Aminophosphonates in Nanofiltration and Reverse Osmosis Permeates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Solutions and Water Compositions
2.3. Membranes and Filtration Experiments
2.4. Analytical Methods
3. Results
3.1. Rejection and Phosphonate Concentration in Permeates under Standard Conditions
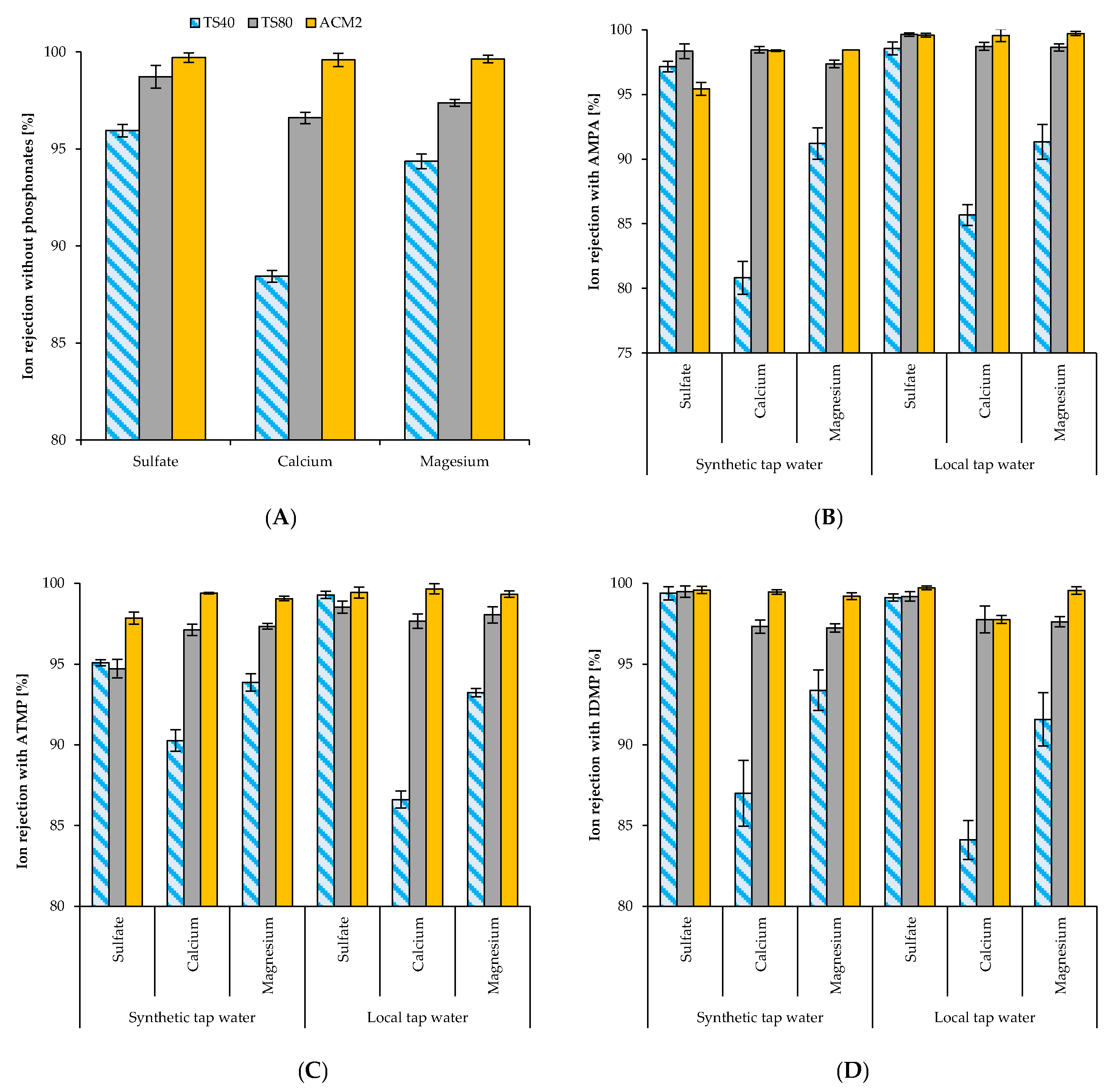
3.2. Ion Rejection with and without Phosphonates
3.3. Rejection Phosphonate of Technical Formulation Containing Antiscalant
4. Discussion
4.1. Influence of Size Exclusion and MWCO during NF and RO with Phosphonates
4.2. Influence of Physico-Chemical Properties during NF and RO with Phosphonates
4.3. Influence on Divalent Ions Rejection in Presence of Phosphonates
4.4. Technical Antiscalant Formulation in Local Tap Water Permeate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Feo-García, J. Antiscalant cost and maximum water recovery in reverse osmosis for different inorganic composition of groundwater. Desalination Water Treat. 2017, 73, 46–53. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Mitrouli, S.T.; Kostoglou, M. Scaling in reverse osmosis desalination plants: A perspective focusing on development of comprehensive simulation tools. Desalination 2020, 474, 114193. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Reverse Osmosis, Chapter 8—RO Membrane Fouling; Elsevier: Amsterdam, The Netherlands. [CrossRef]
- Studnik, H.; Liebsch, S.; Forlani, G.; Wieczorek, D.; Kafarski, P.; Lipok, J. Amino polyphosphonates—chemical features and practical uses, environmental durability and biodegradation. New Biotechnol. 2015, 32, 1–6. [Google Scholar] [CrossRef]
- Armbruster, D.; Müller, U.; Happel, O. Characterization of phosphonate-based antiscalants used in drinking water treatment plants by anion-exchange chromatography coupled to electrospray ionization time-of-flight mass spectrometry and inductively coupled plasma mass spectrometry. J. Chrom. A 2019, 1601, 189–204. [Google Scholar] [CrossRef]
- Cruz, J.M.; Murray, J.A. Determination of glyphosate and AMPA in oat products for the selection of candidate reference material. Food Chem. 2021, 342, 128213. [Google Scholar] [CrossRef]
- Grandcoin, A.; Piel, S.; Braurès, E. Aminomethylphosphonic acid (AMPA) in natural waters: Its source, behaviour and environmental fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, Glyphosate and AMPA in Drinking-Water, 3rd ed.; Chapter 12, Background document for preparation of WHO; (WHO/SDE/WSH/03.04/97); WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Kwiatkowska, M.; Huras, B.; Bukowska, B. The effect of metabolites and impurities of glyphosate on human erythrocytes (in vitro). Pestic. Biochem. Physiol. 2014, 109, 34–43. [Google Scholar] [CrossRef]
- Mañas, F.; Peralta, L.; Raviolo, J.; Ovando, H.G.; Weyers, A.; Ugnia, L.; Cid, M.G.; Larripa, I.; Gorla, N. Genotoxicity of glyphosate assessed by the omet assay and cytogenetic tests. Environ. Toxicol. Pharmacol. 2009, 28, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Benachor, N.; Séralini, G. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxical. 2009, 22, 97–105. [Google Scholar] [CrossRef]
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A critical review of glyphosate findings in human urine samples and comparison with the exposure og operators and consumers. J. für Verbrauch. Lebensm. 2015, 10, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, H.W. Determination of Glyphosate Residues in Human Urine Samples from 18 European Countries; unpublished test report MLHB-2013-06-06 of 12 June 2013, kindly provided to the German Federal Institute for Risk Assessment (BfR); Medical Laboratory Bremen: Bremen, Germany, 2013; pp. 22–23. [Google Scholar]
- Van Stempvoort, D.R.; Spoelstra, J.; Senger, N.D.; Brown, S.J.; Post, R.; Struger, J. Glyphosate residues in rural groundwater, Nottawasaga river watershed, Ontario, Canada. Pest Manag. Sci. 2016, 72, 1862–1872. [Google Scholar] [CrossRef]
- Piel, S.; Baurès, E.; Thomas, O. Contribution to surface water contamination understanding by pesticides and pharmaceuticals, at a watershed scale. Int. J. Environ. Res. Public Health 2012, 9, 4433–4451. [Google Scholar] [CrossRef] [Green Version]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effects of pH and salt on nanofiltration—A critical review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I. Performance assessment of SWRO spiral-wound membrane modules with different feed spacer dimensions. Processes 2020, 8, 692. [Google Scholar] [CrossRef]
- Kovács, Z.; Samhaber, W. Contribution of pH dependent osmotic pressure to amino acid transport through nanofiltration membranes. Sep. Purif. Technol. 2008, 61, 243–248. [Google Scholar] [CrossRef]
- Hoang, T.; Stevens, G.; Kentish, S. The effect of the feed pH on the performance of a reverse osmosis membrane. Desalination 2010, 261, 99–103. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Hu, J.; Gao, W. Chemisorption of estrone in nylon microfiltration membranes: Adsorption mechanism and potential use for estrone removal from water. Water Res. 2012, 46, 873–881. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Amy, G.L.; Van der Bruggen, B.; van Dijk, J.C. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci. 2008, 322, 52–66. [Google Scholar] [CrossRef]
- Donnan, F.G. Theory of membrane equilibria and membrane potential in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. Z. Elektrochem. Angew. Phys. Chem. 1911, 17, 52, reprinted in J. Membr. Sci. 1995, 100, 45–55. [Google Scholar] [CrossRef]
- Chang, F.F.; Liu, W.J.; Wang, X.M. Comparison of polyamide nanofiltration and low-pressure reverse osmosis membranes on As(III) rejection und various operational conditions. Desalination 2014, 334, 10–16. [Google Scholar] [CrossRef]
- Bandini, S.; Drei, J.; Vezzani, D. The role of pH and concentration on the ion rejection in polyamide nanofiltration membranes. J. Membr. Sci. 2005, 264, 65–74. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Effect of pH on hydrophilicity and charge and their effect on the filtration efficiency of NF membranes at different pH. J. Membr. Sci. 2006, 280, 311–320. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E.; Bruening, M.L.; Zholkovskiy, E. Modelling nanofiltration of electrolyte solutions. Adv. Colloid Interface Sci. 2019, 268, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Yaroshchuk, A.E. Rejection mechanisms of nanofiltration membranes. Membr. Technol. 1998, 100, 9–12. [Google Scholar] [CrossRef]
- Oatley, D.L.; Llenas, L.; Pérez, R.; William, P.M.; Martínez-Lladó, X.; Rovira, M. Review of the dielectric properties of nanofiltration membranes and verification of the single oriented layer approximation. Adv. Colloid Interface Sci. 2012, 173, 1–11. [Google Scholar] [CrossRef]
- Cancino-Madariaga, B.; Hurtado, C.F.; Ruby, R. Effects of pressure and pH in ammonium retention for nanofiltration and reverse osmosis membrane to be used in recirculation aquaculture systems (RAS). Aquacult. Eng. 2011, 45, 103–108. [Google Scholar] [CrossRef]
- Lipp, P.; Gimbel, R.; Frimmel, F.H. Parameters influencing the rejection properties of FT30 membranes. J. Membr. Sci. 1994, 95, 185–197. [Google Scholar] [CrossRef]
- Hall, M.S.; Lloyd, D.R.; Starov, V.M. Reverse osmosis of multicomponent electrolyte solutions. Part II. Experimental verification. J. Membr. Sci. 1997, 128, 39–53. [Google Scholar] [CrossRef]
- Nanda, D.; Tung, K.L.; Li, Y.L.; Lin, N.J.; Chuang, C.J. Effect of pH on membrane morphology, fouling potential, and filtration performance of nanofiltration membrane for water softening. J. Membr. Sci. 2010, 349, 411–420. [Google Scholar] [CrossRef]
- Bandini, S.; Morelli, V. Effects of temperature, pH and composition on nanofiltration of mono/disaccharides: Experiments and modelling assessment. J. Membr. Sci. 2017, 533, 57–74. [Google Scholar] [CrossRef]
- Yu, S.; Liu, M.; Ma, M.; Qi, M.; Lü, Z.; Gao, C. Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration. J. Membr. Sci. 2010, 350, 83–91. [Google Scholar] [CrossRef]
- Hagmeyer, G.; Gimbel, R. Modelling the rejection of nanofiltration membranes using zeta potential measurements. Sep. Purif. Technol. 1999, 15, 19–30. [Google Scholar] [CrossRef]
- Elimelech, M.; Chen, W.H.; Waypa, J.J. Measuring the zeta (electrokinetic) potential of reverse osmosis membranes by a streaming potential analyzer. Desalination 1994, 95, 269–286. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Kim, S.; Ozaki, H.; Kim, J. Effect of pH on the rejection of inorganic salts and organic compound using nanofiltration membrane. Korean J. Chem. Eng. 2006, 23, 28–33. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Testa, F.; Lawler, D.F.; Feeman, B.D.; Moulin, P. Effect of antiscalants on precipitation of an RO concentrate: Metal precipitated and characteristics for several water compositions. Water Res. 2010, 44, 2672–2684. [Google Scholar] [CrossRef] [PubMed]



| Feature | TS40 | TS80 | ACM2 |
|---|---|---|---|
| Application | Demineralisation and concentrate organic solutes | Water softening, high rejection of salts and uncharged organic solutes | Standard high rejection |
| Membrane material | Thin film, piperazine | Thin film, polyamide | Thin film, polyamide |
| Active membrane area (cm2) | 84 | 84 | 84 |
| Backing material | Non-woven polyester | Non-woven polyester | Non-woven polyester |
| Thickness (µm) | 130–170 | 130–170 | 130–170 |
| Feed spacer height (mil) * | 44 | 44 | 44 |
| pH range | 1.0–12.0 | 1.0–12.0 | 1.0–12.0 |
| MWCO (Da) | 200–300 | 100–200 | - |
| NaCl rejection (%) | 40.0 | 80.0 | 99.5 |
| MgSO4 rejection (%) | 98.5 | 98.5 | n.a. * |
| Applied pressure (bar) | 9.0 | 9.0 | 15.0 |
| Operational flux (L h−1 m−2) | 34.6–51.8 | 41.8–69.2 | 41.8–60.5 |
| Membrane | AMPA | IDMP | ATMP | |||
|---|---|---|---|---|---|---|
| TP | LC/MS | TP | LC/MS | TP | LC/MS | |
| TS40 | 512.9 ± 29.8 | 205.1 ± 3.9 | 339.9 ± 4.7 | 192.2 ± 18.8 | 318.6 ± 13.3 | 184.0 ± 16.9 |
| TS80 | 185.0 ± 8.8 | 191.0 ± 15.3 | 104.3 ± 8.2 | 44.3 ± 6.8 | 136.0 ± 12.6 | n.d.* |
| ACM2 | 116.8 ± 18.3 | 102.3 ± 14.5 | 39.6 ± 11.9 | n.d. | 38.1 ± 13.4 | n.d. |
| Synthetic Tap Water | Local tap Water | |||||
|---|---|---|---|---|---|---|
| Membrane | AMPA | IDMP | ATMP | AMPA | IDMP | ATMP |
| TS40 | 234.1 ± 25.1 | 114.0 ± 11.3 | 65.6 ± 14.9 | 80.6 ± 32.5 | 69.4 ± 11.5 | 11.5 ± 0.01 |
| TS80 | 146.8 ± 27.3 | 115.1 ± 2.5 | 71.3 ± 0.01 | 27.7 ± 7.8 | 57.9 ± 11.3 | 67.1 ± 8.3 |
| ACM2 | 126.2 ± 9.1 | 74.8 ± 20.0 | 22.4 ± 2.6 | 36.6 ± 12.3 | 39.9 ± 7.2 | 40.9 ± 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, R.; Vornholt, C.; Preuß, V.; Bryant, I.M.; Martienssen, M. Aminophosphonates in Nanofiltration and Reverse Osmosis Permeates. Membranes 2021, 11, 446. https://doi.org/10.3390/membranes11060446
Kuhn R, Vornholt C, Preuß V, Bryant IM, Martienssen M. Aminophosphonates in Nanofiltration and Reverse Osmosis Permeates. Membranes. 2021; 11(6):446. https://doi.org/10.3390/membranes11060446
Chicago/Turabian StyleKuhn, Ramona, Carsten Vornholt, Volker Preuß, Isaac Mbir Bryant, and Marion Martienssen. 2021. "Aminophosphonates in Nanofiltration and Reverse Osmosis Permeates" Membranes 11, no. 6: 446. https://doi.org/10.3390/membranes11060446