Degradation of Components in Cars Due to Bimetallic Corrosion
Abstract
:1. Introduction
- for an aggressive environment, the difference of <150 mV is allowed;
- for a slightly aggressive environment, the difference of <250 mV is allowed;
- for a non-aggressive environment, the difference of <500 mV is allowed.
2. Materials and Methods
2.1. Materials and Test Environments
2.2. Measurement of Basic Corrosion Characteristics
- measurement of the open corrosion potential ESCE samples during their exposure in the environment;
- determination of the kinetics of corrosion of the exposed samples according to Tafel and Stern;
- determination of corrosion potential and current density using Evans polarization diagrams.
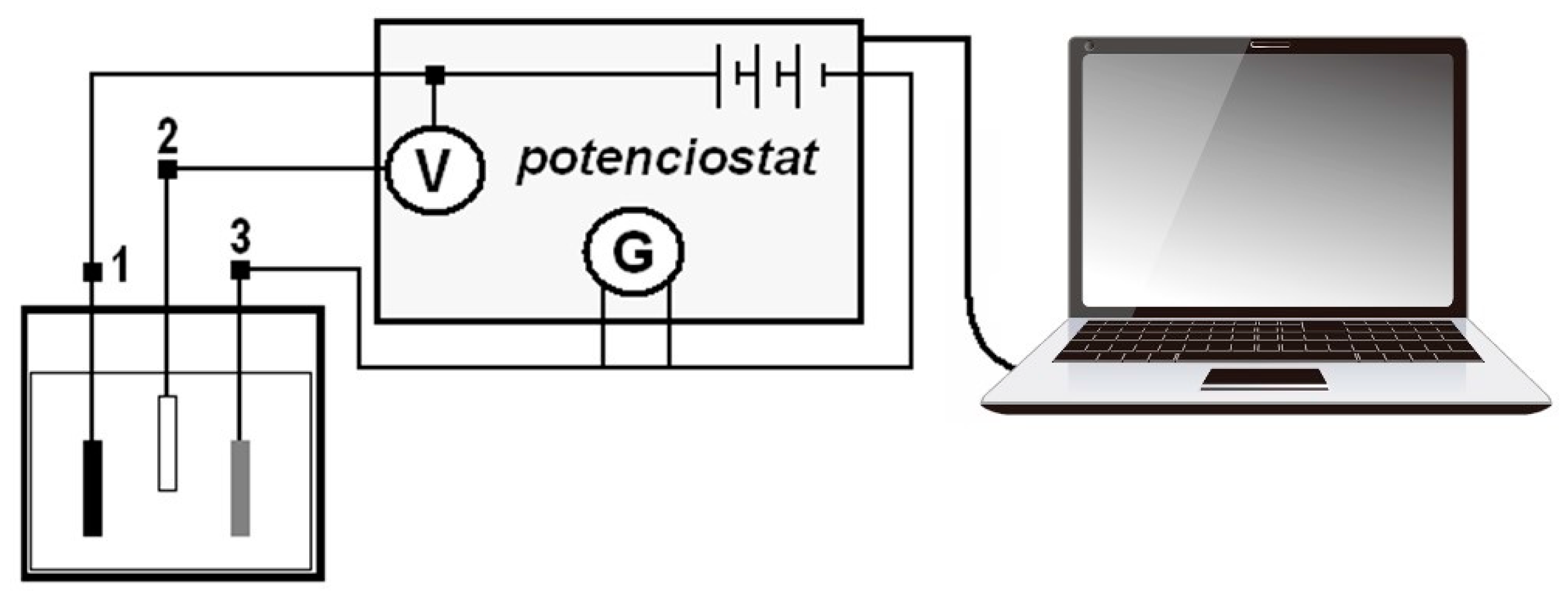
2.2.1. Measurement of the Open Corrosion Potential ESCE
2.2.2. Potentiodynamic Measurements
3. Results and Discussion
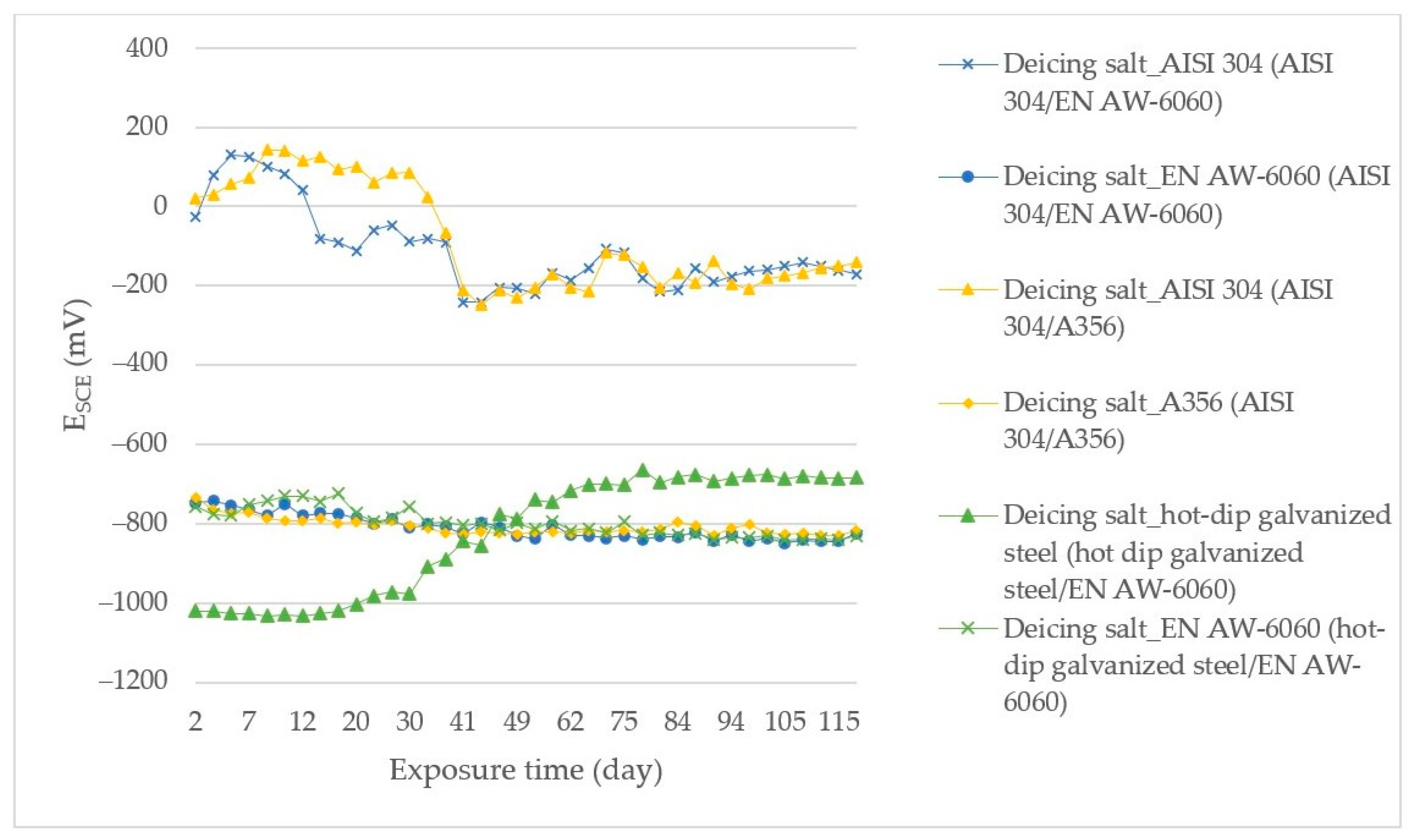
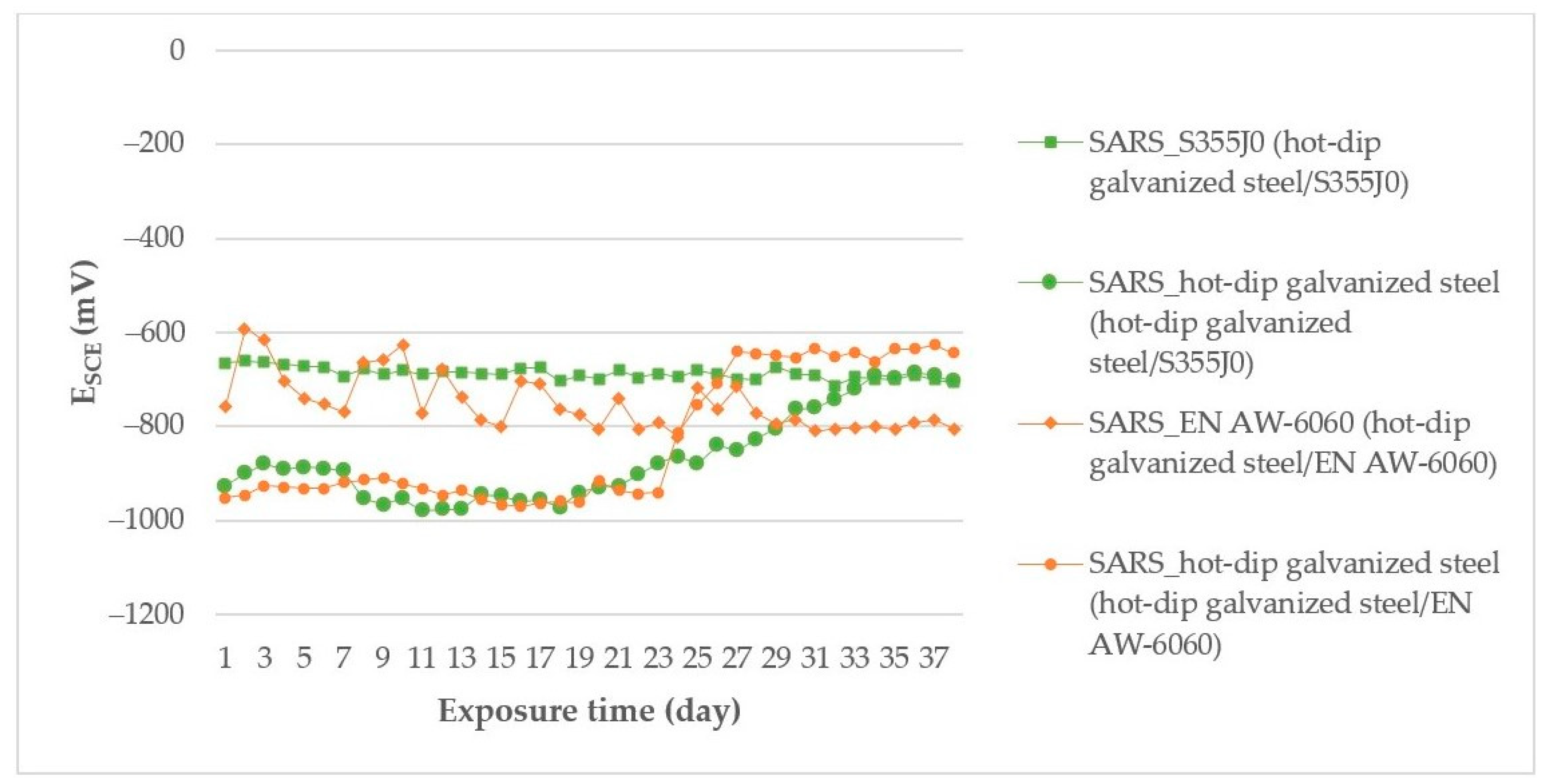
3.1. Measurement of the Open Corrosion Potential of ESCE Samples during Their Exposure in the Environment
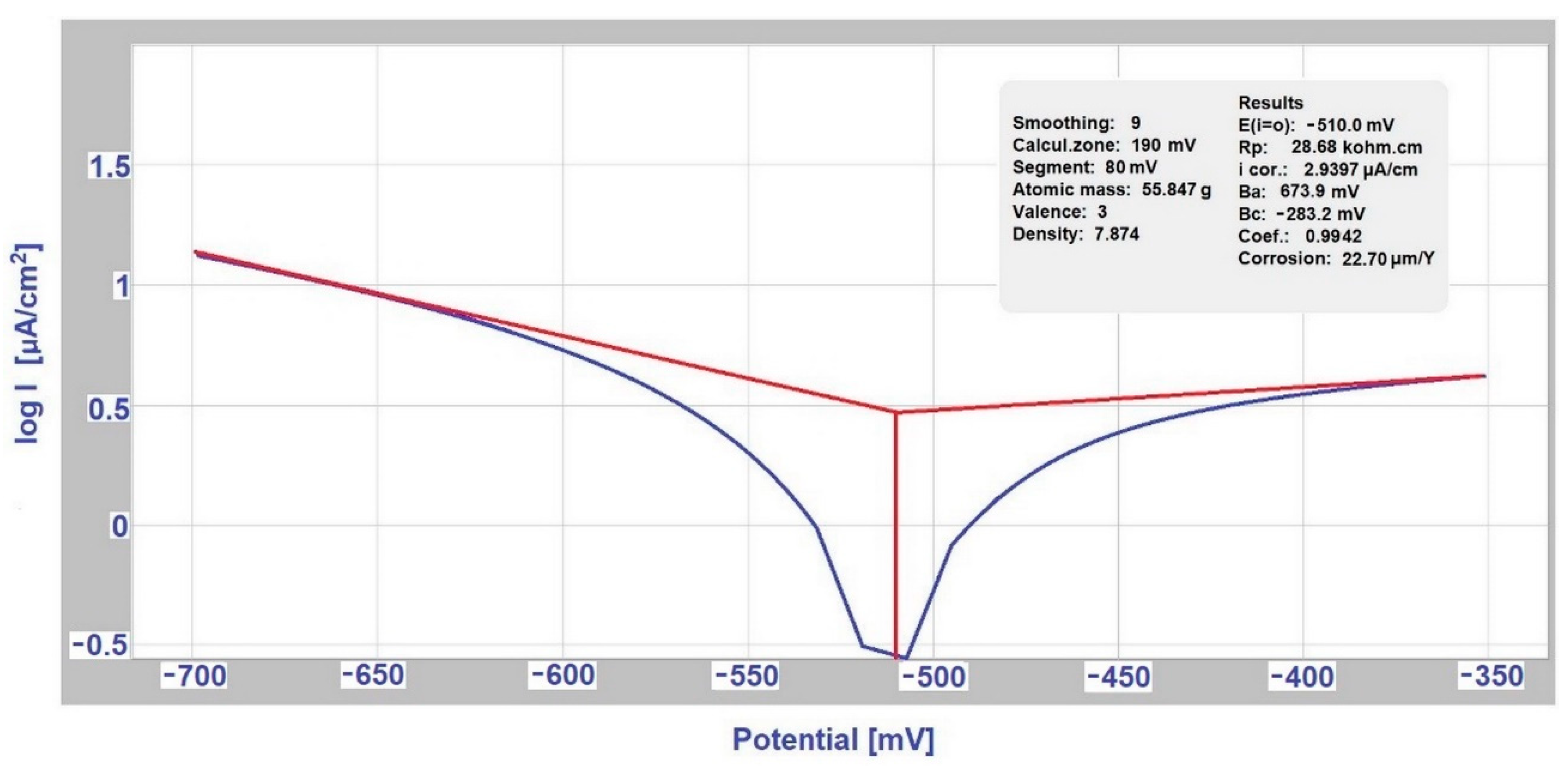
3.2. Determination of the Exposed Samples Corrosion Kinetics according to Tafel and Stern
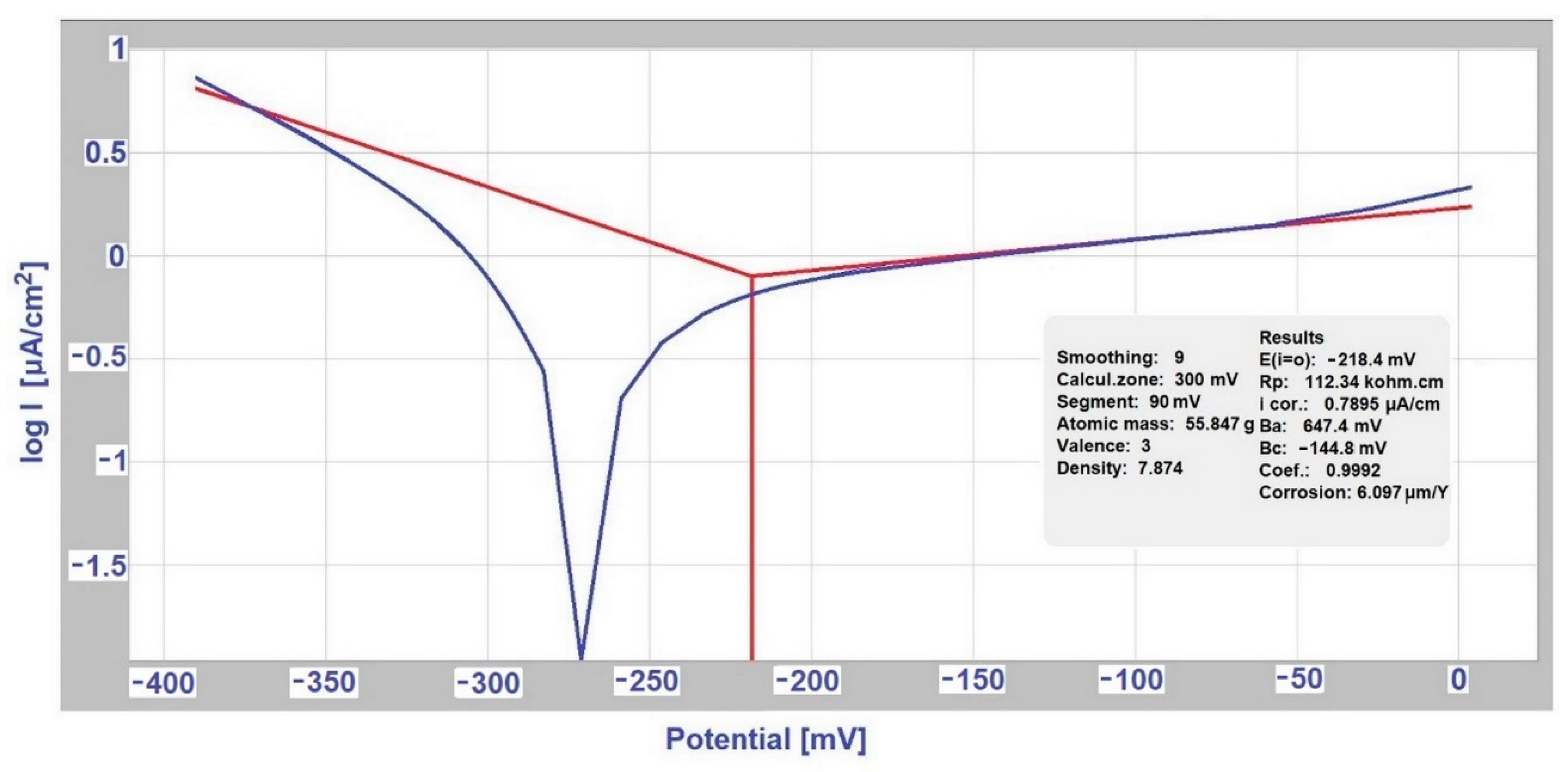
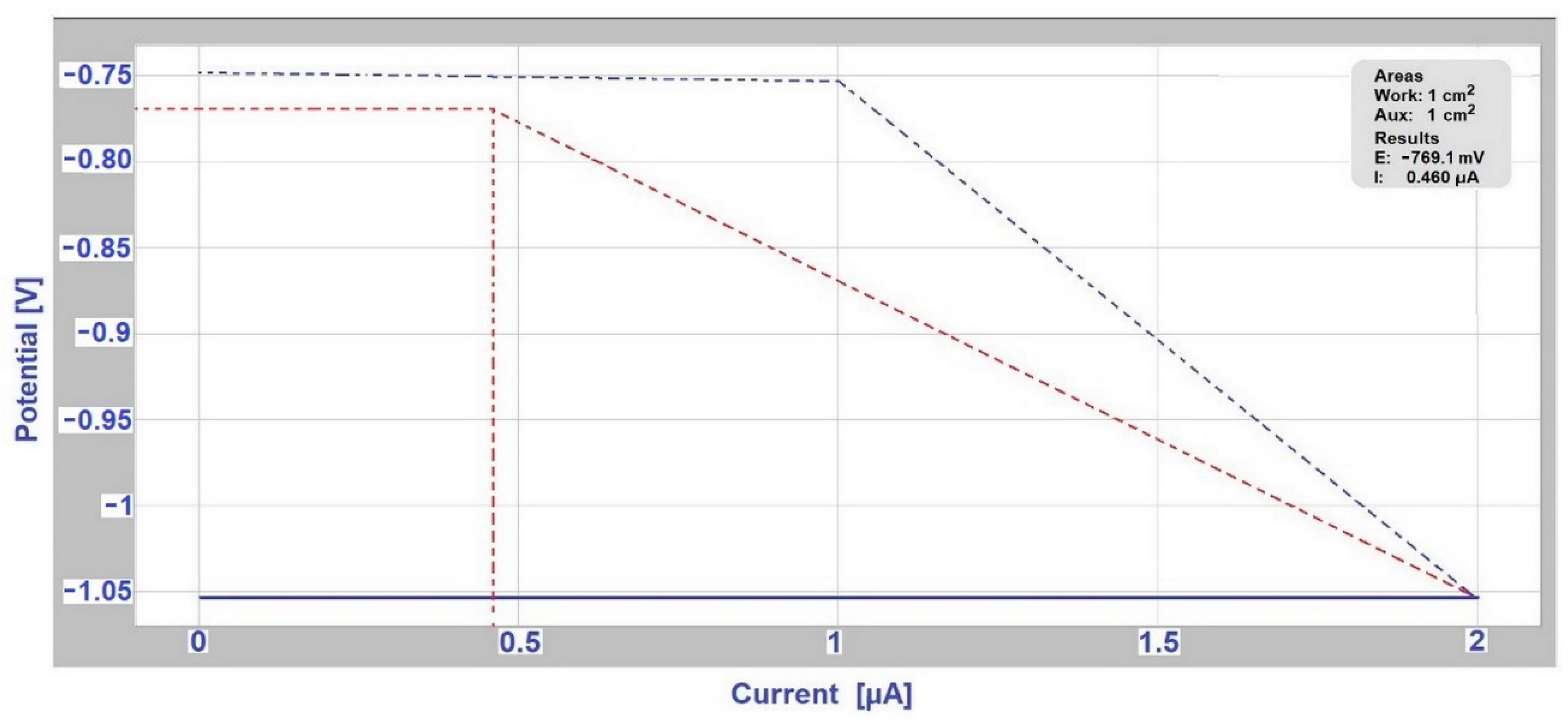
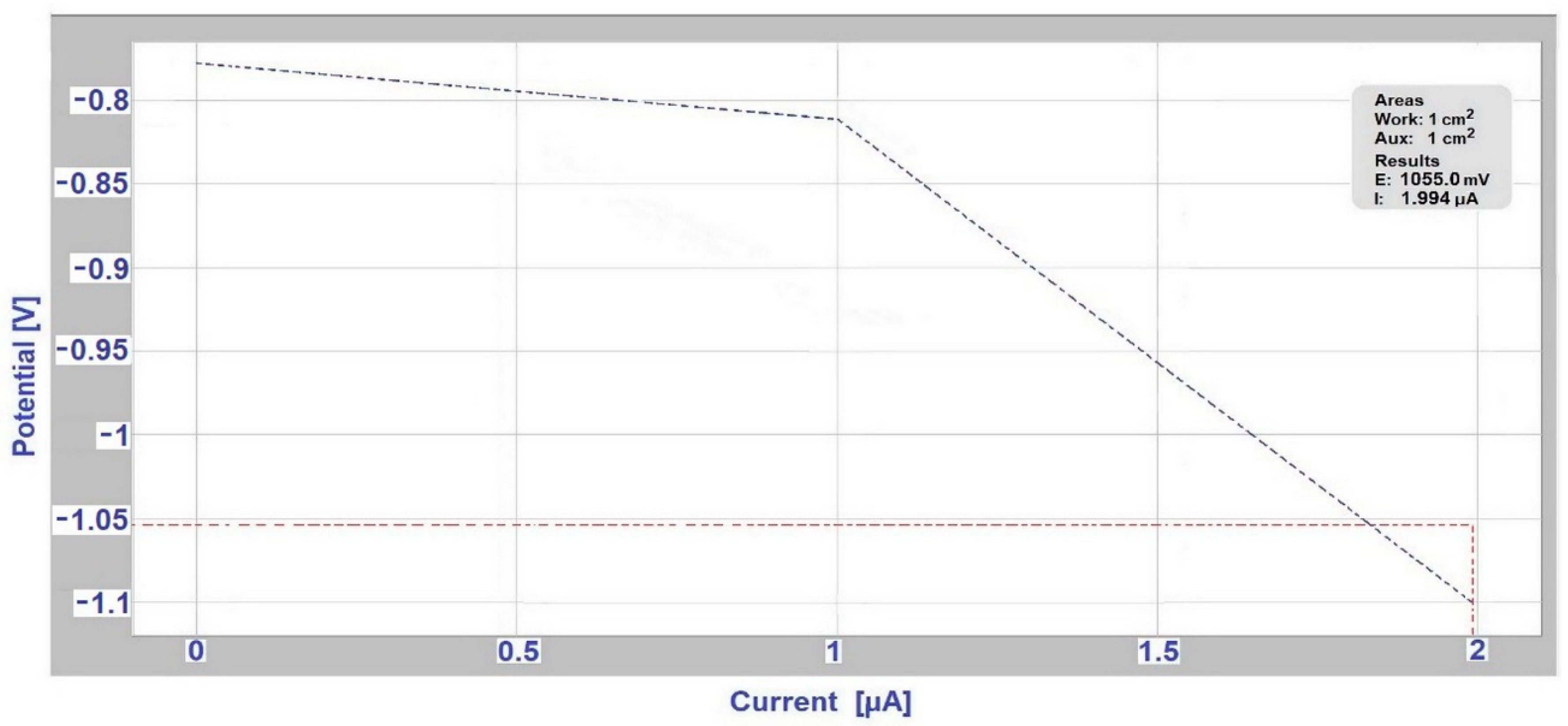
3.3. Determination of Corrosion Potential and Current Density Using Evans Polarization Diagrams
4. Conclusions
- bimetallic couples of hot-dip galvanized steel/EN AW-6060 in deicing salt and hot-dip galvanized steel/S355J0 in SARS proved to be the safest and most usable even for more aggressive environments;
- bimetallic couple of hot-dip galvanized steel/EN AW-6060 in SARS solution is suitable for slightly aggressive environments;
- bimetallic couple of AISI 304/EN AW-6060 in deicing salt and SEG, and AISI 304/A356 couples in deicing salt represent the risk of bimetallic corrosion even in a non-aggressive environment.
- hot-dip galvanized steel/EN AW-6060 in SARS solution;
- AISI 304/EN AW-6060 in SEG;
- A356/AISI 304 in deicing salt.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrosion Protection for Car Parts and Safety. 2017. Available online: https://www.automotive-iq.com/car-body-and-materials/articles/corrosion-protection-car-parts-and-safety (accessed on 30 May 2021).
- Liu, M.; Guo, Y.; Wang, J.; Yergin, M. Corrosion avoidance in lightweight materials for automotive applications. NPJ Mater. Degrad. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Viňáš, J.; Brezinová, J.; Brezina, J.; Maruschak, P.O. Structural and Mechanical Features of Laser-Welded Joints of Zinc-Coated Advanced Steel Sheets. Mater. Sci. 2019, 55, 46–51. [Google Scholar] [CrossRef]
- Gullino, A.; Matteis, P.; D’Aiuto, F. Review of Aluminum-To-Steel Welding Technologies for Car-Body Applications. Metals 2019, 9, 315. [Google Scholar] [CrossRef] [Green Version]
- Setyarini, P.H.; Soenoko, R.; Irawan-Purnomo, Y.S. Corrosion characterization of anodized AA 6061. MM Sci. J. 2018, 6, 2415–2420. [Google Scholar] [CrossRef]
- Fentahun, M.A.; Savas, M.A. Materials Used in Automotive Manufacture and Material Selection Using Ashby Charts. Int. J. Mater. Eng. 2018, 8, 40–54. [Google Scholar] [CrossRef]
- Tisza, M.; Czinege, I. Comparative study of the application of steels and aluminium in lightweight production of automotive parts. Int. J. Lightweight Manuf. 2018, 1, 229–238. [Google Scholar] [CrossRef]
- Reif, K. Fundamentals of Automotive and Engine Technology, 1st ed.; Springer Vieweg Fachmedien Wiesbaden: Friedrichshafen, Germany, 2014; ISBN 978-3-658-03972-1. [Google Scholar]
- Lim, Y.C.; Squires, L.; Pan, T.-Y.; Miles, M.; Song, G.-L.; Wang, Y.; Feng, Z. Study of mechanical joint strength of aluminum alloy 7075-T6 and dual phase steel 980 welded by friction bit joining and weld-bonding under corrosion medium. Mater. Design 2015, 69, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Hodkinson, R.; Fenton, J. Lightweight Electric/Hybrid Vehicle Design, 1st ed.; Butterworth-Heinemann: Woburn, UK, 2001; ISSN 9780-08-0535-517. [Google Scholar]
- Beretta, J. Automotive Electricity: Electric Drive, 1st ed.; Wiley-ISTE: London, UK, 2010; ISBN 978-1-848-21095-0. [Google Scholar]
- Zhang, X.G. Galvanic Corrosion. In Uhling’s Corrosion Handbook, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Policastro, S.A.; Anderson, R.M.; Hangarter, C.M. Analysis of Galvanic Corrosion Current between an Aluminum Alloy and Stainless-Steel Exposed to an Equilibrated Droplet Electrolyte. J. Electrochem. Soc. 2021, 168, 041507. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Meena, B.N.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Badea, G.E.; Caraban, A.; Sebesan, M.; Dzitac, S.; Cret, P.; Setel, A. Polarisation Measurements Used for Corrosion Rates Determination. J. Sustenable Energy 2010, 1, 1–4. [Google Scholar]
- Hagarová, M.; Jakubéczyová, D.; Baranová, G.; Šimko, R. Determination of bimetallic corrosion risk using an electrochemical method. Mater. Sci. Forum 2019, 960, 62–69. [Google Scholar] [CrossRef]
- Stapleton, D. MG Midget & Austin Healey Sprite High-Performance Manual, 3rd ed.; Veloce Publishing Ltd.: Bristol, UK, 2013. [Google Scholar]
- Meschut, G.; Janzen, V.; Olfermann, T. Innovative and Highly Productive Joining Technologies for Multi-Material Lightweight Car Body Structures. J. Mater. Eng. Perform. 2014, 23, 1515–1523. [Google Scholar] [CrossRef]
- Naito, J.; Suzuki, R. Multi-material Automotive Bodies and Dissimilar Joining Technology to Realize Multi-material. Kobelco Technol. Rev. 2020, 38, 32–37. [Google Scholar]
- Mori, K.; Abe, Y. A review on mechanical joining of aluminium and high strength steel sheets by plastic deformation. Int. J. Lightweight Mater. Manuf. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Leitman, S.; Brant, B. Build Your Own Electric Vehicle, 2nd ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Yoshida, H.; Hoshino, K.; Ogihara, Y. Hot-Dip Galvanized (GI) Steel Sheets for Automotive Use. JFE GIHO 2018, 41, 28–33. [Google Scholar]
- Li, G.; Long, X. Mechanical Behavior and Damage of Zinc Coating for Hot Dip Galvanized Steel Sheet DP600. Coatings 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]

| Element | Hot-Dip Galvanized Steel—Substrate | S255J0 | EN AW-6060 | AISI 304 | AlSiMg0.3 |
|---|---|---|---|---|---|
| C | 0.0024 | 0.024 | - | 0.05 | - |
| Mn | 0.140 | 0.470 | 0.10 | 1.65 | 0.08 |
| Si | 0.007 | 0.094 | 0.46 | 0.84 | 7.25 |
| Cr | 0.009 | - | 0.05 | 18.2 | - |
| Ti | 0.061 | 0.025 | 0.10 | - | 0.19 |
| S | 0.014 | 0.003 | - | 0.012 | - |
| P | 0.009 | 0.002 | - | 0.034 | - |
| N | 0.002 | 0.002 | - | - | - |
| Nb | <0.002 | 0.0072 | - | - | - |
| Cu | 0.028 | - | 0.11 | - | 0.01 |
| Mg | - | - | 0.48 | - | 0.42 |
| Sr | - | - | - | - | 0.012 |
| Ni | - | - | - | 8.61 | - |
| Zn | - | - | 0.15 | - | 0.01 |
| Al | - | 0.042 | bal. | - | bal. |
| Fe | - | bal. | 0.37 | bal. | 0.09 |
| Environment | Chemical Composition in MmoL L−1 | Model Environment for: |
|---|---|---|
| SARS (simulated acid rain solution) | 0.01 mmoL L−1 HNO3; 1.0 mmoL L−1 NaCl; 1.0 mmoL L−1 (NH4)2SO4 | industrial environment |
| deicing salt | 3 % NaCl | deicing salt |
| SEG (simulated exhaust gas environment) | 52.1 mmoL L−1 (NH4)2SO4; 2.8 mmoL L−1 NH4Cl; 1.6 mmoL L−1 NH4NO3; 2.2 mmoL L−1 HCOOH | condensed exhaust gas |
| Bimetallic Couple | Part of the Car Construction | Resource |
|---|---|---|
| hot-dip galvanized steel/EN AW-6060 | roof, hood, or doors | Meschut G.; Janzen, V.; Olfermann, T., 2014 |
| AISI 304/EN AW-6060 | EASW (Element Arc Spot Welding) mechanism rivers, screws/Al sheet layers of steel with aluminum bars in the rotor | Naito, J.; Suzuki, R. 2020 Mori, K., Abe, Y., 2018 |
| AISI 304/A356 | exhaust systems (catalytic converter „cat „) oil pan, valve cover, hera gasket/cylinder head | Leitman, S., Brant, 2009 |
| hot-dip galvanized steel/S355J0 | body plates/roof suspension system components/shock absorbers | Shibli, S. M. A.; Meena, B. N.; Remya, R., 2015 Li, G., Long, X., 2020 |
| Sample Couples | Environment | ||
|---|---|---|---|
| SARS | Deicing Salt | SEG Solution | |
| hot-dip galvanized steel/EN AW-6060 | X | X | |
| AISI 304/EN AW-6060 | X | X | |
| AISI 304/A356 | X | ||
| hot-dip galvanized steel/S355J0 | X | ||
| Anode Index According to [15,16] | Environment | Usability of Couple of Metals in Environment | Anode Index According to the Measurement ESCE | Tested Couple |
|---|---|---|---|---|
| ΔE < 500 mV | Non-aggressive environment | not | ΔE = 678 mV | AISI 304/A356 in deicing salt |
| not | ΔE = 654 mV | AISI 304/EN AW-6060 in deicing salt | ||
| not | ΔE = 580 mV | AISI 304/EN AW-6060 in SEG | ||
| ΔE < 250 mV | slightly environment | yes | ΔE = 161 mV | hot-dip galvanized steel/EN AW-6060 in SARS |
| ΔE < 150 mV | aggressive environment | yes | ΔE = 147 mV | hot-dip galvanized steel/EN AW-6060 in deicing salt |
| yes | ΔE = 2 mV | hot-dip galvanized steel /S355J0 in SARS |
| Environment | Sample | Measurement (1st Week) | Measurement (18th Week) | ||
|---|---|---|---|---|---|
| Jcorr (μA·cm−2) | Rp (kΩ·cm2) | Jcorr (μA·cm−2) | Rp (kΩ·cm2) | ||
| deicing salt | AISI 304 | 0.79 | 60.85 | 2.9397 | 21.2 |
| EN AW-6060 | 0.0636 | 1380 | 0.6896 | 61.58 | |
| SEG solution | AISI 304 | 22.7 | 4.02 | 10.9742 | 3.99 |
| EN AW-6060 | 0.1426 | 396.6 | 4.6835 | 8.34 | |
| deicing salt | AISI 304 | 0.79 | 60.85 | 3.4158 | 15.7 |
| A356 | 38.37 | 1.91 | 76.5935 | 0.50216 | |
| SARS | hot-dip galvanized steel | 23.378 | 3.48 | 6.1731 | 7.2 |
| S355J0 | 0.4489 | 50.08 | 45.7841 | 1.12 | |
| SARS | hot-dip galvanized steel | 23.378 | 3.48 | 5.8018 | 8.53 |
| EN AW-6060 | 0.146 | 435.58 | 0.144 | 434.93 | |
| deicing salt | hot-dip galvanized steel | 2268.3 | 0.04032 | 2.1851 | 8.85 |
| EN AW-6060 | 0.0636 | 1380 | 0.3173 | 14.8 | |
| Sample | Exhibition | Jmax (Evans) | Jcorr (Tafel) |
|---|---|---|---|
| (μA·cm−2) | |||
| hot-dip galvanized steel sheet | separately (exposed with EN AW-6060 sample in deicing) | - | 2.1851 |
| in couple with EN AW-6060 (in deicing salt) | 24.321 | - | |
| separately (exposed with S355J0 steel in SARS solution) | - | 6.1731 | |
| in couple with S355J0 (in SARS solution) | 59.570 | - | |
| separately (exposed to EN AW-6060 sample in SARS solution) | - | 5.8018 | |
| in couple with EN AW-6060 (in SARS solution) | 0.636 | - | |
| Al alloy EN AW-6060 | separately (exposed with hot-dip galvanized steel sample in deicing salt) | - | 0.3173 |
| in couple with hot-dip galvanized steel (in deicing salt) | 24.321 | - | |
| separately (exposed with AISI 304 sample in deicing salt) | - | 0.6896 | |
| in couple with AISI 304 (in deicing salt) | 20.151 | - | |
| separately (exposed with AISI 304 in SEG solution) | - | 4.6835 | |
| in couple with AISI 304 (in SEG solution) | 4.845 | - | |
| separately (exposed with hot-dip galvanized steel in SARS solution) | - | 0.144 | |
| in couple with hot-dip galvanized steel (in SARS solution) | 0.636 | - | |
| stainless steel AISI 304 | separately (exposed with EN AW-6060 in deicing salt) | - | 2.9397 |
| in couple with EN AW-6060 (in deicing salt) | 20.151 | - | |
| separately (exposed with A356 in deicing salt) | - | 3.4158 | |
| in couple with A356 (in deicing salt) | 40.349 | - | |
| separately (exposed with EN AW-6060 in SEG solution) | - | 10.9742 | |
| in couple with EN AW-6060 (in SEG solution) | 4.845 | - | |
| silumin A356 | separately (exposed with AISI 304 in deicing salt) | - | 76.5935 |
| in couple with AISI 304 (in deicing salt) | 40.349 | - | |
| steel S355J0 | separately (exposed with hot-dip galvanized steel in SARS solution) | - | 45.7841 |
| in couple with hot-dip galvanized steel (in SARS solution) | 59.570 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagarová, M.; Brezinová, J.; Baranová, G.; Viňáš, J.; Maruschak, P. Degradation of Components in Cars Due to Bimetallic Corrosion. Materials 2021, 14, 3323. https://doi.org/10.3390/ma14123323
Hagarová M, Brezinová J, Baranová G, Viňáš J, Maruschak P. Degradation of Components in Cars Due to Bimetallic Corrosion. Materials. 2021; 14(12):3323. https://doi.org/10.3390/ma14123323
Chicago/Turabian StyleHagarová, Mária, Janette Brezinová, Gabriela Baranová, Ján Viňáš, and Pavlo Maruschak. 2021. "Degradation of Components in Cars Due to Bimetallic Corrosion" Materials 14, no. 12: 3323. https://doi.org/10.3390/ma14123323








