Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-β1 Signaling Pathway and Macrophage Polarization
Abstract
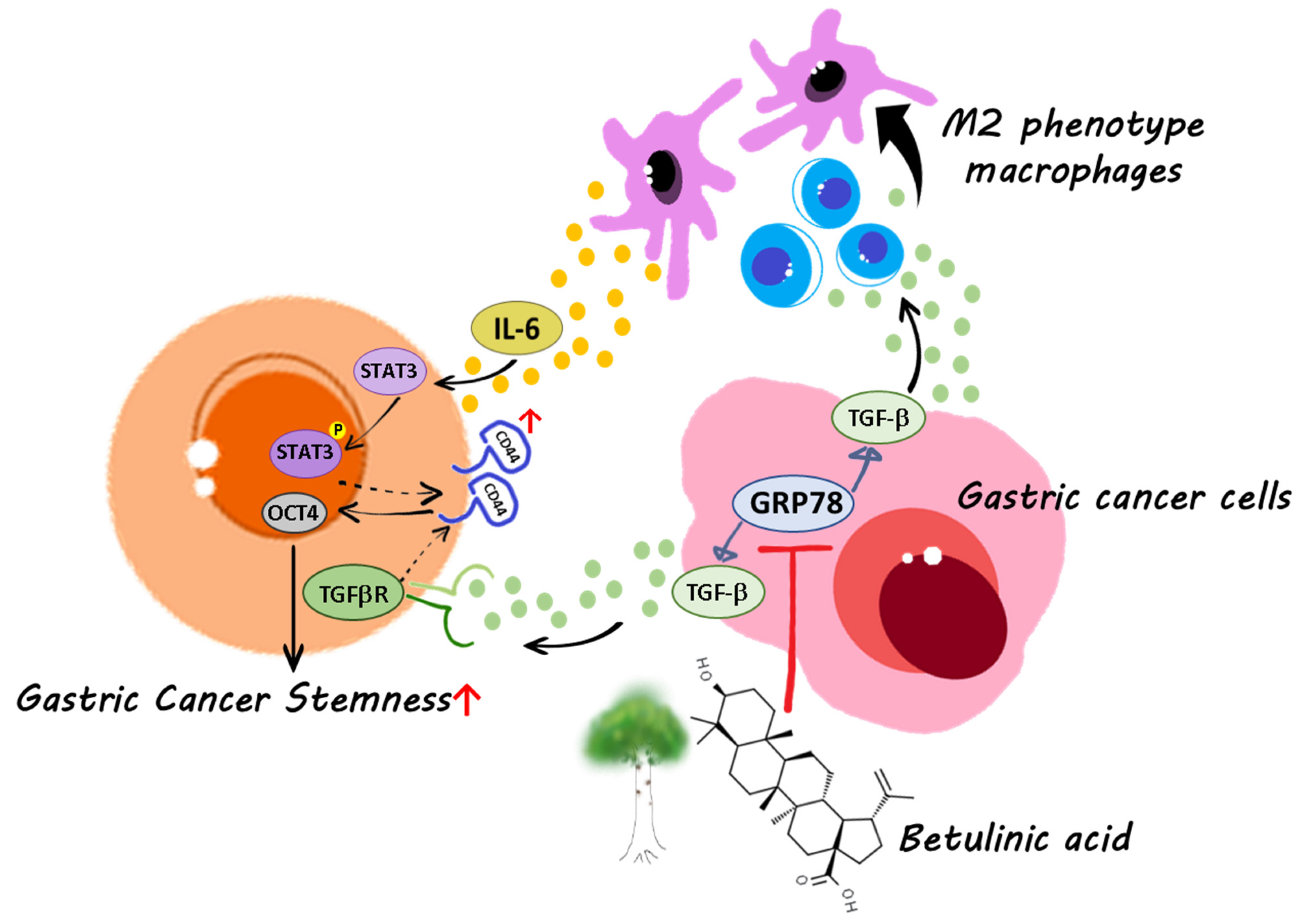
1. Introduction
2. Results
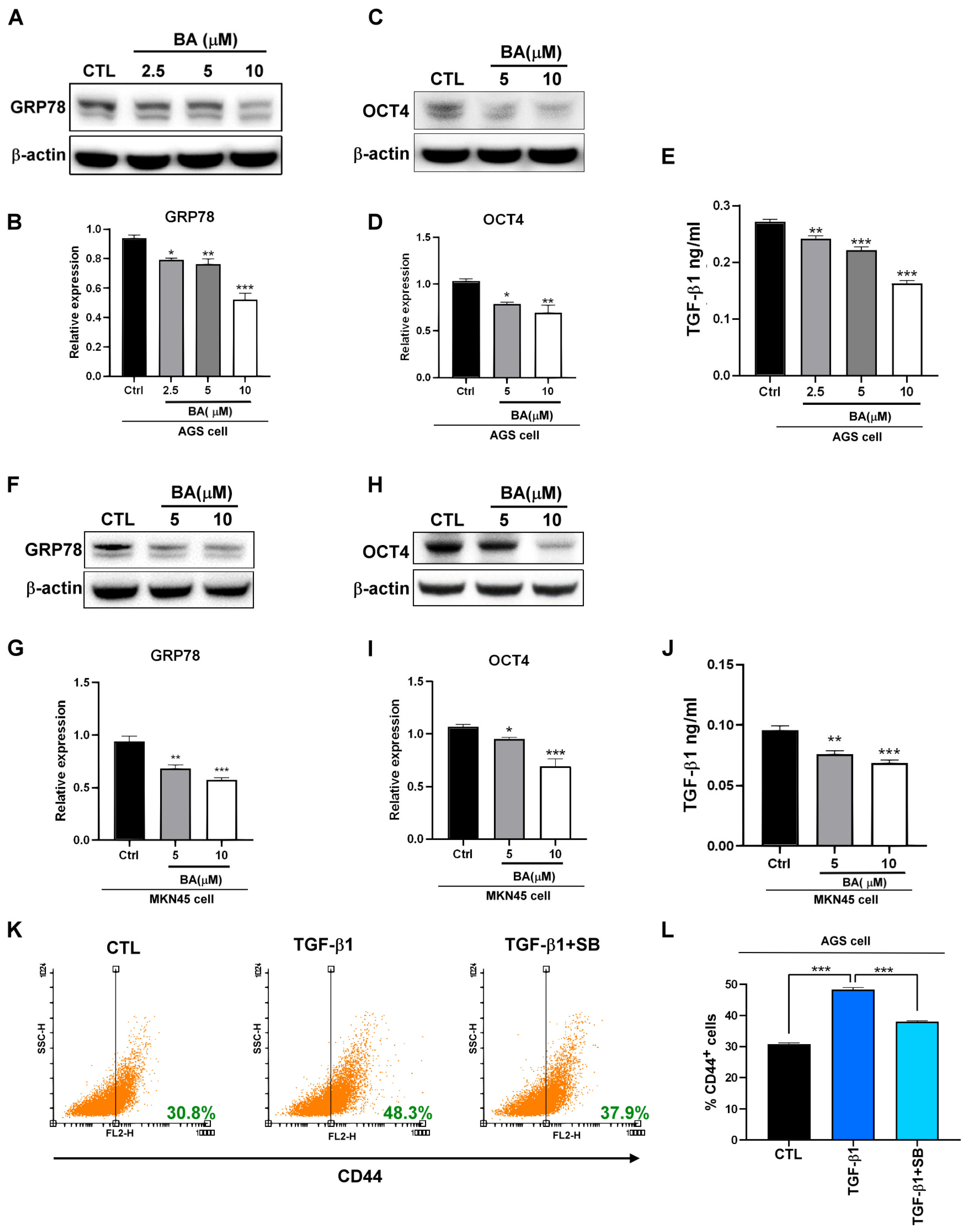
2.1. BA Inhibits the Expression of GRP78, TGF-β1, and Stemness Markers in Human Gastric Cancer Cells
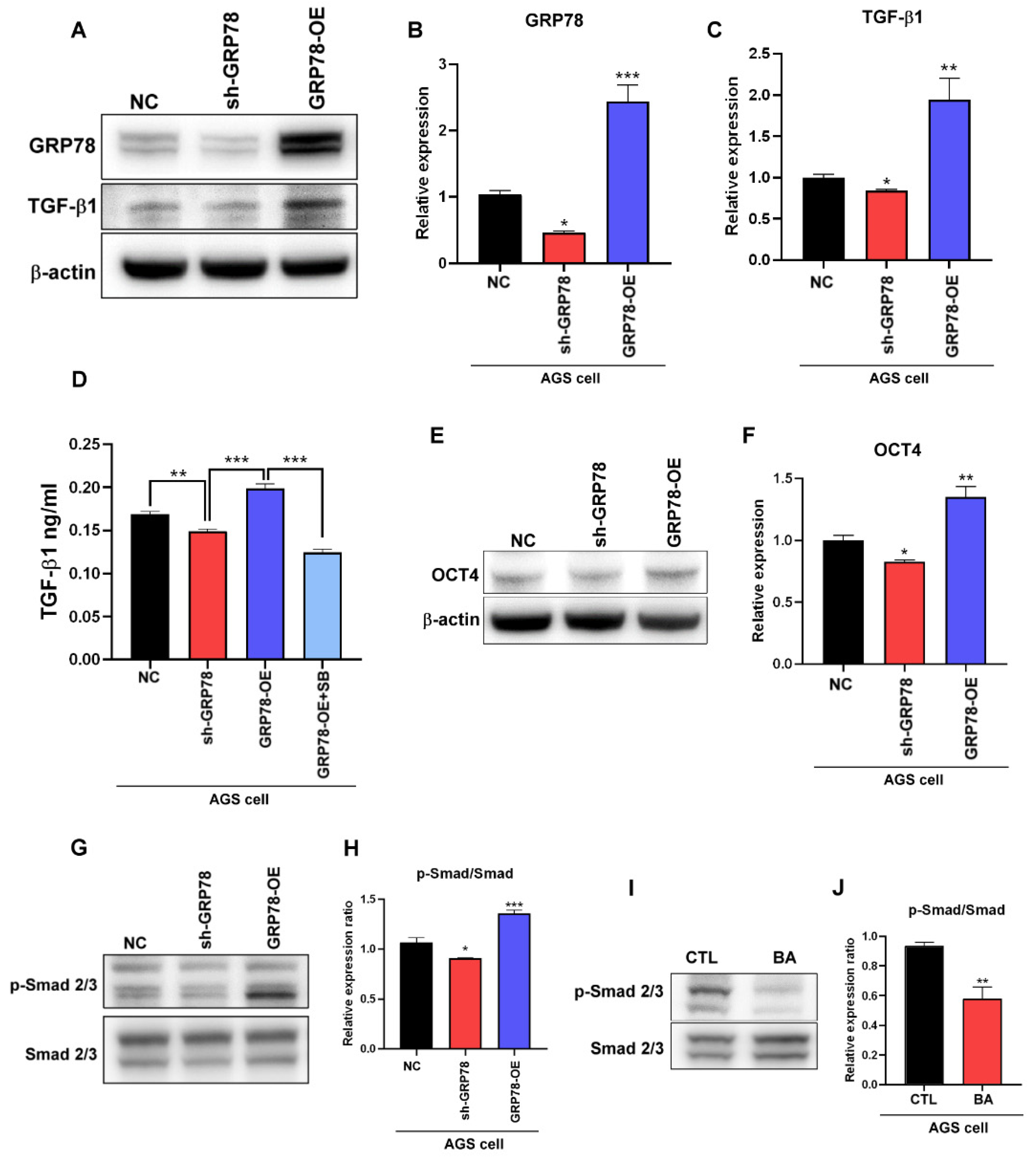
2.2. BA Contributes to the GRP78-TGF-β1-Mediated Inhibition of Gastric Cancer Stemness
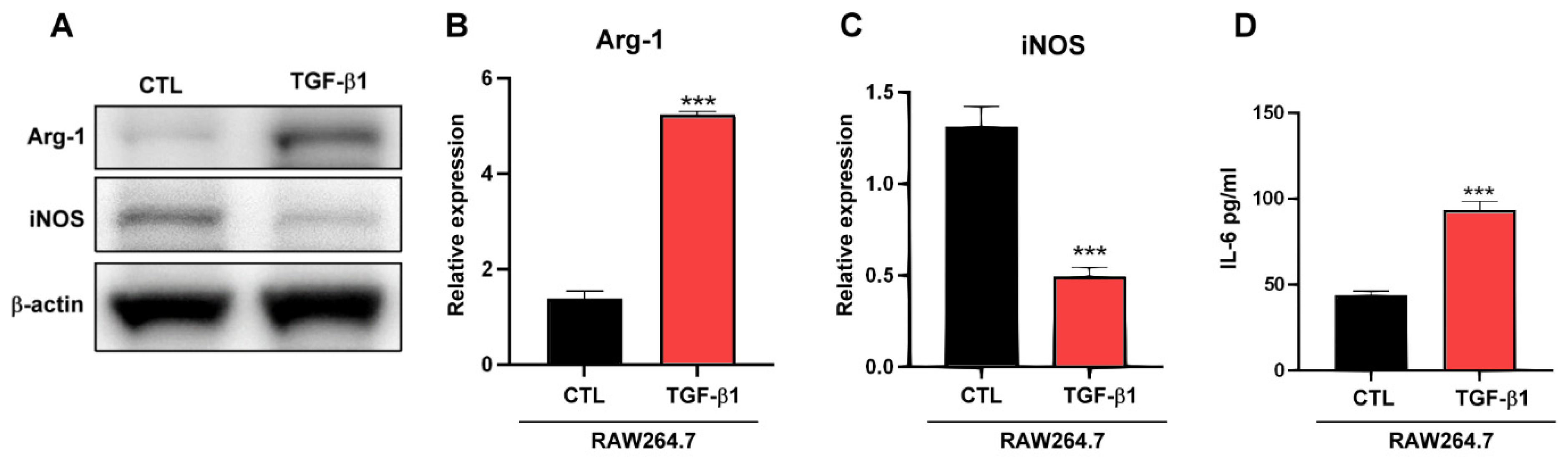
2.3. TGF-β1 Regulates Macrophages TAM-Type Polarization
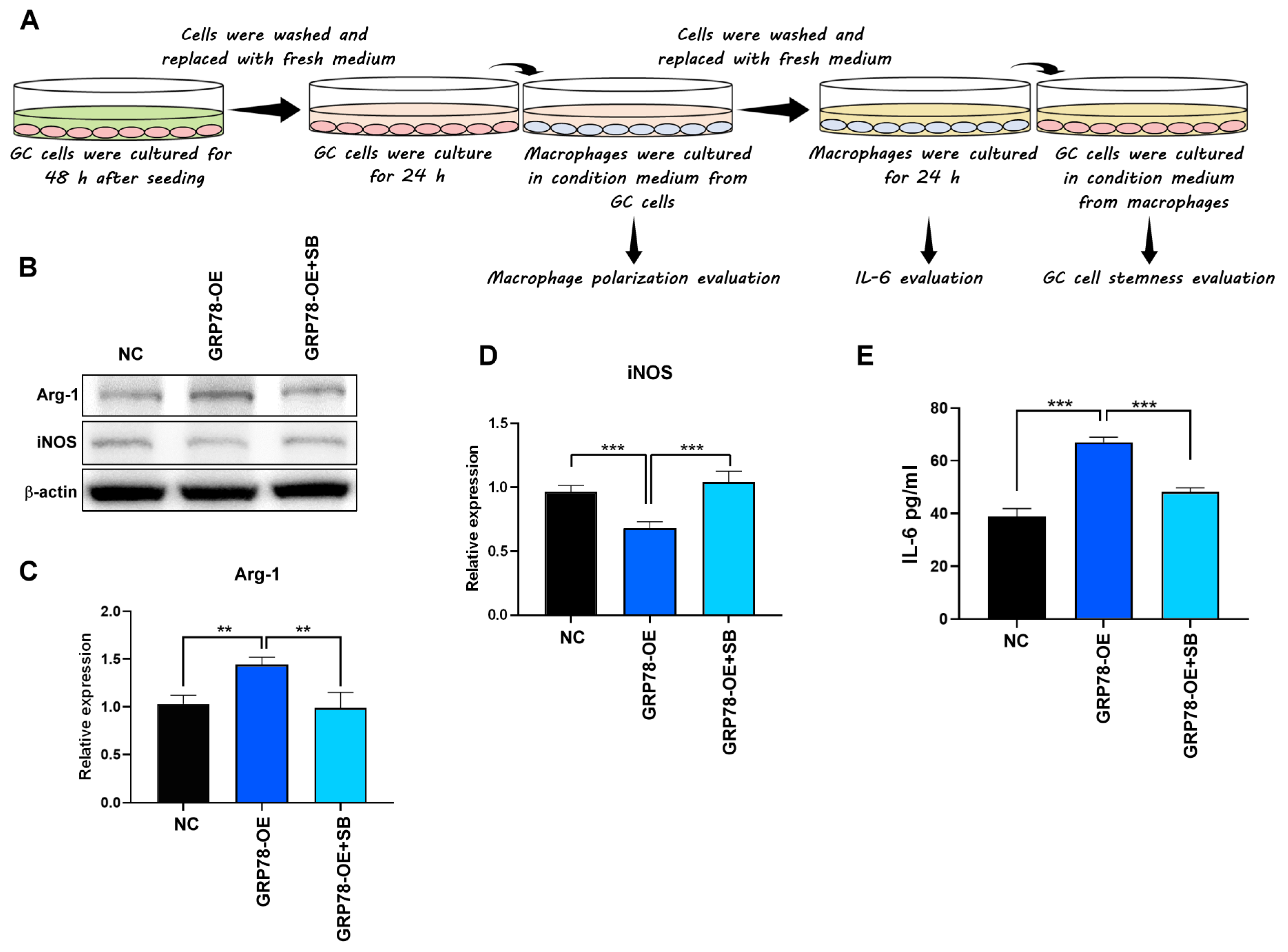
2.4. Gastric Cancer Cells Overexpressing GRP78 Induce M2-Type Macrophages through the TGF-β1 Signaling Pathway
2.5. IL-6 Promotes Gastric Cancer Stemness
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagent
4.2. Western Blot Analysis
4.3. Measurement of TGF-β1 Level
4.4. Measurement of IL-6 Level
4.5. Stimulation of Cells with Conditioned Media
4.6. Flow Cytometry Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sonbol, M.B.; Ahn, D.H.; Bekaii-Saab, T. Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies. Biomedicines 2019, 7, 17. [Google Scholar] [CrossRef]
- Das, M.; Law, S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018, 103, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Memar, B.; Behzadi, R.; Aliakbarian, M.; Jangjoo, A.; Bahar, M.M.; Talebi, S.; Gholamin, M.; Abbaszadegan, M.R. Isolation and identification of chemotherapy-enriched sphere-forming cells from a patient with gastric cancer. J. Cell Physiol. 2018, 233, 7036–7046. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, W.; Zhang, Y.Y.; Wang, D.L.; Wu, X.L. CD44+ gastric cancer cells with stemness properties are chemoradioresistant and highly invasive. Oncol. Lett. 2013, 5, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Wong, G.; Earle, C.; Chen, L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 2012, 287, 32800–32824. [Google Scholar] [CrossRef]
- Zhu, G.; Lee, A.S. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J. Cell Physiol. 2015, 230, 1413–1420. [Google Scholar] [CrossRef]
- Lee, C.H.; Tsai, H.Y.; Chen, C.L.; Chen, J.L.; Lu, C.C.; Fang, Y.P.; Wu, D.C.; Huang, Y.B.; Lin, M.W. Isoliquiritigenin Inhibits Gastric Cancer Stemness, Modulates Tumor Microenvironment, and Suppresses Tumor Growth through Glucose-Regulated Protein 78 Downregulation. Biomedicines 2022, 10, 1350. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Arlauckas, S.P.; Garren, S.B.; Garris, C.S.; Kohler, R.H.; Oh, J.; Pittet, M.J.; Weissleder, R. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics 2018, 8, 5842–5854. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- Morales, V.; Soto-Ortiz, L. Modeling macrophage polarization and its effect on cancer treatment success. Open J. Immunol. 2018, 8, 36–80. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Costa, J.F.; Meira, C.S.; Neves, M.V.G.D.; Dos-Reis, B.P.Z.C.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Quadros, H.; Meira, C.; Silva, L.; Carvalho, D.; Hodel, K.; Moreira, D.; Soares, M. Potential of Triterpenic Natural Compound Betulinic Acid for Neglected Tropical Diseases New Treatments. Biomedicines 2022, 10, 831. [Google Scholar] [CrossRef]
- Aswathy, M.; Vijayan, A.; Daimary, U.D.; Girisa, S.; Radhakrishnan, K.V.; Kunnumakkara, A.B. Betulinic acid: A natural promising anticancer drug, current situation, and future perspectives. J. Biochem. Mol. Toxicol. 2022, 36, e23206. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, X.N.; Zhang, Z.; Gong, P.J.; Yin, W.N.; Jiang, Q.; Xu, J.; Xu, X.L.; Gao, Y.; Chen, W.L.; et al. Betulinic acid inhibits cell proliferation and migration in gastric cancer by targeting the NF-κB/VASP pathway. Eur. J. Pharmacol. 2020, 889, 173493. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, X.; Liu, C.; Zhou, Y. Betulinic acid triggers apoptosis and inhibits migration and invasion of gastric cancer cells by impairing EMT progress. Cell Biochem. Funct. 2020, 38, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Dan, Z.; Nie, Y.Q. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J. Gastroenterol. 2014, 20, 5420–5426. [Google Scholar] [CrossRef] [PubMed]
- Villodre, E.S.; Kipper, F.C.; Pereira, M.B.; Lenz, G. Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat. Rev. 2016, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Munoz-Sagredo, L.; Streule, K.; Muschong, P.; Bayer, E.; Walter, R.J.; Gutjahr, J.C.; Greil, R.; Concha, M.L.; Müller-Tidow, C.; et al. CD44 loss of function sensitizes AML cells to the BCL-2 inhibitor venetoclax by decreasing CXCL12-driven survival cues. Blood 2021, 138, 1067–1080. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Fan, Y.; Li, H.; Li, Z.; Li, Y. Overexpressed GRP78 affects EMT and cell-matrix adhesion via autocrine TGF-β/Smad2/3 signaling. Int. J. Biochem Cell Biol. 2015, 64, 202–211. [Google Scholar] [CrossRef]
- Au, H.K.; Chang, J.H.; Wu, Y.C.; Kuo, Y.C.; Chen, Y.H.; Lee, W.C.; Chang, T.S.; Lan, P.C.; Kuo, H.C.; Lee, K.L.; et al. TGF-βI Regulates Cell Migration through Pluripotent Transcription Factor OCT4 in Endometriosis. PLoS ONE 2015, 10, e0145256. [Google Scholar] [CrossRef]
- Pirozzi, G.; Tirino, V.; Camerlingo, R.; Franco, R.; La Rocca, A.; Liguori, E.; Martucci, N.; Paino, F.; Normanno, N.; Rocco, G. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non-small cell lung cancer cell line. PLoS ONE 2011, 6, e21548. [Google Scholar] [CrossRef]
- Gifford, C.C.; Tang, J.; Costello, A.; Khakoo, N.S.; Nguyen, T.Q.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin. Sci. (Lond) 2021, 135, 275–303. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Sun, B.; Sun, H.; Zhao, X.; Zhang, D.; Liu, T.; Zhao, N.; Gu, Q.; Dong, X.; Liu, F. Nodal signaling activates the Smad2/3 pathway to regulate stem cell-like properties in breast cancer cells. Am. J. Cancer Res. 2017, 7, 503–517. [Google Scholar] [PubMed]
- Beyer, T.A.; Weiss, A.; Khomchuk, Y.; Huang, K.; Ogunjimi, A.A.; Varelas, X.; Wrana, J.L. Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep. 2013, 5, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Maldonado, L.A.G.; Nascimento, C.R.; Rodrigues- Fernandes, N.A.; Silva, A.L.P.; D’Silva, N.J.; Rossa, C. Influence of tumor cell-derived TGF-β on macrophage phenotype and macrophage-mediated tumor cell invasion. Int. J. Biochem Cell Biol. 2022, 153, 106330. [Google Scholar] [CrossRef]
- Radharani, N.N.V.; Yadav, A.S.; Nimma, R.; Kumar, T.V.S.; Bulbule, A.; Chanukuppa, V.; Kumar, D.; Patnaik, S.; Rapole, S.; Kundu, G.C. Tumor-associated macrophage derived IL-6 enriches cancer stem cell population and promotes breast tumor progression via Stat-3 pathway. Cancer Cell Int. 2022, 22, 122. [Google Scholar] [CrossRef]
- Jeong, S.K.; Kim, J.S.; Lee, C.G.; Park, Y.S.; Kim, S.D.; Yoon, S.O.; Han, D.H.; Lee, K.Y.; Jeong, M.H.; Jo, W.S. Tumor associated macrophages provide the survival resistance of tumor cells to hypoxic microenvironmental condition through IL-6 receptor-mediated signals. Immunobiology 2017, 222, 55–65. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, G.Z.; Wang, Y.; Huang, H.Z.; Li, W.T.; Qu, X.D. Hypoxia-induced HMGB1 expression of HCC promotes tumor invasiveness and metastasis via regulating macrophage-derived IL-6. Exp. Cell Res. 2018, 367, 81–88. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, W.; Wang, C. Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial-mesenchymal transition. Onco Targets Ther. 2018, 11, 3817–3826. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, S.; Hu, Y.; Feng, Y.; Li, M.; Bian, Z.; Zhang, J.; Qin, Y.; Qi, X.; Zhou, L.; et al. The Immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin Cancer Res. 2017, 23, 7375–7387. [Google Scholar] [CrossRef]
- Xu, X.; Ye, J.; Huang, C.; Yan, Y.; Li, J. M2 macrophage-derived IL6 mediates resistance of breast cancer cells to hedgehog inhibition. Toxicol. Appl. Pharmacol. 2019, 364, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef]
- Yang, L.; Dong, Y.; Li, Y.; Wang, D.; Liu, S.; Wang, D.; Gao, Q.; Ji, S.; Chen, X.; Lei, Q.; et al. IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-κB/Notch1 pathway in non-small cell lung cancer. Int. J. Cancer 2019, 145, 1099–1110. [Google Scholar] [CrossRef]
- Lv, J.; Liu, C.; Chen, F.K.; Feng, Z.P.; Jia, L.; Liu, P.J.; Yang, Z.X.; Hou, F.; Deng, Z.Y. M2-like tumour-associated macrophage-secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2021, 24, 604. [Google Scholar] [CrossRef]
- Liu, J.; Ma, L.; Xu, J.; Liu, C.; Zhang, J.; Liu, J.; Chen, R.; Zhou, Y. Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int. J. Oncol. 2013, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hermann, P.C.; Liebau, S.; Weidgang, C.; Seufferlein, T.; Kleger, A.; Perkhofer, L. The role of pluripotency factors to drive stemness in gastrointestinal cancer. Stem Cell Res. 2016, 16, 349–357. [Google Scholar] [CrossRef]
- Yoon, C.; Park, D.J.; Schmidt, B.; Thomas, N.J.; Lee, H.J.; Kim, T.S.; Janjigian, Y.Y.; Cohen, D.J.; Yoon, S.S. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014, 20, 3974–3988. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, Y.; Yang, S.; Zeng, T.; Hua, Y.; Bao, S.; Yang, F.; Duan, N.; Sun, C.; Liang, Y.; et al. A positive feedback loop: RAD18-YAP-TGF-β between triple-negative breast cancer and macrophages regulates cancer stemness and progression. Cell Death Discov. 2022, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Viji, V.; Shobha, B.; Kavitha, S.K.; Ratheesh, M.; Kripa, K.; Helen, A. Betulinic acid isolated from Bacopa monniera (L.) Wettst suppresses lipopolysaccharide stimulated interleukin-6 production through modulation of nuclear factor-kappaB in peripheral blood mononuclear cells. Int. Immunopharmacol. 2010, 10, 843–849. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-L.; Tai, Y.-S.; Tsai, H.-Y.; Hsieh, C.-Y.; Chen, C.-L.; Liu, C.-J.; Wu, D.-C.; Huang, Y.-B.; Lin, M.-W. Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-β1 Signaling Pathway and Macrophage Polarization. Molecules 2023, 28, 1725. https://doi.org/10.3390/molecules28041725
Chen J-L, Tai Y-S, Tsai H-Y, Hsieh C-Y, Chen C-L, Liu C-J, Wu D-C, Huang Y-B, Lin M-W. Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-β1 Signaling Pathway and Macrophage Polarization. Molecules. 2023; 28(4):1725. https://doi.org/10.3390/molecules28041725
Chicago/Turabian StyleChen, Jen-Lung, Yun-Shen Tai, Hsin-Yi Tsai, Chia-Yuan Hsieh, Chun-Lin Chen, Chung-Jung Liu, Deng-Chyang Wu, Yaw-Bin Huang, and Ming-Wei Lin. 2023. "Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-β1 Signaling Pathway and Macrophage Polarization" Molecules 28, no. 4: 1725. https://doi.org/10.3390/molecules28041725
APA StyleChen, J.-L., Tai, Y.-S., Tsai, H.-Y., Hsieh, C.-Y., Chen, C.-L., Liu, C.-J., Wu, D.-C., Huang, Y.-B., & Lin, M.-W. (2023). Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-β1 Signaling Pathway and Macrophage Polarization. Molecules, 28(4), 1725. https://doi.org/10.3390/molecules28041725





