Flow-Induced Shear Stress Combined with Microtopography Inhibits the Differentiation of Neuro-2a Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Microfluidic System
2.2. Fabrication of Laser-Microstructured Polyethylene Terephthalate (PET) Substrates
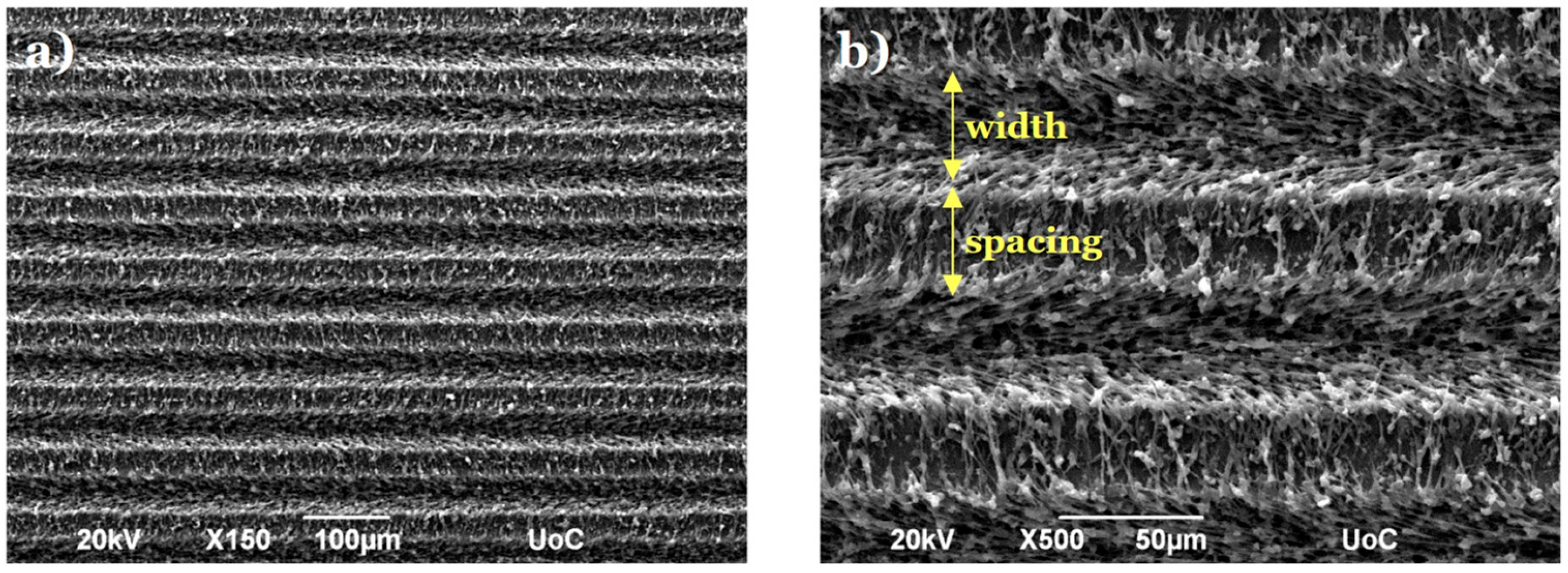
2.3. Morphological and Geometrical Characterization of Laser-Microstructured Polyethylene Terephthalate (PET) Substrates by Scanning Electron Microscopy (SEM)
2.4. Static and Dynamic Cell Cultures
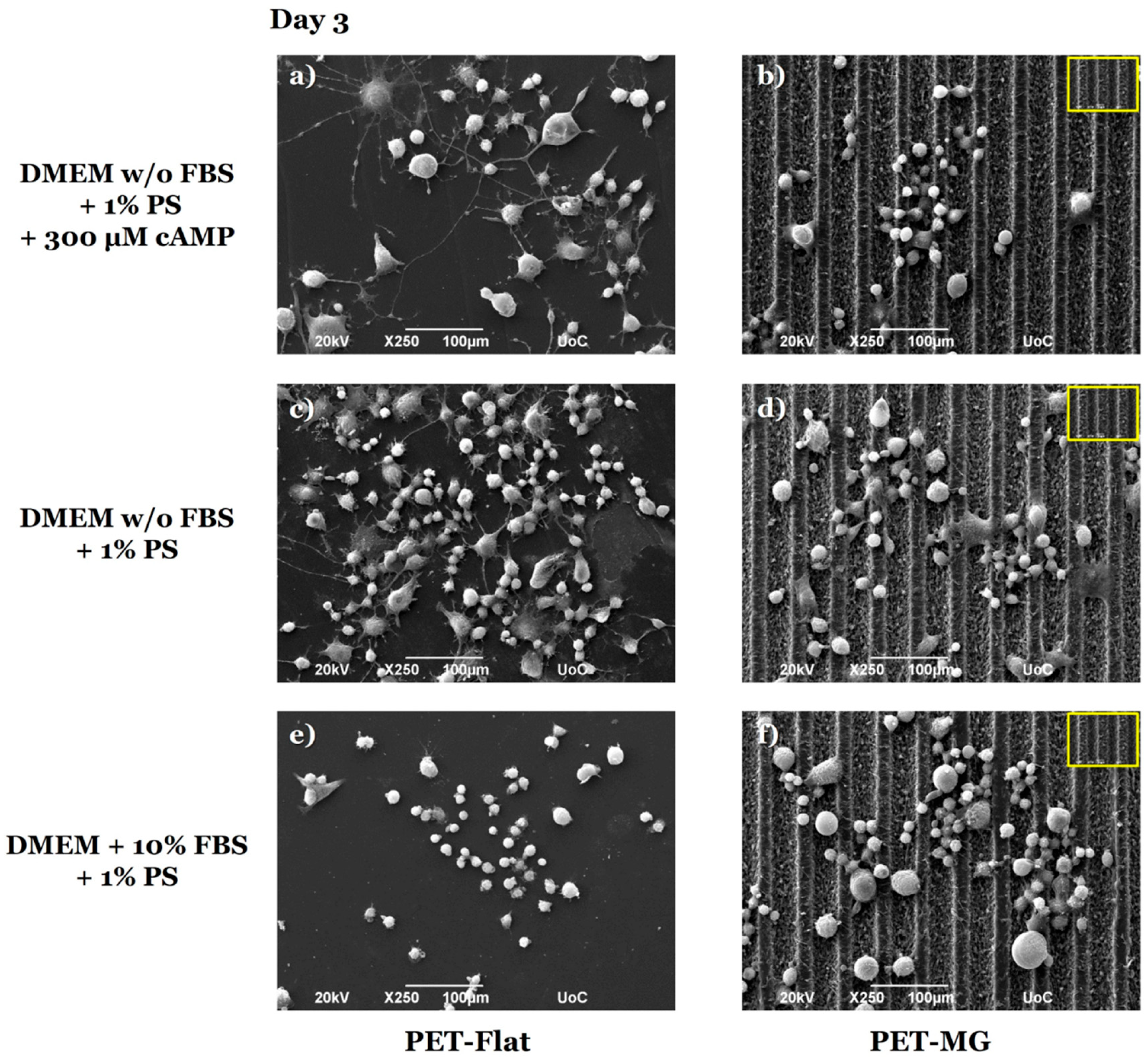
2.5. Morphological Characterization of N2a Cells by Scanning Electron Microscopy (SEM)
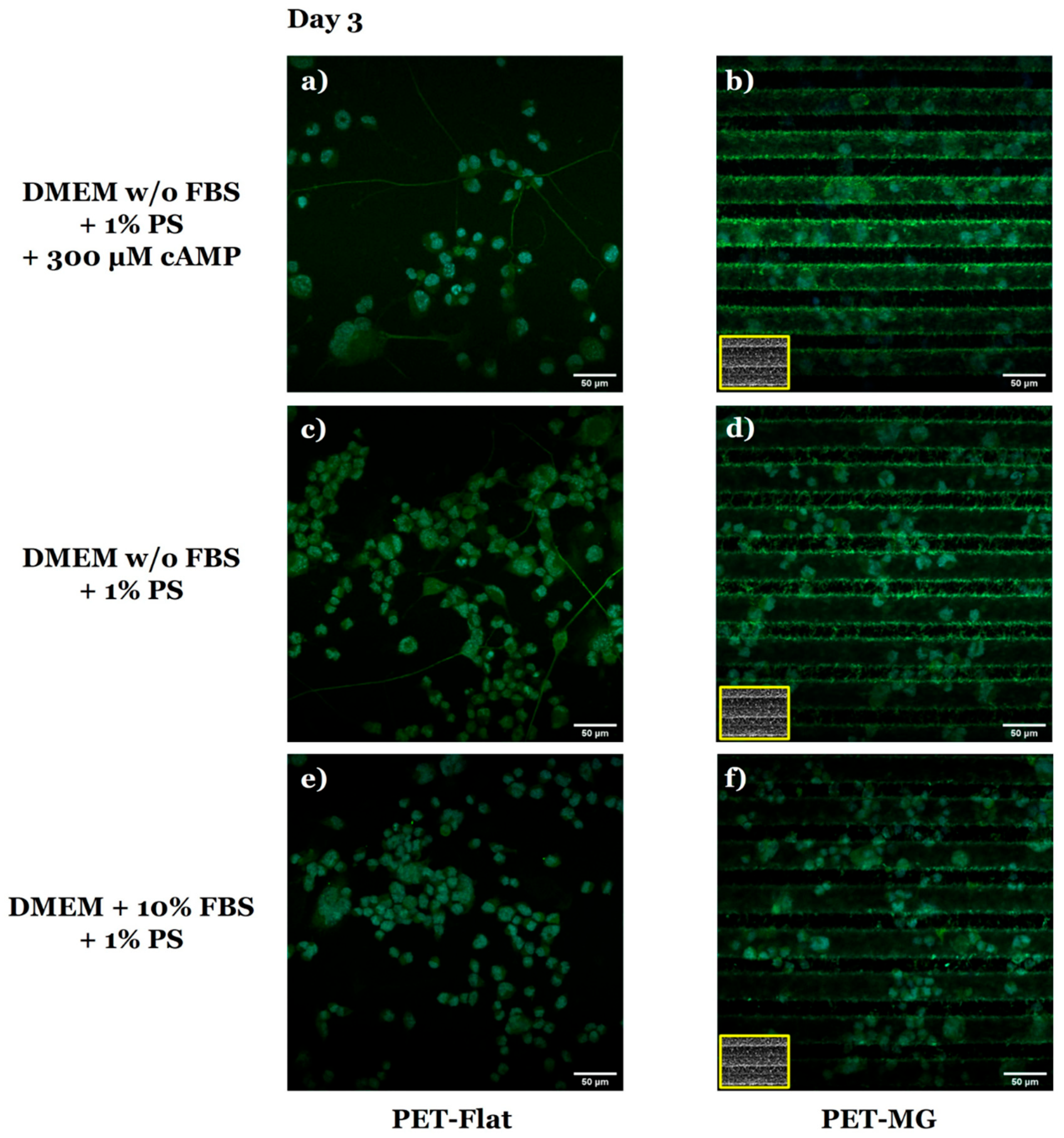
2.6. Immunocytochemical Assays
2.7. Live Cell Imaging Assay
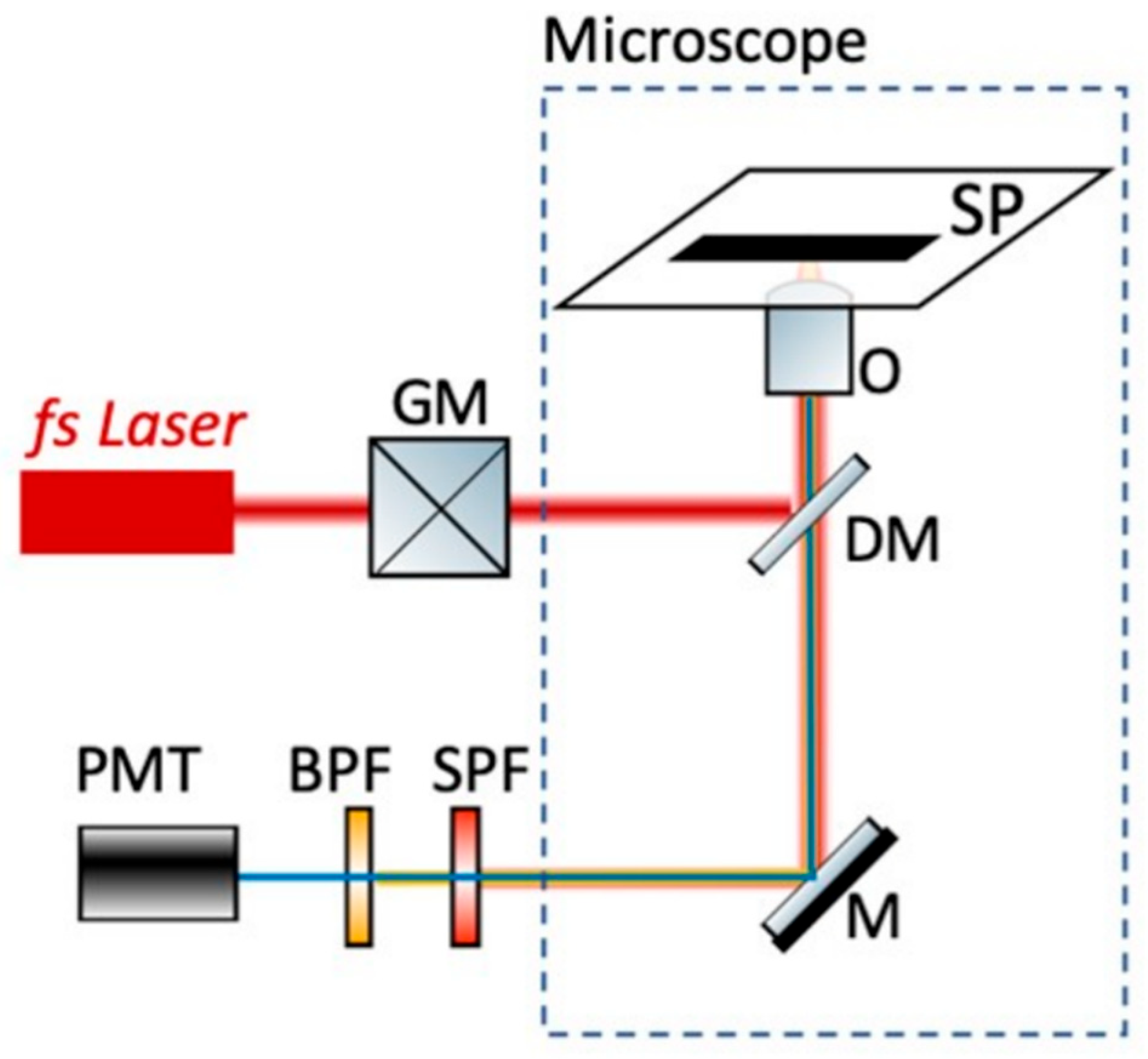
2.8. Non-Linear Microscope
3. Results
3.1. Morphological Characterization of Laser-Microstructured Polyethylene Terephthalate (PET) Substrates
3.2. Growth and Differentiation of N2a Cells on the Laser-Microstructured Polyethylene Terephthalate (PET) Substrates Under Static Conditions
3.3. Growth and Differentiation of N2a Cells on the Laser-Microstructured Polyethylene Terephthalate (PET) Substrates Under Dynamic Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pedrosa, S.S.; Caseiro, A.R.; Santos, J.D.; Maurício, A.C. Scaffolds for Peripheral Nerve Regeneration, the Importance of In Vitro and In Vivo Studies for the Development of Cell-Based Therapies and Biomaterials: State of the Art. In Scaffolds in Tissue Engineering Materials, Technologies and Clinical Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for Neural Tissue Engineering Applications. Mater. Today Bio. 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.; Fawcett, J. Repair in the Central Nervous System. J. Bone Jt. Surg. Ser. B 2007, 89, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Willerth, S.M. 3-D Bioprinting of Neural Tissue for Applications in Cell Therapy and Drug Screening. Front. Bioeng. Biotechnol. 2017, 5, 304216. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Santhosh, M.; Choi, J.W. In Vitro Blood–Brain Barrier-Integrated Neurological Disorder Models Using a Microfluidic Device. Micromachines 2019, 11, 21. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2004; ISBN 0125824637. [Google Scholar]
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering: Fourth Edition; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123983589. [Google Scholar]
- Chow, W.N.; Simpson, D.G.; Bigbee, J.W.; Colello, R.J. Evaluating Neuronal and Glial Growth on Electrospun Polarized Matrices: Bridging the Gap in Percussive Spinal Cord Injuries. Neuron Glia Biol. 2007, 3, 119–126. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of Glial Cell Migration and Axonal Growth on Electrospun Nanofibers of Poly-ε-Caprolactone and a Collagen/Poly-ε-Caprolactone Blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef]
- Yao, L.; Wang, S.; Cui, W.; Sherlock, R.; O’Connell, C.; Damodaran, G.; Gorman, A.; Windebank, A.; Pandit, A. Effect of Functionalized Micropatterned PLGA on Guided Neurite Growth. Acta Biomater. 2009, 5, 580–588. [Google Scholar] [CrossRef]
- Simitzi, C.; Ranella, A.; Stratakis, E. Controlling the Morphology and Outgrowth of Nerve and Neuroglial Cells: The Effect of Surface Topography. Acta Biomater. 2017, 51, 21–52. [Google Scholar] [CrossRef]
- Chen, W.; Shao, Y.; Li, X.; Zhao, G.; Fu, J. Nanotopographical Surfaces for Stem Cell Fate Control: Engineering Mechanobiology from the Bottom. Nano Today 2014, 9, 759–784. [Google Scholar] [CrossRef]
- Manganas, P.; Kavatzikidou, P.; Kordas, A.; Babaliari, E.; Stratakis, E.; Ranella, A. The Role of Mechanobiology on the Schwann Cell Response: A Tissue Engineering Perspective. Front. Cell. Neurosci. 2022, 16, 948454. [Google Scholar] [CrossRef]
- Reis, R.L. Encyclopedia of Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2019; Volume 3, ISBN 978-0-12-813700-0. [Google Scholar]
- Coluccio, M.L.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.M.; Cuda, G.; Candeloro, P.; et al. Microfluidic Platforms for Cell Cultures and Investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Babaliari, E.; Ranella, A.; Stratakis, E. Microfluidic Systems for Neural Cell Studies. Bioengineering 2023, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Van Noort, D. Cells in Microfluidics. Top. Curr. Chem. 2011, 304, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M. Microfluidic Cell Culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef]
- Kim, J.H.; Sim, J.; Kim, H.J. Neural Stem Cell Differentiation Using Microfluidic Device-Generated Growth Factor Gradient. Biomol. Ther. 2018, 26, 380–388. [Google Scholar] [CrossRef]
- Futai, N.; Tamura, M.; Ogawa, T.; Tanaka, M. Microfluidic Long-Term Gradient Generator with Axon Separation Prototyped by 185 Nm Diffused Light Photolithography of SU-8 Photoresist. Micromachines 2018, 10, 9. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Folch, A. Large-Scale Microfluidic Gradient Arrays Reveal Axon Guidance Behaviors in Hippocampal Neurons. Microsystems Nanoeng. 2017, 3, 17003. [Google Scholar] [CrossRef]
- Park, M.G.; Jang, H.; Lee, S.H.; Justin Lee, C. Flow Shear Stress Enhances the Proliferative Potential of Cultured Radial Glial Cells Possibly via an Activation of Mechanosensitive Calcium Channel. Exp. Neurobiol. 2017, 26, 71–81. [Google Scholar] [CrossRef]
- Ristola, M.; Sukki, L.; Azevedo, M.M.; Seixas, A.I.; Relvas, J.B.; Narkilahti, S.; Kallio, P. A Compartmentalized Neuron-Oligodendrocyte Co-Culture Device for Myelin Research: Design, Fabrication and Functionality Testing. J. Micromechanics Microengineering 2019, 29, 065009. [Google Scholar] [CrossRef]
- Hesari, Z.; Soleimani, M.; Atyabi, F.; Sharifdini, M.; Nadri, S.; Warkiani, M.E.; Zare, M.; Dinarvand, R. A Hybrid Microfluidic System for Regulation of Neural Differentiation in Induced Pluripotent Stem Cells. J. Biomed. Mater. Res. Part A 2016, 104, 1534–1543. [Google Scholar] [CrossRef]
- Kim, I.A.; Park, S.A.; Kim, Y.J.; Kim, S.-H.; Shin, H.J.; Lee, Y.J.; Kang, S.G.; Shin, J.-W. Effects of Mechanical Stimuli and Microfiber-Based Substrate on Neurite Outgrowth and Guidance. J. Biosci. Bioeng. 2006, 101, 120–126. [Google Scholar] [CrossRef]
- Jeon, K.J.; Park, S.H.; Shin, J.W.; Kang, Y.G.; Hyun, J.-S.; Oh, M.J.; Kim, S.Y.; Shin, J.-W. Combined Effects of Flow-Induced Shear Stress and Micropatterned Surface Morphology on Neuronal Differentiation of Human Mesenchymal Stem Cells. J. Biosci. Bioeng. 2014, 117, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Babaliari, E.; Kavatzikidou, P.; Mitraki, A.; Papaharilaou, Y.; Ranella, A.; Stratakis, E. Combined Effect of Shear Stress and Laser-Patterned Topography on Schwann Cell Outgrowth: Synergistic or Antagonistic? Biomater. Sci. 2021, 9, 1334. [Google Scholar] [CrossRef] [PubMed]
- Babaliari, E.; Kavatzikidou, P.; Angelaki, D.; Chaniotaki, L.; Manousaki, A.; Siakouli-Galanopoulou, A.; Ranella, A.; Stratakis, E. Engineering Cell Adhesion and Orientation via Ultrafast Laser Fabricated Microstructured Substrates. Int. J. Mol. Sci. 2018, 19, 2053. [Google Scholar] [CrossRef] [PubMed]
- Salto, R.; Vílchez, J.D.; Girón, M.D.; Cabrera, E.; Campos, N.; Manzano, M.; Rueda, R.; López-Pedrosa, J.M. β-Hydroxy-β-Methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS ONE 2015, 10, e0135614. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, M.L.; An, H.K.; Kim, K.S.; Ko, M.J.; Kim, C.M.; Choi, Y.W.; Lee, Y.C. Emodin Induces Neurite Outgrowth through PI3K/Akt/GSK-3β-Mediated Signaling Pathways in Neuro2a Cells. Neurosci. Lett. 2015, 588, 101–107. [Google Scholar] [CrossRef]
- Wu, P.Y.; Lin, Y.C.; Chang, C.L.; Lu, H.T.; Chin, C.H.; Hsu, T.T.; Chu, D.; Sun, S.H. Functional Decreases in P2X7 Receptors Are Associated with Retinoic Acid-Induced Neuronal Differentiation of Neuro-2a Neuroblastoma Cells. Cell. Signal. 2009, 21, 881–891. [Google Scholar] [CrossRef]
- Yanaka, N.; Nogusa, Y.; Fujioka, Y.; Yamashita, Y.; Kato, N. Involvement of Membrane Protein GDE2 in Retinoic Acid-Induced Neurite Formation in Neuro2A Cells. FEBS Lett. 2007, 581, 712–718. [Google Scholar] [CrossRef]
- Ma’ayan, A.; Jenkins, S.L.; Barash, A.; Iyengar, R. Neuro2A Differentiation by Gαi/o Pathway. Sci. Signal. 2009, 2, cm1. [Google Scholar]
- Arsenault, J.; Cuijpers, S.A.G.; Ferrari, E.; Niranjan, D.; Rust, A.; Leese, C.; O’Brien, J.A.; Binz, T.; Davletov, B. Botulinum Protease-Cleaved SNARE Fragments Induce Cytotoxicity in Neuroblastoma Cells. J. Neurochem. 2014, 129, 781–791. [Google Scholar] [CrossRef]
- Chollet, C.; Chanseau, C.; Remy, M.; Guignandon, A.; Bareille, R.; Labrugère, C.; Bordenave, L.; Durrieu, M.-C. The Effect of RGD Density on Osteoblast and Endothelial Cell Behavior on RGD-Grafted Polyethylene Terephthalate Surfaces. Biomaterials 2009, 30, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, T.; Yang, S.-T.; Kniss, D.A. Thermal Compression and Characterization of Three-Dimensional Nonwoven PET Matrices as Tissue Engineering Scaffolds. Biomaterials 2001, 22, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Swar, S.; Zajícová, V.; Rysová, M.; Lovětinská-Šlamborová, I.; Voleský, L.; Stibor, I. Biocompatible Surface Modification of Poly(Ethylene Terephthalate) Focused on Pathogenic Bacteria: Promising Prospects in Biomedical Applications. J. Appl. Polym. Sci. 2017, 134, 44990. [Google Scholar] [CrossRef]
- Baker, M.; Wayland, H. On-Line Volume Flow Rate and Velocity Profile Measurement for Blood in Microvessels. Microvasc. Res. 1974, 7, 131–143. [Google Scholar] [CrossRef]
- Frangos, J.A.; McIntire, L.V.; Eskin, S.G. Shear Stress Induced Stimulation of Mammalian Cell Metabolism. Biotechnol. Bioeng. 1988, 32, 1053–1060. [Google Scholar] [CrossRef]
- Sorkin, A.M.; Dee, K.C.; Knothe Tate, M.L. “Culture Shock” from the Bone Cell’s Perspective: Emulating Physiological Conditions for Mechanobiological Investigations. Am. J. Physiol. Physiol. 2004, 287, C1527–C1536. [Google Scholar] [CrossRef]
- Babaliari, E.; Petekidis, G.; Chatzinikolaidou, M. A Precisely Flow-Controlled Microfluidic System for Enhanced Pre-Osteoblastic Cell Response for Bone Tissue Engineering. Bioengineering 2018, 5, 66. [Google Scholar] [CrossRef]
- Fröhlich, E.; Bonstingl, G.; Höfler, A.; Meindl, C.; Leitinger, G.; Pieber, T.R.; Roblegg, E. Comparison of Two in Vitro Systems to Assess Cellular Effects of Nanoparticles-Containing Aerosols. Toxicol. Vitr. 2013, 27, 409–417. [Google Scholar] [CrossRef]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of Mouse Neuro 2A Cells into Dopamine Neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef]
- Ladt, K.; Ganguly, A.; Roy, S. Axonal Actin in Action: Imaging Actin Dynamics in Neurons. Methods Cell Biol. 2016, 131, 91–106. [Google Scholar] [CrossRef]
- Xydias, D.; Ziakas, G.; Psilodimitrakopoulos, S.; Lemonis, A.; Bagli, E.; Fotsis, T.; Gravanis, A.; Tzeranis, D.S.; Stratakis, E. Three-Dimensional Characterization of Collagen Remodeling in Cell-Seeded Collagen Scaffolds via Polarization Second Harmonic Generation. Biomed. Opt. Express 2021, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Baričević, Z.; Ayar, Z.; Leitao, S.M.; Mladinic, M.; Fantner, G.E.; Ban, J. Label-Free Long-Term Methods for Live Cell Imaging of Neurons: New Opportunities. Biosensors 2023, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Yu, W.-M.; Strickland, S. Peripheral Regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Huebner, E.A.; Strittmatter, S.M. Axon Regeneration in the Peripheral and Central Nervous Systems. Results Probl. Cell Differ. 2009, 48, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Cecchini, M.; Dhawan, A.; Micera, S.; Tonazzini, I.; Stabile, R.; Pisignano, D.; Beltram, F. Nanotopographic Control of Neuronal Polarity. Nano Lett. 2011, 11, 505–511. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Feng, J.; Duan, Y.; Xing, D.; Gao, C. Micropatterned Biodegradable Polyesters Clicked with CQAASIKVAV Promote Cell Alignment, Directional Migration, and Neurite Outgrowth. Acta Biomater. 2018, 74, 143–155. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zhang, L.; Yang, J.; Cui, W.; Yang, Y.; Zhang, H. Anisotropic Ridge/Groove Microstructure for Regulating Morphology and Biological Function of Schwann Cells. Appl. Mater. Today 2020, 18, 100468. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925–12942. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Kuppuswamy, A.A.; Sethuraman, S.; Subramanian, A. Topographic Cue from Electrospun Scaffolds Regulate Myelin-Related Gene Expressions in Schwann Cells. J. Biomed. Nanotechnol. 2015, 11, 512–521. [Google Scholar] [CrossRef]
- Xia, H.; Sun, X.; Liu, D.; Zhou, Y.; Zhong, D. Oriented Growth of Rat Schwann Cells on Aligned Electrospun Poly(Methyl Methacrylate) Nanofibers. J. Neurol. Sci. 2016, 369, 88–95. [Google Scholar] [CrossRef]
- Simitzi, C.; Efstathopoulos, P.; Kourgiantaki, A.; Ranella, A.; Charalampopoulos, I.; Fotakis, C.; Athanassakis, I.; Stratakis, E.; Gravanis, A. Laser Fabricated Discontinuous Anisotropic Microconical Substrates as a New Model Scaffold to Control the Directionality of Neuronal Network Outgrowth. Biomaterials 2015, 67, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Simitzi, C.; Stratakis, E.; Fotakis, C.; Athanassakis, I.; Ranella, A. Microconical Silicon Structures Influence NGF-Induced PC12 Cell Morphology. J. Tissue Eng. Regen. Med. 2015, 9, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Angelaki, D.; Kavatzikidou, P.; Fotakis, C.; Stratakis, E.; Ranella, A. Laser-Induced Topographies Enable the Spatial Patterning of Co-Cultured Peripheral Nervous System Cells. Mater. Sci. Eng. C 2020, 115, 111144. [Google Scholar] [CrossRef] [PubMed]
- Angelaki, D.; Kavatzikidou, P.; Fotakis, C.; Stratakis, E.; Ranella, A. Laser-Structured Si and PLGA Inhibit the Neuro2a Differentiation in Mono- and Co-Culture with Glia. Tissue Eng. Regen. Med. 2023, 20, 111–125. [Google Scholar] [CrossRef]
- Stoll, H.; Kwon, I.K.; Lim, J.Y. Material and Mechanical Factors: New Strategy in Cellular Neurogenesis. Neural Regen. Res. 2014, 9, 1810–1813. [Google Scholar]
- Franze, K. The Mechanical Control of Nervous System Development. Development 2013, 140, 3069–3077. [Google Scholar] [CrossRef]
- Chafik, D.; Bear, D.; Bui, P.; Patel, A.; Jones, N.F.; Kim, B.T.; Hung, C.T.; Gupta, R. Optimization of Schwann Cell Adhesion in Response to Shear Stress in an in Vitro Model for Peripheral Nerve Tissue Engineering. Tissue Eng. 2003, 9, 233–241. [Google Scholar] [CrossRef]
- Mitra, J.; Jain, S.; Sharma, A.; Basu, B. Patterned Growth and Differentiation of Neural Cells on Polymer Derived Carbon Substrates with Micro/Nano Structures in Vitro. Carbon 2013, 65, 140–155. [Google Scholar] [CrossRef]
- Béduer, A.; Vaysse, L.; Flahaut, E.; Seichepine, F.; Loubinoux, I.; Vieu, C. Multi-Scale Engineering for Neuronal Cell Growth and Differentiation. Microelectron. Eng. 2011, 88, 1668–1671. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, L.; Li, B.; Dawood, M.K.; Wan, G.; Lai, C.Q.; Cheng, H.; Leong, K.C.; Rajagopalan, R.; Too, H.P.; et al. Creation of Nanostructures by Interference Lithography for Modulation of Cell Behavior. Nanoscale 2011, 3, 2723–2729. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.S.; Cho, N.; Ju, B.K.; Lee, K.B.; Lee, S.H. Topographical Guidance of Mouse Neuronal Cell on SiO2 Microtracks. Sens. Actuators B Chem. 2007, 128, 252–257. [Google Scholar] [CrossRef]
- Marcus, M.; Baranes, K.; Park, M.; Choi, I.S.; Kang, K.; Shefi, O. Interactions of Neurons with Physical Environments. Adv. Healthc. Mater. 2017, 6, 1700267. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of Integrin Function by Nanopatterned Adhesive Interfaces. ChemPhysChem 2004, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, V.; Maiorano, G.; Rizzello, L.; Sorce, B.; Sabella, S.; Cingolani, R.; Pompa, P.P. Neurons Sense Nanoscale Roughness with Nanometer Sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 6264–6269. [Google Scholar] [CrossRef]
- Curtis, A.S.G.; Dolby, M.; Gadegaand, N. Cell Signaling Arising from Nanotopography: Implications for Nanomedical Devices. Nanomedicine 2006, 1, 67–72. [Google Scholar] [CrossRef]
- Yim, E.K.F.; Pang, S.W.; Leong, K.W. Synthetic Nanostructures Inducing Differentiation of Human Mesenchymal Stem Cells into Neuronal Lineage. Exp. Cell Res. 2007, 313, 1820–1829. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, M.; Wu, G.; Lai, Y.; Greber, B.; Schöler, H.R.; Chi, L. Topographic Effect on Human Induced Pluripotent Stem Cells Differentiation towards Neuronal Lineage. Biomaterials 2013, 34, 8131–8139. [Google Scholar] [CrossRef]
- Brown, T.D. Techniques for Mechanical Stimulation of Cells in Vitro: A Review. J. Biomech. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Shemesh, J.; Jalilian, I.; Shi, A.; Heng Yeoh, G.; Knothe Tate, M.L.; Ebrahimi Warkiani, M. Flow-Induced Stress on Adherent Cells in Microfluidic Devices. Lab Chip 2015, 15, 4114–4127. [Google Scholar] [CrossRef]



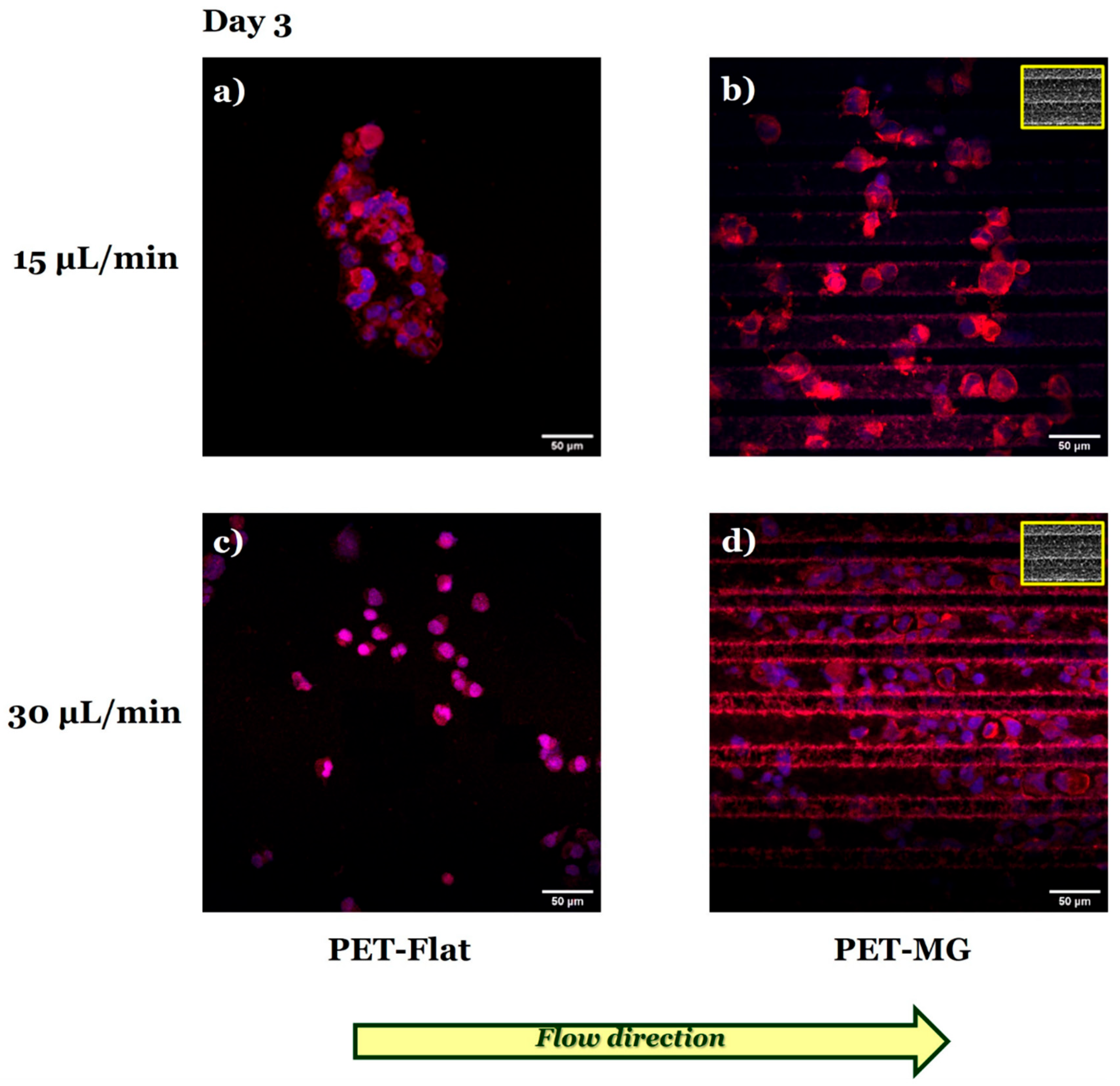
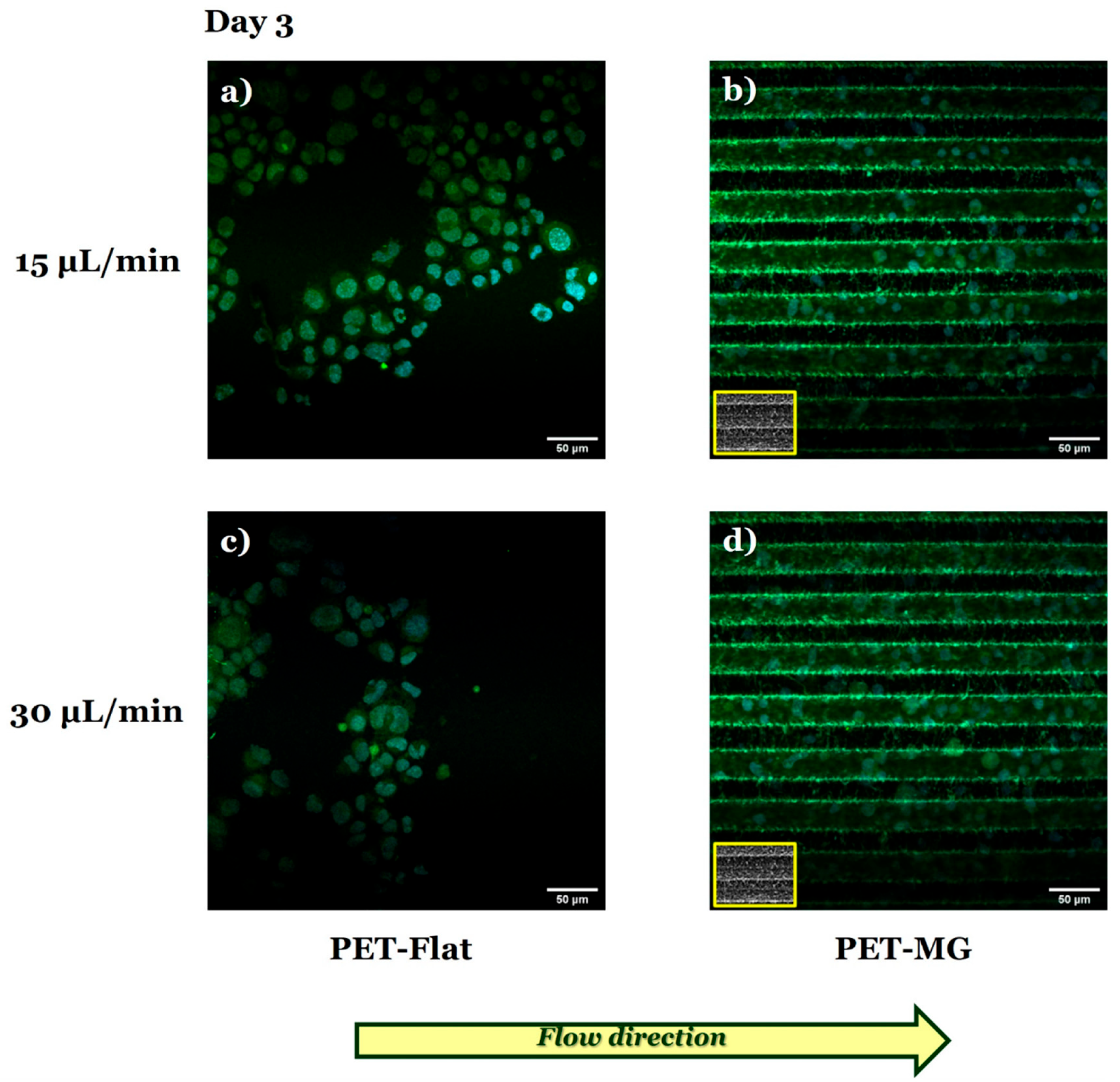

| Q (μL/Min) | (m/s) | σ (Pa) |
|---|---|---|
| 15 | 0.0013 | 0.01 |
| 30 | 0.0025 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babaliari, E.; Kavatzikidou, P.; Xydias, D.; Psilodimitrakopoulos, S.; Ranella, A.; Stratakis, E. Flow-Induced Shear Stress Combined with Microtopography Inhibits the Differentiation of Neuro-2a Cells. Micromachines 2025, 16, 341. https://doi.org/10.3390/mi16030341
Babaliari E, Kavatzikidou P, Xydias D, Psilodimitrakopoulos S, Ranella A, Stratakis E. Flow-Induced Shear Stress Combined with Microtopography Inhibits the Differentiation of Neuro-2a Cells. Micromachines. 2025; 16(3):341. https://doi.org/10.3390/mi16030341
Chicago/Turabian StyleBabaliari, Eleftheria, Paraskevi Kavatzikidou, Dionysios Xydias, Sotiris Psilodimitrakopoulos, Anthi Ranella, and Emmanuel Stratakis. 2025. "Flow-Induced Shear Stress Combined with Microtopography Inhibits the Differentiation of Neuro-2a Cells" Micromachines 16, no. 3: 341. https://doi.org/10.3390/mi16030341
APA StyleBabaliari, E., Kavatzikidou, P., Xydias, D., Psilodimitrakopoulos, S., Ranella, A., & Stratakis, E. (2025). Flow-Induced Shear Stress Combined with Microtopography Inhibits the Differentiation of Neuro-2a Cells. Micromachines, 16(3), 341. https://doi.org/10.3390/mi16030341











