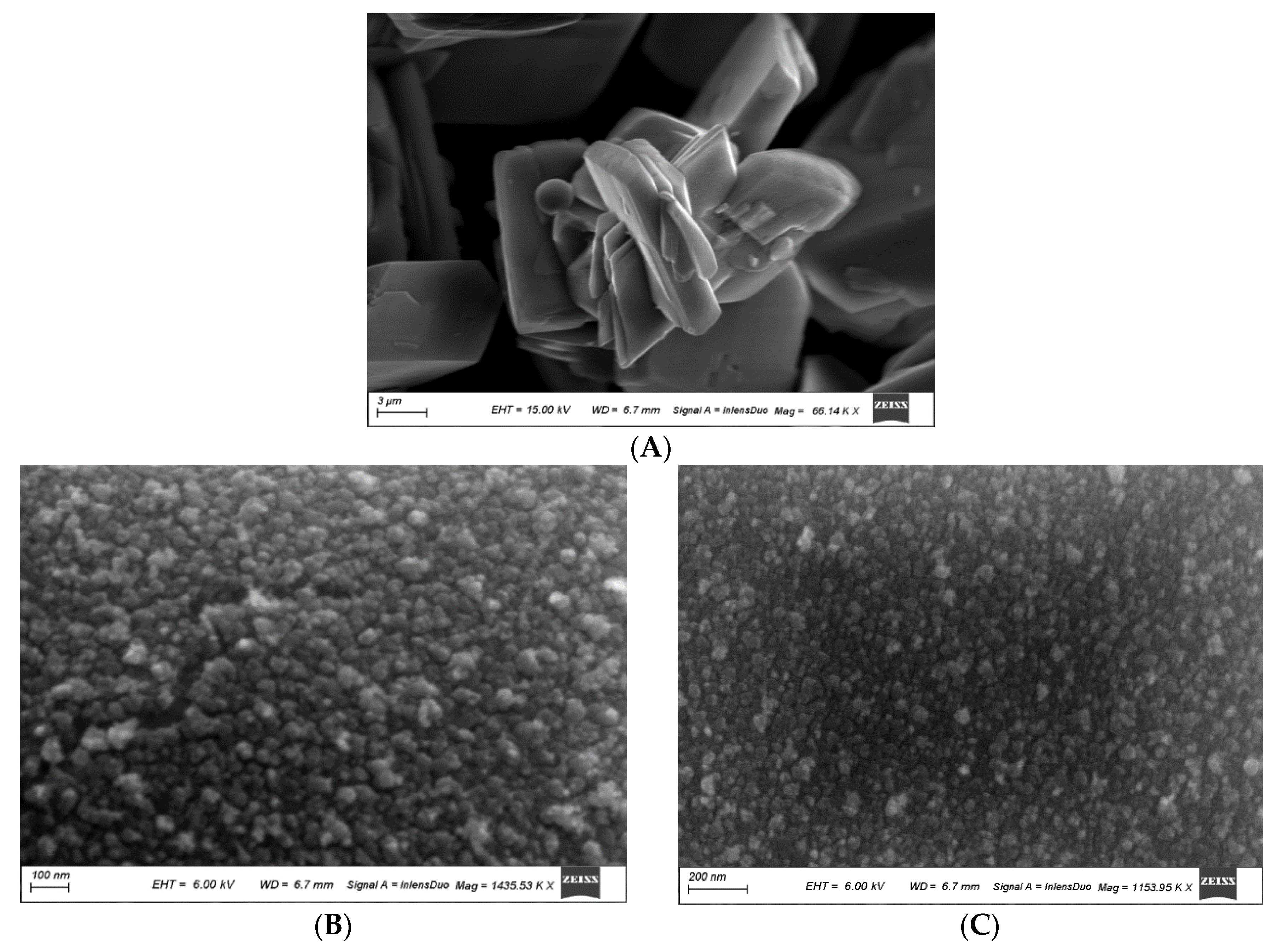
3.1.2. Scanning Electron Microscopy (SEM)
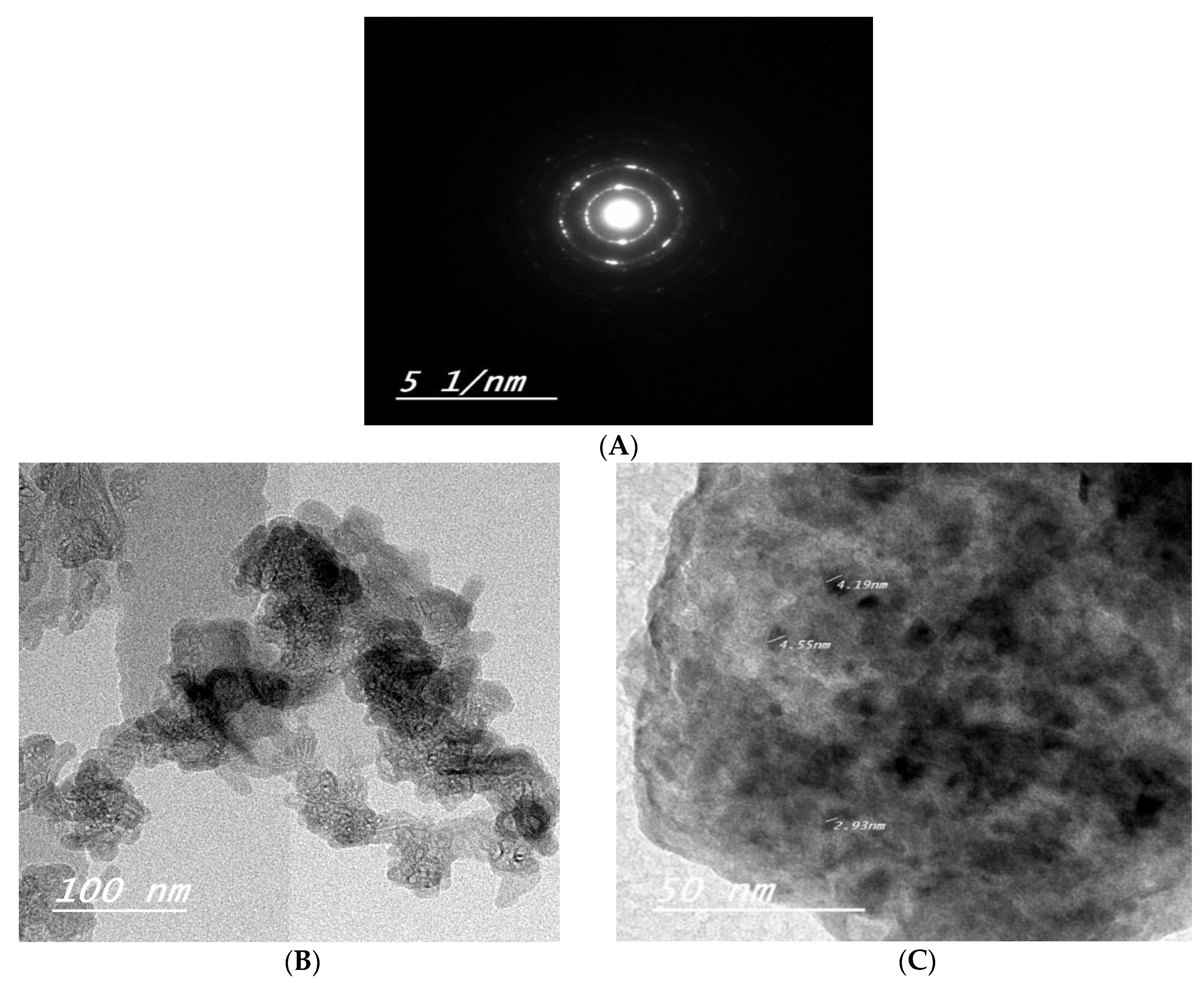
3.1.3. Transmission Electron Microscopy (TEM)
3.1.4. Fourier Transformer Infra-Red (FTIR)
3.2. Pot Experiment
3.2.1. Effect of Traditional and Nano Calcium Phosphate Fertilizers on Shoot and Root Dry Weights of Snap Bean Plants
3.2.2. Effect of Traditional and Nano Calcium Phosphate Fertilizers on the Nutrient (N, P and K) Content of Snap Bean Plants
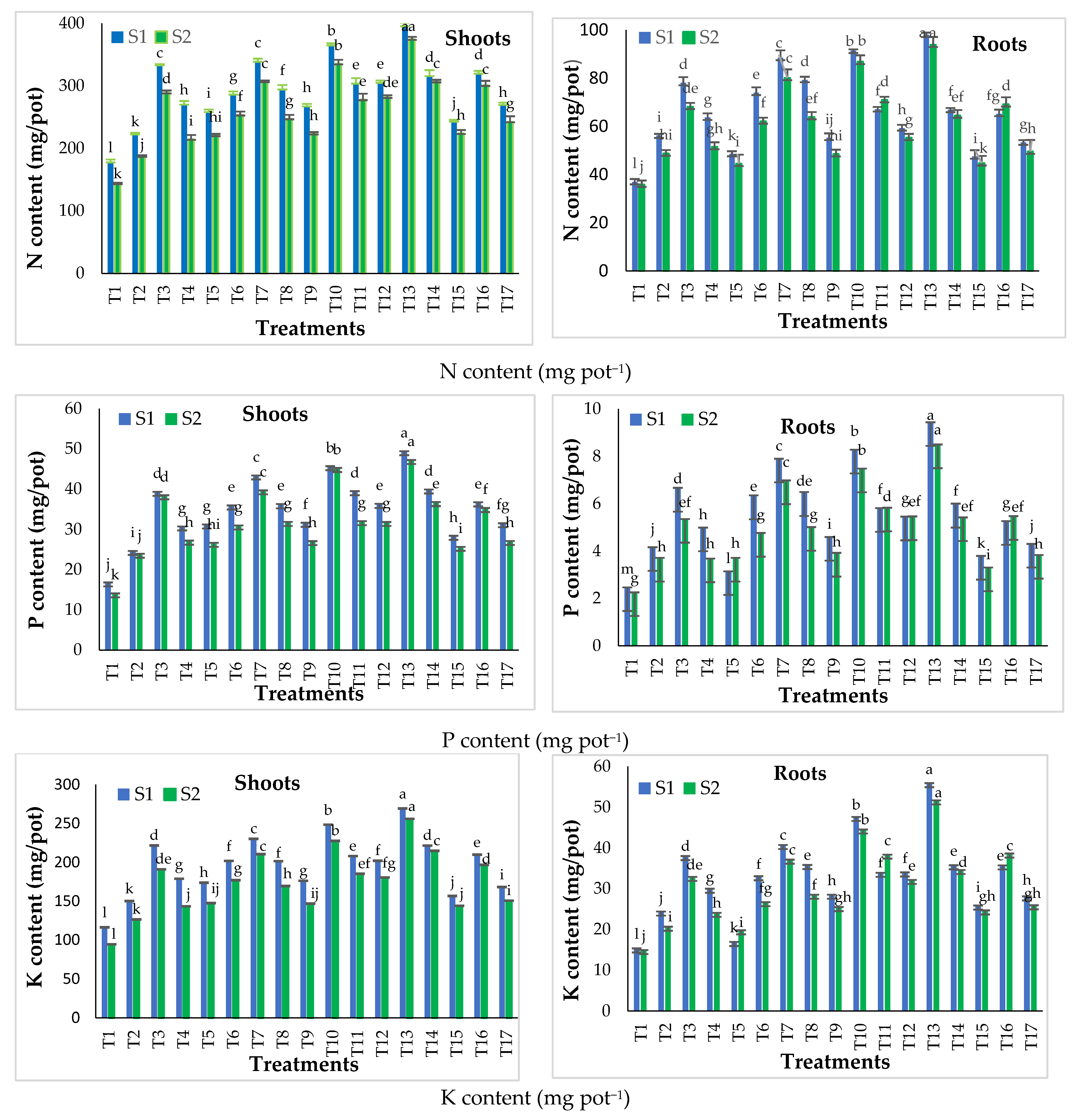
3.3. Field Experiment
3.3.1. Effect of Traditional and Nano Calcium Phosphate Fertilizers on the Yield and Components of Snap Bean Plants
3.3.2. Effect of Traditional and Nano Calcium Phosphate Fertilizers on the Nutrient Concentrations and Crude Protein Percentage in Pods of Snap Bean Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.-K.; Ma, J.-J.; Liu, L.-J. Effects of phosphate-solubilizing bacteria application on soil phosphorus availability in coal mining subsidence area in Shanxi. J. Plant Interact. 2017, 12, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition: Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.; Smit, A. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Hedley, M.; McLaughlin, M. Reactions of phosphate fertilizers and by-products in soils. In Phosphorus: Agriculture and the Environment; Sims, J.T., Sharpley, A.N., Eds.; American Society of Agronomy: Madison, WI, USA, 2005; Volume Phosphorusagric; pp. 181–252. [Google Scholar]
- El-Agrodi, M.W.; Mosa, A.A.; Elsherpiny, M.A. Inorganic phosphorus forms in alluvial and calcareous soils as affected by different phosphorus application levels and incubation periods. J. Soil Sci. Agric. Eng. 2011, 2, 1195–1206. [Google Scholar] [CrossRef]
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Gérard, F. A mechanistic model for understanding root-induced chemical changes controlling phosphorus availability. Ann. Bot. 2010, 105, 1183–1197. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Dashti, S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric. Slov. 2016, 107, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Naderi, M.R.; Danesh-Shahraki, A. Nanofertilizers and their roles in sustainable agriculture. Int. J. Agric. Crop. Sci. 2013, 5, 2229–2232. [Google Scholar]
- Rameshaiah, G.N.; Jpallavi, S. Nano fertilizers and nano sensors-an attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015, 3, 314–320. [Google Scholar]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2015, 4, srep05686. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total. Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Tulasi, G.; Veronica, N.; Ramesh, T.; Narender, S. Crop nutrition management with nano fertilizers. Int. J. Environ. Sci. Technol. 2015, 1, 4–6. [Google Scholar]
- Singh, M.D.; Chirag, G.; Prakash, P.O.; Mohan, M.H.; Prakasha, G. Nano-fertilizers is a new way to increase nutrients use efficiency in crop production. Int. J. Agric. Sci. 2017, 9, 3831–3833. [Google Scholar]
- Marzouk, N.M.; Abd-Alrahman, H.A.; El-Tanahy, A.M.M.; Mahmoud, S.H. Impact of foliar spraying of nano micronutrient fertilizers on the growth, yield, physical quality, and nutritional value of two snap bean cultivars in sandy soils. Bull. Natl. Res. Cent. 2019, 43, 84. [Google Scholar] [CrossRef]
- Rosen, C.J.; Kelling, K.A.; Stark, J.C.; Porter, G.A. Optimizing Phosphorus Fertilizer Management in Potato Production. Am. J. Potato Res. 2014, 91, 145–160. [Google Scholar] [CrossRef]
- Rane, M.; Bawskar, M.; Rathod, D.; Nagaonkar, D.; Rai, M. Influence of calcium phosphate nanoparticles, Piriformospora indica and Glomus mosseae on growth of Zea mays. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045014. [Google Scholar] [CrossRef]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.-M. Interaction and Regulation of Carbon, Nitrogen, and Phosphorus Metabolisms in Root Nodules of Legumes. Front. Plant Sci. 2018, 9, 1860. [Google Scholar] [CrossRef] [Green Version]
- FAO. World Food and Agriculture—Statistical Pocket Book; FAO: Rome, Italy, 2018; p. 103, Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Abd El-Hakim, W.M. Response of some vegetable legume plants to foliar application of some antioxidants. J. Amer. Sci. 2014, 10, 1–12. [Google Scholar]
- Bargaz, A.; Nassar, R.M.A.; Rady, M.M.; Gaballah, M.S.; Thompson, S.M.; Brestic, M.; Schmidhalter, U.; Abdelhamid, M.T. Improved salinity tolerance by phosphorus fertilizer in two Phaseolus vulgaris recombinant inbred lines contrasting in their P-efficiency. J. Agron. Crop. Sci. 2016, 202, 497–507. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants, and Waters; University of California: Riverside, CA, USA, 1961. [Google Scholar]
- Mansour, S.; El-Dek, S.I.; Ahmed, M.A.; Abd-Elwahab, S.M. Effect of preparation conditions on the nanostructure of hydroxyapatite and brushite phases. Appl. Nanosci. 2016, 6, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State Univ. Press: Iowa City, IA, USA, 1989. [Google Scholar]
- Freed, R.S.P.; Einensmith, S.; Gutez, D.; Reicosky, V.; Smail, W.; Wolberg, P. User’s Guide to MSTAT-C Analysis of Agronomic Research Experiments; Michigan State University: East Lansing, MI, USA, 1989. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Singh, S.; Singh, V.; Aggarwal, S.; Mandal, U.K. Synthesis of brushite nanoparticles at different temperatures. Chem. Pap. 2010, 64, 491–498. [Google Scholar] [CrossRef]
- Mandel, S.; Tas, A.C. Brushite (CaHPO4·2H2O) to octacalcium phosphate (Ca8(HPO4)2(PO4)4·5H2O) transformation in DMEM solutions at 36.5°C. Mater. Sci. Eng. C 2010, 30, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Lin, T.J.; Cheng, H.H.; Chen, C.; Reddy, A.S.; Atla, S.B.; Chang, Y.; Chen, H.; Chen, C. Synthesis of brushite particles in reverse micro emulsions of the biosurfactant surfactin. Int. J. Mol. Sci. 2011, 12, 3821–3830. [Google Scholar] [CrossRef] [Green Version]
- Turuko, M.; Mohammed, A. Effect of Different Phosphorus Fertilizer Rates on Growth, Dry Matter Yield and Yield Components of Common Bean (Phaseolus vulgaris L.). World J. Agric. Res. 2014, 2, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.M.; El-Shewy, A.A.; El-Yazal, M.A.S.; El-Gawwad, I.F.M.A. Integrative Application of Soil P-Solubilizing Bacteria and Foliar Nano P Improves Phaseolus vulgaris Plant Performance and Antioxidative Defense System Components under Calcareous Soil Conditions. J. soil Sci. Plant Nutr. 2020, 20, 820–839. [Google Scholar] [CrossRef]
- Soliman, A.S.; Hassan, M.; Abou-Elella, F.; Ahmed, A.H.; El-Feky, S.A. Effect of nano and molecular phosphorus fertilizers on growth and chemical composition of baobab (Adansonia digitata L.). J. Plant Sci. 2016, 11, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, M. Implications of Applying Nano-Hydroxyapatite and Nano-Iron Oxide on Faba Bean (Vicia faba L.) Productivity. J. Soil Sci. Agric. Eng. 2018, 9, 543–548. [Google Scholar] [CrossRef]
- Uarrota, V.G. Response of Cowpea (Vigna unguiculata L. Walp.) to Water Stress and Phosphorus Fertilization. J. Agron. 2010, 9, 87–91. [Google Scholar] [CrossRef]
- Priya, B.; Srinivasarao, M.; Mukherjee, S. Screening of Phosphorus Nanoparticle Concentration Based on their Effects at Germination & Seedling Level in Mung, Urd and Cowpea. Vegetos Int. J. Plant Res. 2015, 28, 169. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Begum, L.; Dey, B.; Nath, P.K.; Panda, S.K. Impact of Calcium Phosphate Nanoparticles on Rice Plant. J. Plant Sci. Phytopathol. 2017, 1, 001–010. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.; Nisar, S.; Gul, I.; Nawaz, U.; Irum, S.; Ahmad, S.; Sadat, H.; Mina, I.A.; Ali, S.; Rizwan, M.; et al. Multi-element uptake and growth responses of Rice (Oryza sativa L.) to TiO2 nanoparticles applied in different textured soils. Ecotoxicol. Environ. Saf. 2021, 215, 112149. [Google Scholar] [CrossRef]
- Waani, S.P.T.; Irum, S.; Gul, I.; Yaqoob, K.; Khalid, M.U.; Ali, M.A.; Manzoor, U.; Noor, T.; Ali, S.; Rizwan, M.; et al. TiO2 nanoparticles dose, application method and phosphorous levels influence genotoxicity in Rice (Oryza sativa L.), soil enzymatic activities and plant growth. Ecotoxicol. Environ. Saf. 2021, 213, 111977. [Google Scholar] [CrossRef] [PubMed]
- El-Azizy, F.A.; Habib, A.A.M.; Abd-El baset, A.M. Effect of nano phosphorus and potassium fertilizers on productivity and mineral content of broad bean in north Sinai. J. Soil Sci. Agric. Eng. Mansoura Univ. 2021, 12, 239–246. [Google Scholar]
- Youssef, S.; Riad, G.S.; Elhady, S.A.A. Effect of Phosphorus Sources and Arbuscular Mycorrhizal Inoculation on Growth and Productivity of Snap Bean (Phaseolus vulgaris L.). Gesunde Pflanz. 2017, 69, 139–148. [Google Scholar] [CrossRef]
- Zahra, Z.; Maqbool, T.; Arshad, M.; Badshah, M.A.; Choi, H.-K.; Hur, J. Changes in fluorescent dissolved organic matter and their association with phytoavailable phosphorus in soil amended with TiO2 nanoparticles. Chemosphere 2019, 227, 17–25. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.; White, P. Genetic Responses to Phosphorus Deficiency. Ann. Bot. 2004, 94, 323–332. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, M.; Murphy, A. Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, CT, USA, 2015. [Google Scholar]
- Ehiagiator, J.O.; Ariyo, A.D.; Imasuen, E.E. Soil fertility and nutritional studies on citrus, fruit and vegetable crops in Nigeria. In Proceedings of the 29th Annual National Conference of Horticultural Society of Nigeria, Kano, Nigeria, 24–29 July 2011. [Google Scholar]
- El-Kereti, M.A.; El-feky, S.A.; Khater, M.S.; Osman, Y.A.; El-sherbini, E.S.A. ZnO nanofertilizer and He Ne laser irradiation for promoting growth and yield of sweet basil plant. Recent Patents Food Nutr. Agric. 2013, 5, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and Their Smart Delivery System. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Taşkin, M.B.; Şahin, Ö.; Taskin, H.; Atakol, O.; Inal, A.; Gunes, A. Effect of synthetic nano-hydroxyapatite as an alternative phosphorus source on growth and phosphorus nutrition of lettuce (Lactuca sativa L.) plant. J. Plant Nutr. 2018, 41, 1148–1154. [Google Scholar] [CrossRef]
- Burhan, S.A.; AL-Hassan, M.G. Impact of nano NPK fertilizers to correlation between productivity, quality and flag leaf of some bread wheat varieties. Iraqi J. Agric. Sci. 2019, 50, 1–7. [Google Scholar]
- Kaviani, B.; Ghaziani, M.V.F.; Negahdar, N. Effect of application methods and different concentrations of biologic nanofertilizer, especial for ornamental plants on some morphological, physiological and proliferation traits and enhancing the quality of Buxus hyrcana pojark. Int. J. Adv. Sci. Eng. Tech. 2016, 2, 206–212. [Google Scholar]
- Rajonee, A.A.; Zaman, S.; Huq, S.M.I. Preparation, Characterization and Evaluation of Efficacy of Phosphorus and Potassium Incorporated Nano Fertilizer. Adv. Nanoparticles 2017, 06, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Dhansil, A.; Zalawadia, N.; Prajapat, B.S.; Yadav, K. Effect of Nano Phosphatic Fertilizer on Nutrient Content and Uptake by Pearl Millet (Pennisetum glaucum L.) Crop. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2327–2337. [Google Scholar] [CrossRef]
- Desnos, T. Root branching responses to phosphate and nitrate. Curr. Opin. Plant Biol. 2008, 11, 82–87. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Hussain, R.M. The Effect of Phosphorus in Nitrogen Fixation in Legumes. Agric. Res. Technol. Open Access J. 2017, 5, 1–3. [Google Scholar] [CrossRef]
- Singh, A.; Baoule, A.L.; Ahmed, H.G.; Dikko, A.U.; Aliyu, U.; Sokoto, M.B.; Alhassan, J.; Musa, M.; Haliru, B. Influence of phosphorus on the performance of cowpea (Vigna unguiculata (L) Walp.) varieties in the Sudan savanna of Nigeria. Agric. Sci. 2011, 02, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Hagagg, L.F.; Mustafa, N.S.; Genaidy, E.A.E.; El-Hady, E.S. Effect of spraying nano-NPK on growth performance and nutrients status for (Kalamat cv.) olive seedling. Biosci. Res. 2018, 15, 1297–1303. [Google Scholar]

| Properties | Particle Size Distribution | pH 1:2.5 | ECe dSm−1 | Soluble Anions (meq−1) | Soluble Cations (meq−1) | Available Macronutrients (mg kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Soil Texture | HCO3− | Cl− | SO4− | Na+ | K+ | Ca2+ | Mg2+ | N | P | K | |||
| Pot experiment | ||||||||||||||||
| SI (2016/17) | 77.40 | 15.25 | 7.35 | Sandy loam | 7.70 | 0.96 | 5.90 | 2.87 | 0.83 | 2.75 | 0.90 | 3.50 | 2.45 | 25.00 | 6.30 | 194.00 |
| SII (2017/18) | 76.80 | 14.75 | 8.45 | Sandy loam | 7.60 | 0.94 | 6.90 | 1.90 | 1.20 | 2.40 | 0.80 | 3.70 | 2.30 | 27.00 | 7.00 | 210.00 |
| Field experiment Season (2018–2019) | ||||||||||||||||
| 77.20 | 15.00 | 7.80 | Sandy loam | 7.80 | 0.95 | 6.50 | 2.20 | 0.80 | 2.51 | 0.75 | 3.54 | 2.70 | 23.00 | 5.30 | 187.00 | |
| * P-Fertilizer | Treatment | Rate |
|---|---|---|
| SSP | T1 | 30 kg P2O5 fed−1 (recommended dose) as soil application (control) |
| T2 | T1 +2.5 g/L MAP as foliar application | |
| T3 | T1 + 5% NCaP as foliar application | |
| T4 | T1 + 10% NCaP as foliar application | |
| T5 | 60 kg P2O5 fed−1 as soil application | |
| T6 | T5 + 2.5 g/L MAP as foliar application | |
| T7 | T5 + 5% NCaP as foliar application | |
| T8 | T5 + 10% NCaP as foliar application | |
| NCaP | T9 | 10% NCaP (of the recommended rate) as soil application |
| T10 | T9 + 5% NCaP as foliar application | |
| T11 | T9 + 10% NCaP as foliar application | |
| T12 | 20% NCaP (of the recommended rate) as soil application | |
| T13 | T12 + 5% NCaP as foliar application | |
| T14 | T12 + 10% NCaP as foliar application | |
| T15 | 30% NCaP (of the recommended rate) as soil application | |
| T16 | T15 + 5% NCaP as foliar application | |
| T17 | T15 + 10% NCaP as foliar application |
| Pos. [°2Th.] | d-Spacing [Å] | Rel. Int. [%] | (hkl) |
|---|---|---|---|
| 11.6415 | 7.60166 | 100 | (020) |
| 20.9303 | 4.24437 | 6.56 | (−121) |
| 23.3968 | 3.80222 | 11.11 | (040) |
| 29.2588 | 3.05242 | 14.30 | (−112) |
| 30.4671 | 2.93406 | 2.64 | (121) |
| 33.5449 | 2.67157 | 1.15 | (051) |
| 34.0854 | 2.63043 | 2.54 | (022) |
| 35.4079 | 2.53516 | 2.09 | (−231) |
| 39.7037 | 2.27021 | 2.19 | (−161) |
| 41.5395 | 2.17402 | 2.34 | (−152) |
| 42.0094 | 2.15079 | 2.43 | (240) |
| 42.8994 | 2.1082 | 0.50 | (−251) |
| 44.7010 | 2.02734 | 0.55 | (211) |
| 45.1943 | 2.00635 | 3.07 | (−123) |
| 47.8587 | 1.90069 | 4.14 | (080) |
| 49.232 | 1.85084 | 2.27 | (−233) |
| 50.2017 | 1.81583 | 2.46 | (260) |
| 50.6988 | 1.80068 | 1.53 | (−262) |
| 51.2680 | 1.78202 | 1.67 | (−181) |
| 53.0611 | 1.72595 | 2.00 | (−341) |
| 56.9298 | 1.61751 | 1.60 | (−352) |
| 58.7486 | 1.5717 | 0.82 | (222) |
| 60.3067 | 1.53477 | 0.76 | (−114) |
| 63.8810 | 1.45724 | 1.38 | (−291) |
| 68.4063 | 1.37146 | 1.75 | (1101) |
| 70.2666 | 1.33964 | 0.26 | (0111) |
| 74.9556 | 1.26704 | 0.95 | (213) |
| 77.8778 | 1.22665 | 0.56 | (−1121) |
| pH 5 | Assignment [28,29,30] | pH 5 | Assignment [28,29,30] |
|---|---|---|---|
| 570.1 | v4 of P–O–P bending | 1558.48 | Bending mode of H2O |
| 887.11 | Stretching mode of (HPO4 2−) | 1643.89 | Bending mode of H2O |
| 995.75 | v1 stretching vibration of PO4 3− | 2391.00 | Overtone or combination band |
| 1068.31 | v2 (P–O) Stretching of the PO4 3− group | 3199.37 | (O–H) stretching of water |
| 1130.24 | v′6 and v″6 of HPO4 2− group | 3417.01 | Stretching of water (vs (O–H)) |
| 1402.04 | Bending (O–H) in HPO4 2− group |
| Dry Weight (g pot−1) | ||||
|---|---|---|---|---|
| Treatments | SI | SII | ||
| Shoot | Root | Shoot | Root | |
| T1 | 5.26j ± 0.11 | 1.49m ± 0.05 | 4.27k ± 0.05 | 1.41l ± 0.07 |
| T2 | 6.49i ± 0.04 | 2.14j ± 0.03 | 5.49j ± 0.04 | 1.86jk ± 0.05 |
| T3 | 9.14c ± 0.02 | 2.90d ± 0.09 | 7.91d ± 0.10 | 2.55cd ± 0.06 |
| T4 | 7.50g ± 0.14 | 2.42hi ± 0.07 | 5.99i ± 0.19 | 1.94ij ± 0.07 |
| T5 | 7.44g ± 0.09 | 2.03k ± 0.05 | 6.35g ± 0.08 | 1.69k ± 0.14 |
| T6 | 7.99f ± 0.11 | 2.67e ± 0.08 | 7.04f ± 0.15 | 2.17gh± 0.05 |
| T7 | 9.23c± 0.11 | 3.10c ± 0.11 | 8.40c ± 0.05 | 2.79b ± 0.12 |
| T8 | 8.24e ± 0.17 | 2.82d ± 0.05 | 6.93f ± 0.08 | 2.28fg ± 0.07 |
| T9 | 7.36gh ± 0.09 | 2.38i ± 0.08 | 6.16h ± 0.08 | 2.07hi ± 0.08 |
| T10 | 9.72b ± 0.06 | 3.39b ± 0.02 | 8.94b ± 0.17 | 3.25a ± 0.10 |
| T11 | 8.58d ± 0.22 | 2.65ef ± 0.04 | 7.64e ± 0.26 | 2.78b ± 0.05 |
| T12 | 8.26e ± 0.08 | 2.57f ± 0.06 | 7.41e ± 0.09 | 2.38ef ± 0.07 |
| T13 | 10.29a ± 0.12 | 3.51a ± 0.03 | 9.75a ± 0.10 | 3.40a ± 0.12 |
| T14 | 8.67d ± 0.24 | 2.56fg ± 0.03 | 8.44c ± 0.09 | 2.47de ± 0.09 |
| T15 | 6.46i ± 0.06 | 1.90l ± 0.10 | 5.97i ± 0.14 | 1.84j ± 0.12 |
| T16 | 8.34e ± 0.10 | 2.51gh ± 0.07 | 7.85d ± 0.19 | 2.61c ± 0.11 |
| T17 | 7.17h± 0.07 | 2.05jk ± 0.04 | 6.45g ± 0.23 | 1.92ij ± 0.18 |
| Treatments | No. of Pods (plant−1) | Pod Length (cm) | Pod Diameter (mm) | Pod Weight (g plant−1) | Weight of Pod (g) | Pod Yield (ton ha−1) |
|---|---|---|---|---|---|---|
| 30 kg P2O5 fed−1 soil (T1) | 14.69 ± 0.44 | 11.55 ± 0.12 | 6.50 ± 0.07 | 59.66 ± 1.55 | 3.74 ± 0.19 | 11.45 ± 0.30 |
| 60 Kg P2O5 fed−1 soil (T5) | 16.27 ± 0.50 | 12.10 ± 0.01 | 7.00 ± 0.22 | 68.49 ± 1.97 | 3.98 ± 0.11 | 13.11 ± 0.48 |
| T1 + 5% NCaP foliar (T3) | 16.55 ± 0.88 | 12.46 ± 0.10 | 7.20 ± 0.15 | 73.49 ± 2.47 | 4.15 ± 0.21 | 13.78 ± 0.13 |
| T5 + 5% NCaP foliar (T7) | 17.98 ± 0.31 | 12.74 ± 0.12 | 7.25 ± 0.11 | 74.70 ± 0.71 | 4.20 ± 0.17 | 14.34 ± 0.12 |
| 10% NCaP soil + 5%NCaP foliar (T10) | 18.47 ± 0.74 | 13.43 ± 0.10 | 7.40 ± 0.05 | 78.66 ± 2.29 | 4.31 ± 0.12 | 14.67 ± 0.05 |
| 20% NCaP soil + 5%NCaP foliar (T13) | 20.72 ± 0.85 | 14.52 ± 0.38 | 7.60 ± 0.17 | 83.86 ± 4.16 | 4.45 ± 0.04 | 15.33 ± 0.01 |
| L.S.D. | 1.2950 | 0.3335 | 0.2227 | 4.7927 | 0.2960 | 0.3978 |
| Treatments | N% | P% | K% | Crude Protein % |
|---|---|---|---|---|
| 30 kg P2O5 fed−1 soil (T1) | 2.57 ± 0.08 | 0.35 ± 0.02 | 1.73 ± 0.07 | 16.06 ± 0.73 |
| 60 kg P2O5 fed−1 soil (T5) | 3.01 ± 0.13 | 0.42 ± 0.01 | 2.21 ± 0.06 | 18.81 ± 0.81 |
| T1+ 5% NCaP foliar (T3) | 3.28 ± 0.10 | 0.40 ± 0.02 | 2.24 ± 0.07 | 20.50 ± 0.81 |
| T5+ 5% NCaP foliar (T7) | 3.33 ± 0.17 | 0.45 ± 0.02 | 2.33 ± 0.12 | 20.81 ± 1.04 |
| 10% NCaP soil + 5%NCaP foliar (T10) | 3.41 ± 0.10 | 0.49 ± 0.01 | 2.65 ± 0.13 | 21.31 ± 1.06 |
| 20% NCaP soil + 5%NCaP foliar (T13) | 3.48 ± 0.17 | 0.53 ± 0.02 | 2.91 ± 0.08 | 21.75 ± 0.64 |
| LSD | 0.2492 | 0.0319 | 0.1705 | 1.5991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ghany, M.F.A.; El-Kherbawy, M.I.; Abdel-Aal, Y.A.; El-Dek, S.I.; Abd El-Baky, T. Comparative Study between Traditional and Nano Calcium Phosphate Fertilizers on Growth and Production of Snap Bean (Phaseolus vulgaris L.) Plants. Nanomaterials 2021, 11, 2913. https://doi.org/10.3390/nano11112913
El-Ghany MFA, El-Kherbawy MI, Abdel-Aal YA, El-Dek SI, Abd El-Baky T. Comparative Study between Traditional and Nano Calcium Phosphate Fertilizers on Growth and Production of Snap Bean (Phaseolus vulgaris L.) Plants. Nanomaterials. 2021; 11(11):2913. https://doi.org/10.3390/nano11112913
Chicago/Turabian StyleEl-Ghany, Mona F. Abd, Mohamady I. El-Kherbawy, Youssef A. Abdel-Aal, Samaa I. El-Dek, and Tarek Abd El-Baky. 2021. "Comparative Study between Traditional and Nano Calcium Phosphate Fertilizers on Growth and Production of Snap Bean (Phaseolus vulgaris L.) Plants" Nanomaterials 11, no. 11: 2913. https://doi.org/10.3390/nano11112913
APA StyleEl-Ghany, M. F. A., El-Kherbawy, M. I., Abdel-Aal, Y. A., El-Dek, S. I., & Abd El-Baky, T. (2021). Comparative Study between Traditional and Nano Calcium Phosphate Fertilizers on Growth and Production of Snap Bean (Phaseolus vulgaris L.) Plants. Nanomaterials, 11(11), 2913. https://doi.org/10.3390/nano11112913






