Crystal Structure, Spectroscopic Characterization, Antioxidant and Cytotoxic Activity of New Mg(II) and Mn(II)/Na(I) Complexes of Isoferulic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Single-Crystal X-ray Diffraction
2.4. Spectroscopic Studies
2.5. Anti-/Pro-Oxidant Study
2.6. Cell Viability Test
3. Results and Discussion
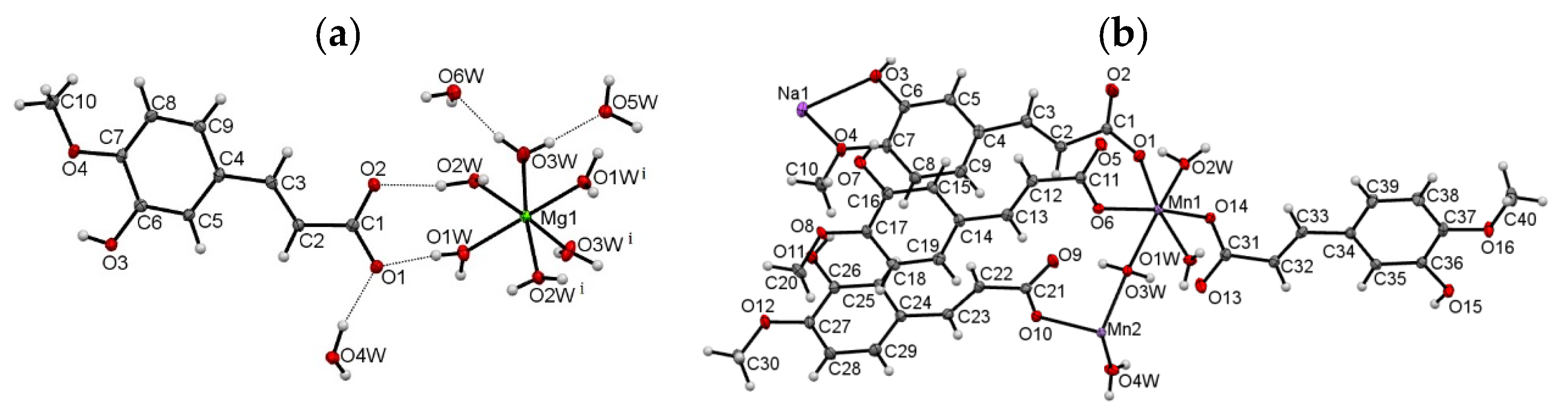
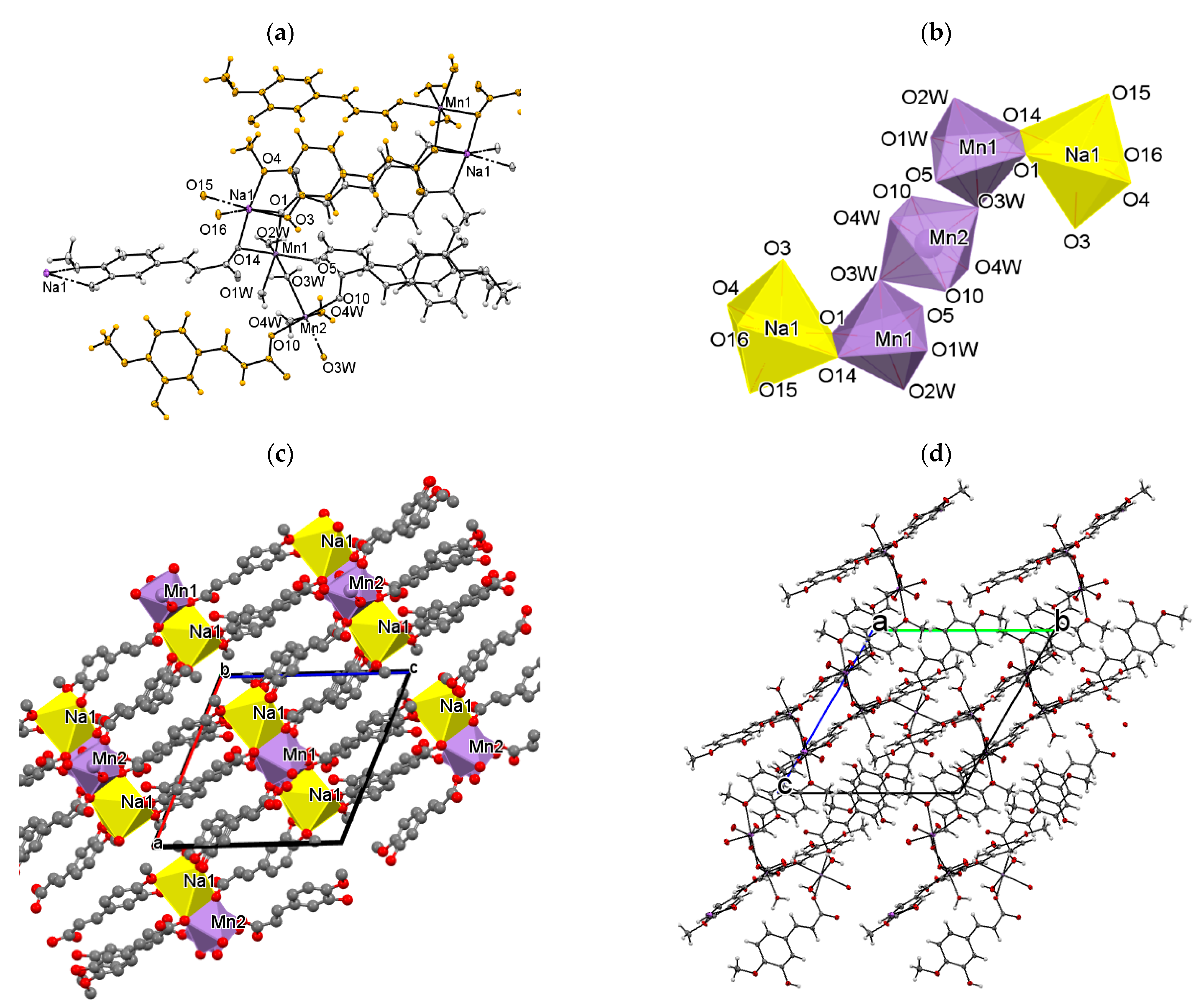
3.1. Molecular and Crystal Structure
3.2. Spectroscopic Studies
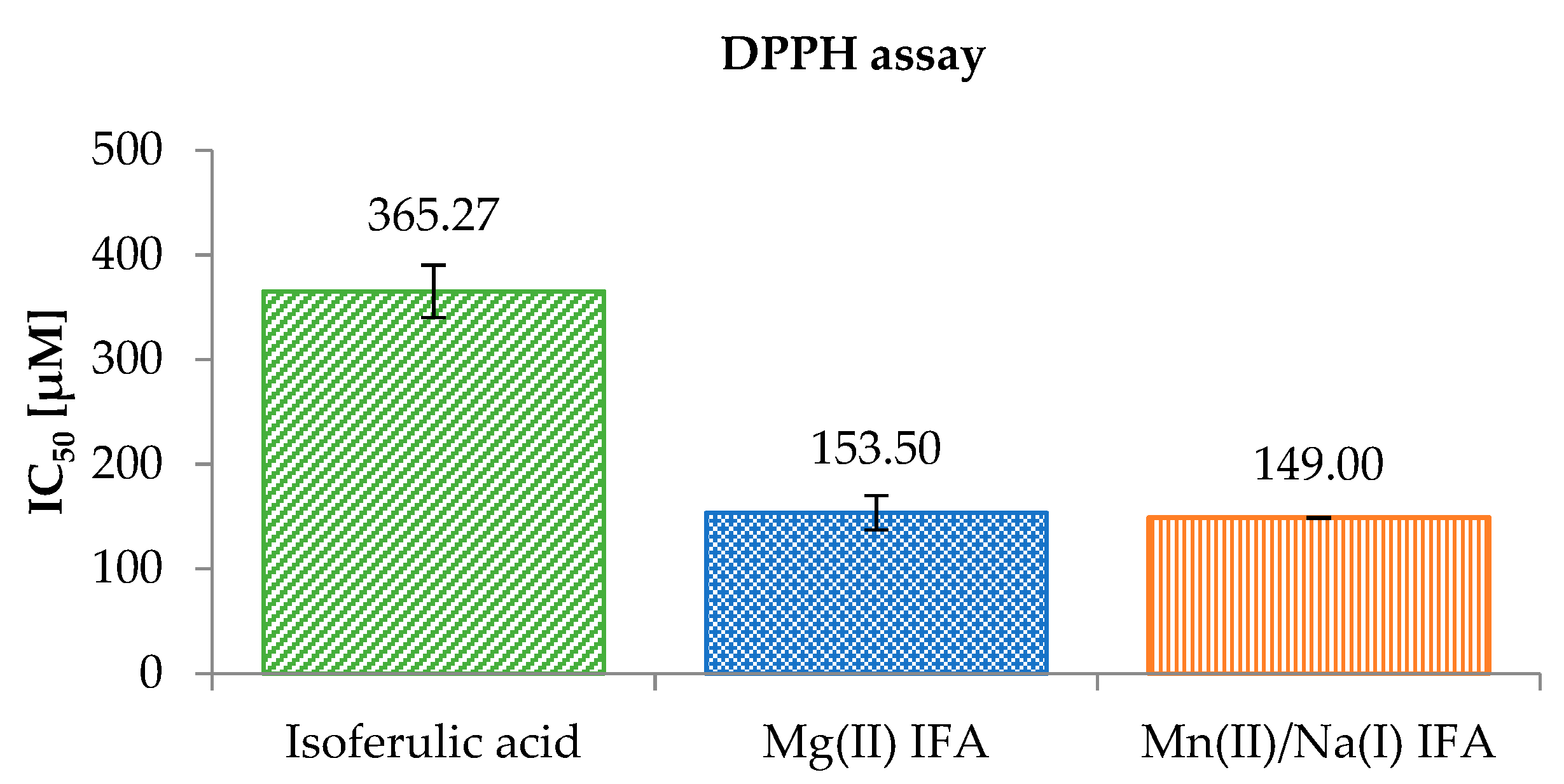
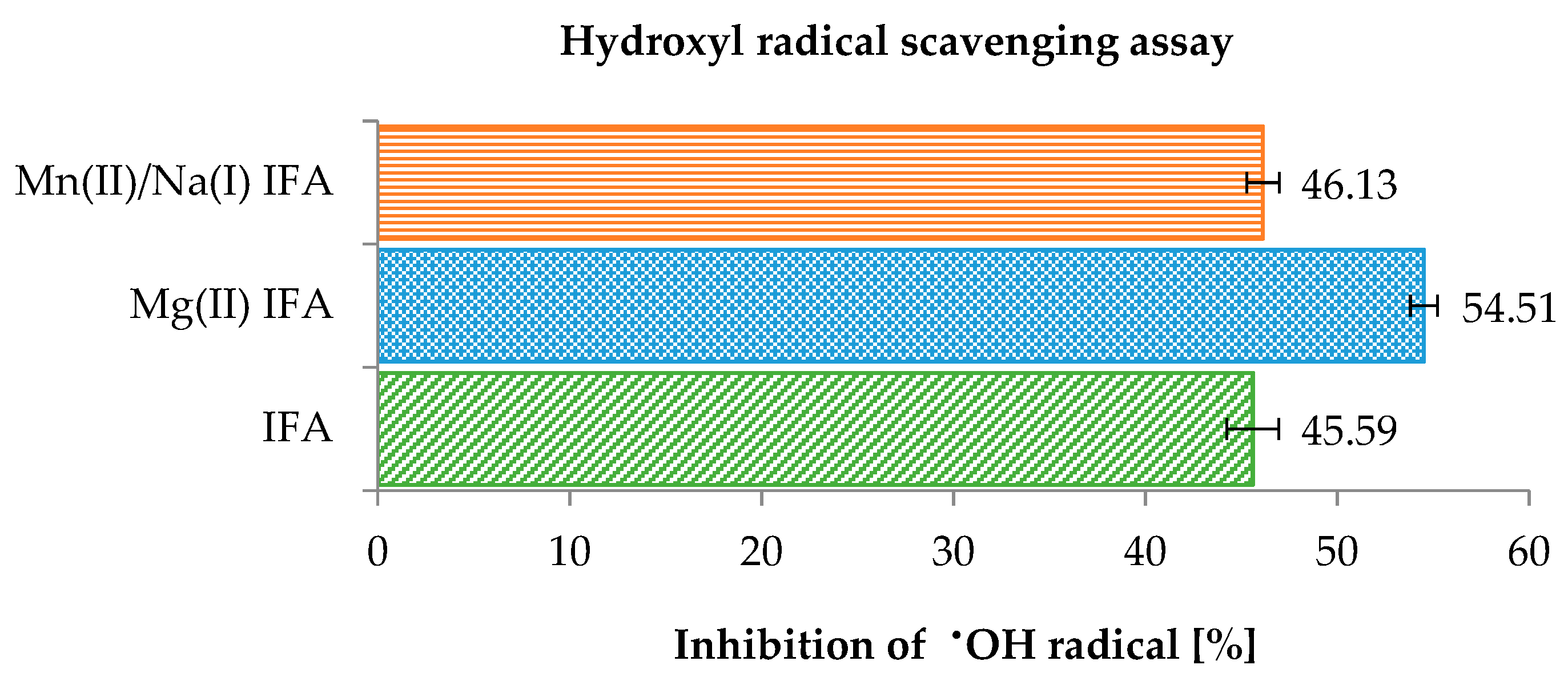
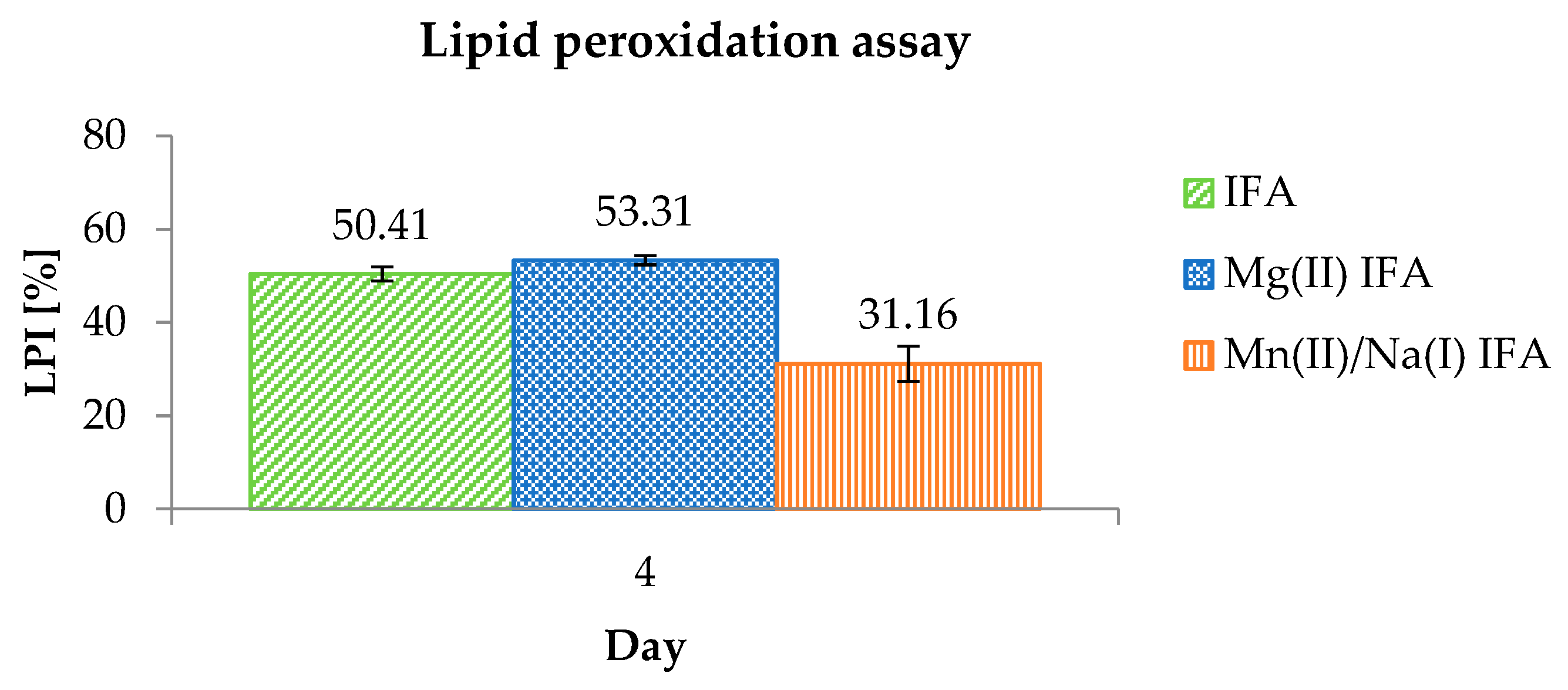
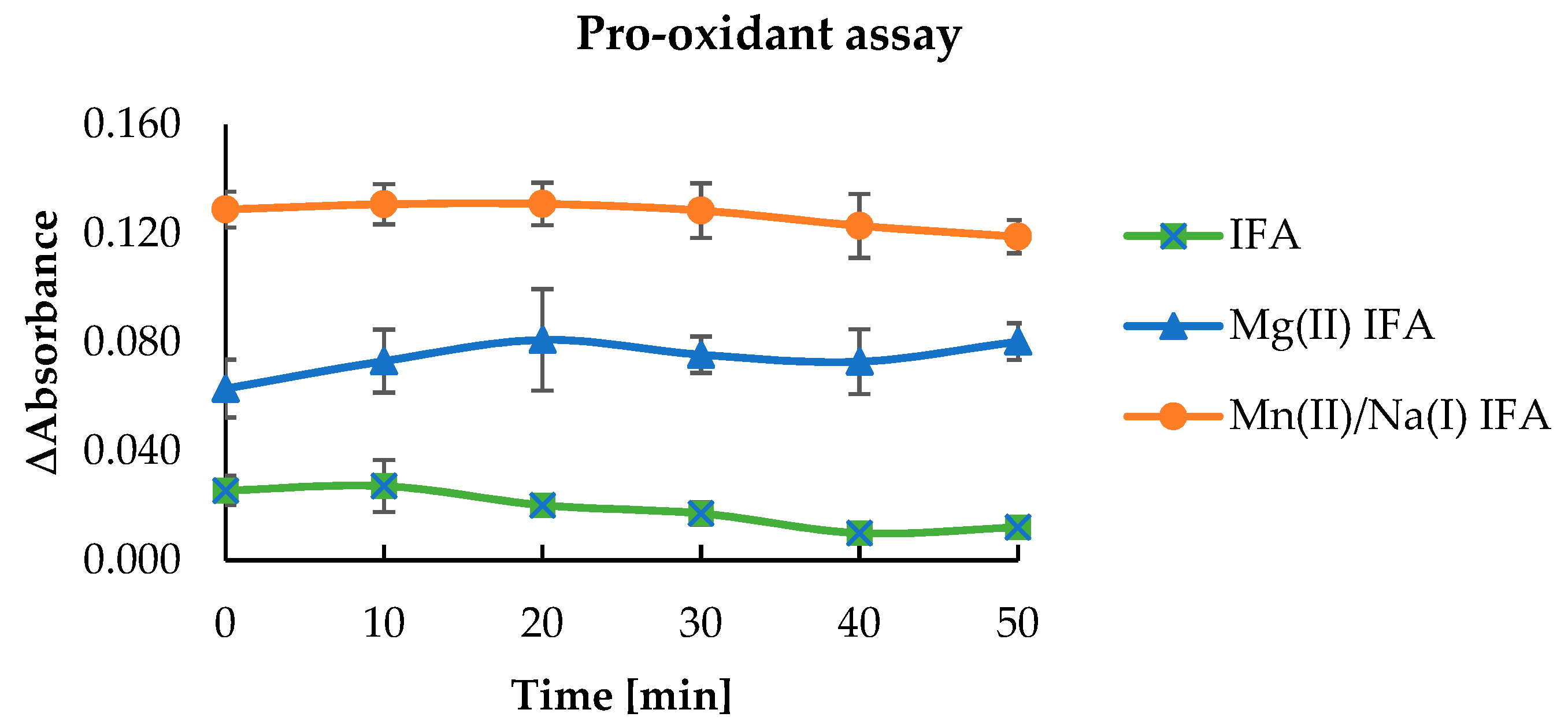
3.3. Anti-/Pro-Oxidant Study
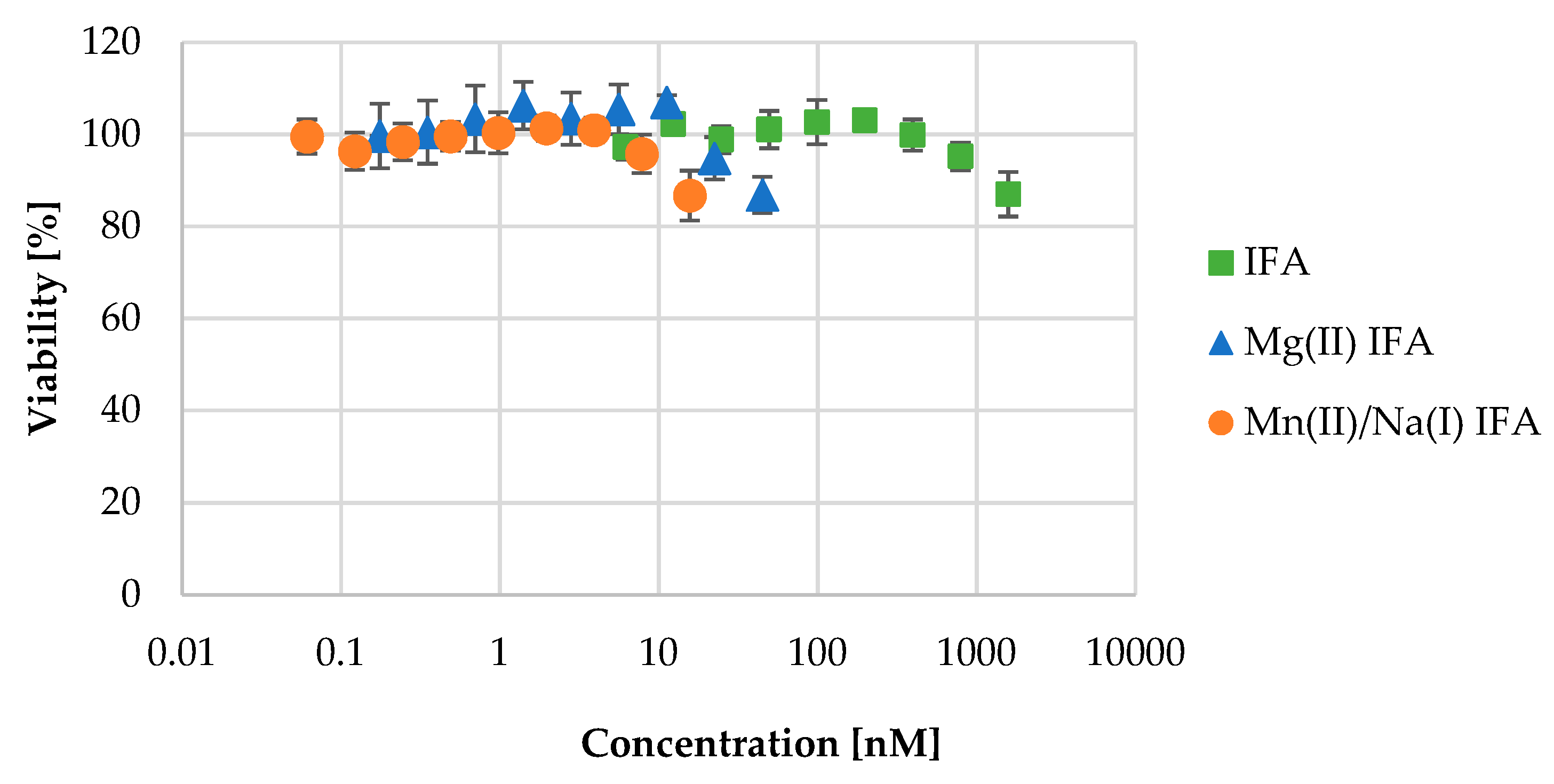
3.4. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anwar, H.; Hussain, G.; Mustafa, I. Antioxidants from Natural Sources. In Antioxidants in Foods and Its Applications; IntechOpen: London, UK, 2018; pp. 1–28. [Google Scholar]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic compounds: Potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar]
- Liu, I.M.; Hsu, F.L.; Chen, C.F.; Cheng, J.T. Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2000, 129, 631–636. [Google Scholar] [CrossRef]
- Liu, I.M.; Chen, W.C.; Cheng, J.T. Mediation of β-Endorphin by Isoferulic Acid to Lower Plasma Glucose in Streptozotocin-Induced Diabetic Rats. J. Pharmacol. Exp. Ther. 2003, 307, 1196–1204. [Google Scholar] [CrossRef]
- Guo, X.; Chen, X.; Cheng, W.; Yang, K.; Ma, Y.; Bi, K. RP-LC determination and pharmacokinetic study of ferulic acid and isoferulic acid in rat plasma after taking traditional Chinese medicinal-preparation: Guanxinning lyophilizer. Chromatographia 2008, 67, 1007–1011. [Google Scholar] [CrossRef]
- Jurgowiak, M.; Oliński, R. Wolne rodniki a starzenie się. Kosmos 1995, 44, 71–88. [Google Scholar]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 1–21. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacol. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska, A. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci. 2005, 55, 165–170. [Google Scholar]
- Arfin, S.; Siddiqui, G.A.; Naeem, A.; Moin, S. Inhibition of advanced glycation end products by isoferulic acid and its free radical scavenging capacity: An in vitro and molecular docking study. Int. J. Biol. Macromol. 2018, 118, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Murakami, Y.; Atsumi, T.; Shoji, M.; Ogiwara, T.; Shibuya, K.; Itō, S.; Yokoe, I.; Fujisawa, S. Ferulic acid dimer inhibits lipopolysaccharide-stimulated cyclooxygenase-2 expression in macrophages. Vivo Brooklyn 2005, 19, 849–853. [Google Scholar]
- Karamać, M.; Koleva, L.; Kancheva, V.; Amarowicz, R. The Structure—Antioxidant Activity Relationship of Ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Feng, G.; Zhao, N.; Wu, L.; Zhu, H. Isoferulic acid inhibits human leukemia cell growth through induction of G2/M-phase arrest and inhibition of Akt/mTOR signaling. Mol. Med. Rep. 2020, 21, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Nuntanakorn, P.; Jiang, B.; Einbond, L.S.; Yang, H.; Kronenberg, F.; Weinstein, I.B.; Kennelly, E.J. Polyphenolic constituents of Actaea racemosa. J. Nat. Prod. 2006, 69, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Wang, Y.; Li, A.; Fu, C.; Wang, Y.; Peng, W. Bioactive Components of Chinese Propolis Water Extract on Antitumor Activity and Quality Control. Evid. Based Complement. Altern. Med. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Zabin, S.A. Antimicrobial, Antiradical Capacity and Chemical Analysis of Conyza incana Essential Oil Extracted from Aerial Parts. J. Essent. Oil-Bear. Plants 2018, 21, 502–510. [Google Scholar] [CrossRef]
- Pj, B.; Shibumon, G.; Sunny, K.; Cincy, G. 2, 3-Dihydroxybenzoic Acid: An Effective Antifungal Agent Isolated from Flacourtia inermis Fruit. Int. J. Pharm. Clin. Res. 2010, 2, 101–105. [Google Scholar]
- Jairajpuri, D.S.; Jairajpuri, Z.S. Isoferulic acid action against glycation-induced changes in structural and functional attributes of human high-density lipoprotein. Biochemistry 2016, 81, 289–295. [Google Scholar] [CrossRef]
- Liu, I.M.; Tsai, C.C.; Lai, T.Y.; Cheng, J.T. Stimulatory effect of isoferulic acid on α1A-adrenoceptor to increase glucose uptake into cultured myoblast C2C12 cell of mice. Auton. Neurosci. Basic Clin. 2001, 88, 175–180. [Google Scholar] [CrossRef]
- Meeprom, A.; Sompong, W.; Chan, C.; Adisakwattana, S. Isoferulic Acid, a New Anti-Glycation Agent, Inhibits Fructose- and Glucose-Mediated Protein Glycation in Vitro. Molecules 2013, 18, 6439–6454. [Google Scholar] [CrossRef] [PubMed]
- Dilshara, M.G.; Lee, K.T.; Jayasooriya, R.G.P.T.; Kang, C.H.; Park, S.R.; Choi, Y.H.; Choi, I.W.; Hyun, J.W.; Chang, W.Y.; Kim, Y.S.; et al. Downregulation of NO and PGE2 in LPS-stimulated BV2 microglial cells by trans-isoferulic acid via suppression of PI3K/Akt-dependent NF-κB and activation of Nrf2-mediated HO-1. Int. Immunopharmacol. 2014, 18, 203–211. [Google Scholar] [CrossRef]
- Schmid, D.; Woehs, F.; Svoboda, M.; Thalhammer, T.; Chiba, P.; Moeslinger, T. Aqueous extracts of Cimicifuga racemosa and phenolcarboxylic constituents inhibit production of proinflammatory cytokines in LPS-stimulated human whole blood. Can. J. Physiol. Pharmacol. 2009, 87, 963–972. [Google Scholar] [CrossRef]
- Sakai, S.; Kawamata, H.; Kogure, T.; Mantani, N.; Terasawa, K.; Umatake, M.; Ochiai, H. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 cells. Mediat. Inflamm. 1999, 8, 173–175. [Google Scholar] [CrossRef]
- You, C.-X.; Guo, S.-S.; Zhang, W.-J.; Geng, Z.-F.; Liang, J.-Y.; Lei, N.; Du, S.-S.; Deng, Z.-W. Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum. Molecules 2017, 22, 1379. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure-activity relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.F.C.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids—A structure-activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Marinova, E.M.; Yanishlieva, N.V.L. Inhibited Oxidation of Lipids II: Comparison of the Antioxidative Properties of Some Hydroxy Derivatives of Benzoic and Cinnamic Acids. Fett Wiss. Technol. Sci. Technol. 1992, 94, 428–432. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 631–638. [Google Scholar] [CrossRef]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) complex of plant phenolic chlorogenic acid: Antioxidant, antimicrobial and structural studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of Antioxidant Activity of Caffeic Acid and p-Coumaric Acid by Using Electrochemical and Spectrophotometric Assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Santos, A.F.; Brotto, D.F.; Favarin, L.R.V.; Cabeza, N.A.; Andrade, G.R.; Batistote, M.; Cavalheiro, A.A.; Neves, A.; Rodrigues, D.C.M.; dos Anjos, A. Study of the antimicrobial activity of metal complexes and their ligands through bioassays applied to plant extracts. Braz. J. Pharmacol. 2014, 24, 309–315. [Google Scholar] [CrossRef]
- Roy, S.; Mallick, S.; Chakraborty, T.; Ghosh, N.; Singh, A.K.; Manna, S.; Majumdar, S. Synthesis, characterisation and antioxidant activity of luteolin-vanadium(II) complex. Food Chem. 2015, 173, 1172–1178. [Google Scholar] [CrossRef]
- Kopacz, M.; Woźnicka, E.; Gruszecka, J. Antibacterial activity of morin and its complexes with La(III), Gd(III) and Lu(III) ions. Acta Pol Pharm. 2005, 65, 65–67. [Google Scholar]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Novel role of Zn(II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem. Biol. Interact. 2012, 197, 31–39. [Google Scholar] [CrossRef]
- Souza, R.F.V.; Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Mazur, L.; Jabłońska-Trypuć, A.; Lewandowski, W. A new calcium 2,5-dihydroxybenzoate: Synthesis, characterization and antioxidant studies and stress mediated cytotoxity in MCF-7 cells. J. Saudi Chem. Soc. 2018, 22, 742–756. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone metal complexes—Molecular structure, antioxidant activity and biological effects. Chem. Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, K.; Bai, J.; Zhang, H.; Chao, J. Synthesis, characterization and free radical scavenging activity of apigenin with or without magnesium(II). Oxid. Antioxid. Med. Sci. 2014, 3, 231–235. [Google Scholar] [CrossRef][Green Version]
- Maitra, R.; Roy, R.; Ghosh, S.; Mallick, S. Synthesis characterization and study of antioxidant activity of luteolin-magnesium complex. UJPSR 2016, 2, 21–26. [Google Scholar]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; He, J.; Cai, S.; Xiao, K.; Zhu, L. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese(II) and its inhibition kinetics on xanthine oxidase. RSC Adv. 2017, 7, 53385–53395. [Google Scholar] [CrossRef]
- Gorgannezhad, L.; Dehghan, G.; Ebrahimipour, S.Y.; Naseri, A.; Nazhad Dolatabadi, J.E. Complex of manganese (II) with curcumin: Spectroscopic characterization, DFT study, model-based analysis and antiradical activity. J. Mol. Struct. 2016, 1109, 139–145. [Google Scholar] [CrossRef]
- Jamil, W.; Solangi, S.; Ali, M.; Khan, K.M.; Taha, M.; Khuhawar, M.Y. Syntheses, characterization, in vitro antiglycation and DPPH radical scavenging activities of isatin salicylhydrazidehydrazone and its Mn (II), Co (II), Ni (II), Cu (II), and Zn (II) metal complexes. Arab. J. Chem. 2019, 12, 2262–2269. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Siddiqui, K.; Bawazeer, N.; Scaria Joy, S. Variation in macro and trace elements in progression of type 2 diabetes. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Davis, C.D. Manganese deficiency and toxicity: Are high or low dietary amounts of manganese cause for concern? BioFactors 1999, 10, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef]
- Li, H.C.; Xu, Q.M.; Liu, L.M.; Wu, L.H.; Tang, Z.T.; Cui, H.; Liu, Y.C. A new magnesium(II) complex of marbofloxacin: Crystal structure, antibacterial activity and acute toxicity. Inorg. Chim. Acta 2021, 516, 120065. [Google Scholar] [CrossRef]
- Wagner, C.C.; Baran, E.J.; Piro, O.E. Characterization of bis (isoorotato) diaquamagnesium (II) dihydrate: A potentially useful complex for magnesium supplementation. J. Inorg. Biochem. 1999, 73, 259–263. [Google Scholar] [CrossRef]
- Schoop, V.M.; Mirancea, N.; Fusenig, N.E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2013. [Google Scholar]
- Sheldrick, G.M. A short history of ShelX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Faruggia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Bisby, R. Techniques in free radical research: (Laboratory techniques in biochemistry and molecular biology, volume 22). FEBS Lett. 1992, 308, 107. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Xiong, S.L.; Li, A.; Huang, N.; Lu, F.; Hou, D. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohydr. Polym. 2011, 86, 1273–1280. [Google Scholar] [CrossRef]
- Mitsuda, H.; Yasumoto, K.; Iwami, K. Antioxidative Action of Indole Compounds during the Autoxidation of Linoleic Acid. Eiyo Shokuryo 1966, 19, 210–214. [Google Scholar] [CrossRef]
- Zeraik, M.L.; Petrônio, M.S.; Coelho, D.; Regasini, L.O.; Silva, D.H.S.; da Fonseca, L.M.; Machado, S.A.S.; Bolzani, V.S.; Ximenes, V.F. Improvement of Pro-Oxidant Capacity of Protocatechuic Acid by Esterification. PLoS ONE 2014, 9, e110277. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Row, T.N.G. Charge Density Analysis of Ferulic Acid: Robustness of Trifurcated C-H⋅⋅⋅O Hydrogen Bond. Cryst. Growth Des. 2012, 12, 6083–6091. [Google Scholar] [CrossRef]
- Zhu, L.-C. Ammonium (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o2953. [Google Scholar] [CrossRef]
- Kula, A.; Mazur, L.; Rzączyńska, Z. Crystal structure, spectroscopic and thermal studies of 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid sodium salt. J. Coord. Chem. 2007, 8, 843–850. [Google Scholar] [CrossRef]
- Shi, F.; Reis, M.S.; Brandao, P.; Souza, A.M.; Felix, V.; Rocha, J. Synthesis, structures and magnetic properties of three metal-organic frameworks containing manganese(II). Trans. Met. Chem. 2010, 35, 779–786. [Google Scholar] [CrossRef]
- Tung, Y.T.; Wu, J.H.; Kuo, Y.H.; Chang, S.T. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour. Technol. 2007, 98, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; Hussein, S.A.M.; Ayoub, N.A.; Hofmann, K.; Linscheid, M.; Harms, M.; Wende, K.; Lindequist, U. Aphyllin, the first isoferulic acid glycoside and other phenolics from Tamarix aphylla flowers. Pharmazie 2009, 64, 342–347. [Google Scholar]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Jabbari, M.; Moallem, H.R. Effect of solute-solvent interactions on DPPH radical scavenging efficiency of some flavonoid antioxidants in various binary water-methanol mixtures. Can. J. Chem. 2014, 93, 558–563. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Effect of pH and metal ions on DPPH radical scavenging activity of tea. Int. J. Food Sci. Nutr. 2015, 66, 58–62. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH.) Tests. JAOCS J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstractions. 1. The reactions of phenols with 2,2-diphenyl-1-picrylhydrazyl (dpph•) in alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hou, D.; Huang, N. Isolation and antioxidant activity of acidic polysaccharide with water-solubility from prunella vulgaris linn. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, ICBBE, Chengdu, China, 18–20 June 2010; pp. 1–5. [Google Scholar]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Trifunschi, S.; Munteanu, M.F. Synthesis, Characterization and Antioxidant Activity of Cooper-Quercetin Complex and Iron-Quercetin Complex. Rev. Chim. 2018, 67, 2422–2424. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Vladykovskaya, E.N.; Korkina, L.G.; Afanas’ev, I.B.A. Influence of metal ions on flavonoid protection against asbestos-induced cell injury. Arch. Biochem. Biophys. 2001, 385, 129–137. [Google Scholar] [CrossRef]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef]
- Guzowska, M.; Kalinowska, M.; Lewandowski, W. “Good Fashion is Evolution, Not Revolution”—Methods to Enhance Existing Anticancer Medicines, primarily with the Use of Transition Metal. Anticancer. Agents Med. Chem. 2018, 18, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phyther. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]


| Antioxidant/Method; Parameter | Ref. |
|---|---|
| DPPH•; IC50 = 4.58 μg/mL; EC50 = 5.65 μM; EC50 = 0.75 μM; IC50 = 40.20 mM | [11,12,13,14] |
| ABTS•+; IC50 = 1.08 μg/mL | [11] |
| FRAP; IC50 = 8.84 μg/mL; FRAP value = 53.83 mM Fe2+; FRAP value = 4.38 mM Fe2+ | [11,13,15] |
| CUPRAC; IC50 = 7.69 μg/mL | [11] |
| •OH; IC50 = 1.57 μg/mL | [11] |
| O2•−; IC50 = 13.33 μg/mL | [11] |
| Anti-lipid peroxidation; IC50 = 7.30 μg/mL | [11] |
| Cytotoxic | |
| the inhibition of cell viability, inducing cell apoptosis and triggered cell cycle arrest in G2/M phase in hematologic cancer cell lines (Raji, K562 and Jurkat) | [16,17,18] |
| Antimicrobial | |
| antibacterial activity against Staphylococcus aureus, Bacillus subtilis | [19] |
| antifungal activity against Candida albicans, Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger | [19,20] |
| Anti-diabetes | |
| lowering plasma glucose levels | [4,5] |
| antiglycation action against aldehyde-associated glycation in high-density lipoprotein (HDL) | [21] |
| protection against human serum albumin (HSA) structural changes induced by ROS and glycation | [13] |
| activation of α1A-AR adrenoceptor by IFA increasing the glucose uptake via PLC–PKC pathway in cultured myoblast C2C12 cell of mice | [22] |
| protection against fructose- and glucose-mediated glycation in vitro | [23] |
| Anti-inflammatory | |
| reducing joint pain, swelling, and inflammation | [24] |
| inhibition of the production of pro-inflammatory cytokines IL-6, TNF-α, and IFN-γ in lipopolysaccharide (LPS)-stimulated human whole blood | [25] |
| Antiviral | |
| elimination of viral infections, e.g., the production of macrophage inflammatory protein-2 (MIR-2) in a murine macrophage cell line, in response to respiratory syncytial virus (RSV) infection | [26] |
| Other properties | |
| repellent activity against the stored grain pest Tribolium castaneum | [27] |
| Mg(II) IFA | Mn(II)/Na(I) IFA | |
|---|---|---|
| Formula | MgC20H42O20 | Mn3Na2C80H88O40 |
| Formula weight | 626.85 | 1900.30 |
| T (K) | 100(2) | 100(2) |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | P-1 |
| a (Å) | 35.329(3) | 12.827(1) |
| b (Å) | 6.9547(4) | 13.425(1) |
| c (Å) | 12.162(2) | 14.566(1) |
| α (°) | 90 | 116.37(1) |
| β (°) | 97.21(1) | 108.22(1) |
| γ (°) | 90 | 95.81(1) |
| V (Å3) | 2964.6(6) | 2047.8(3) |
| Z | 4 | 1 |
| dcalc (g cm−3) | 1.404 | 1.541 |
| θ range (°) | 2.99–27.48 | 2.81–27.48 |
| µ (mm−1) | 0.145 | 0.565 |
| Rint | 0.032 | 0.028 |
| Refl. collected/unique/obs. [I>2σ(I)] | 11134/3389/2059 | 16224/9369/7824 |
| Parameters/restrains | 270/0 | 661/0 |
| Completeness to θmax | 0.999 | 0.998 |
| R1, wR2 [I>2σ(I)] | 0.0328; 0.0861 | 0.0404; 0.0944 |
| R1, wR2 [all data] | 0.0411; 0.0918 | 0.0518; 0.1020 |
| GOF on F2 | 1.056 | 1.092 |
| Max. and min. residual density (e Å−3) | 0.31; −0.22 | 0.45; −0.31 |
| Isoferulic Acid | Mg(II) IFA | Mn(II)/Na(I) IFA | Assignments | |||
|---|---|---|---|---|---|---|
| IR | Raman | IR | Raman | IR | Raman | |
3406s * | 3474vs 3463vs 3415vs 3238s | 3412s 3171s | ν(OH)ar | |||
| 3078vw | ν(OH)COOH | |||||
| 2943w | 2945vw | 2934m | 2935vw | 2944s | 2944vw | ν(CH) |
| 2848m | 2848vw | 2841m | 2844vw | 2838s | 2847vw | ν(CH) |
| 1694s 1671s | ν(C=O) | |||||
| 1629s | 1635vs | 1639s | 1632m | 1636vs | 1634vs | ν(CC)-C=C- |
| 1613s | 1615s | 1617s 1595m | 1615vs | 1593s | 1608m | ν(CC)ar |
| 1556s | 1522vs | νas(COO−) | ||||
| 1538m 1513vs | 1532s | ν(CC)ar | ||||
| 1454m | 1468vw | 1466w | 1462vw | δas(CH3) | ||
| 1443m | 1432m | 1440vw | 1440vs | 1438vw | ν(CC)ar | |
| 1413s | 1413w | 1402vs | 1410w | νas(COO−) | ||
| 1323s | 1300m | 1312vw | 1311vw | ν(C-O) | ||
| 1265vs | 1279m | 1253m | 1275s | 1253vs | 1277m | β(CH) + ν(C-O) |
| 1136s | 1137w | 1126s | 1138vw | 1136vs | 1138vw | β(CH) |
| 1024m | 1031m | 1037vw | 1025s | ν(O-CH3) | ||
| 977m | 977vw | 974m | 980vw | 981s | 985vw | γ(CH)-C=C- |
| 949m | γ(OH)COOH | |||||
| 860w | 880vw | 862s | 874vw | βs(COO−) | ||
| 817m | 818vw | 816m | 810s | γ(CH)ar | ||
| 599m | 602m | γs(COO−) | ||||
| 571w | γ(C=O) | |||||
| 575m | 566vw | 565s | βas(COO−) | |||
| 521m | 535m | 512vw | 513m | βO-(CH3) | ||
| 448vw | 477m | 450w | φ(CC) | |||
| Concentration (mM) | Band (nm) | IFA | Mg(II) IFA | Mn(II)/Na(I) IFA |
|---|---|---|---|---|
| 0.01 | λmax1 | 322 | 316 | 315 |
| λmax2 | 294 | 291 | 290 | |
| λmax3 | 243 | 239 | 235 |
| Compound | ABTS * I% | ABTS ** I% | CUPRAC */CTrolox [μM] | CUPRAC**/CTrolox [μM] |
|---|---|---|---|---|
| IFA | 65.96 ± 1.49 | 64.79 ± 1.37 | 40.92 ± 3.79 | 46.31 ± 5.90 |
| Mg(II) IFA | 65.83 ± 1.33 | 66.81 ± 2.24 | 87.93 ± 3.85 | 191.38 ± 8.30 |
| Mn(II)/Na(I) IFA | 51.95 ± 1.87 | 58.91 ± 1.51 | 105.85 ± 3.72 | 245.14 ± 2.41 |
| Compound | Concentration (μM) | VI (%) | t-Test |
|---|---|---|---|
| IFA | 1582 | 87 ± 5 | 0.010 |
| Mg(II) IFA | 16 | 87 ± 4 | 0.026 |
| Mn(II)/Na(I) IFA | 45 | 87 ± 5 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowska, M.; Gołębiewska, E.; Mazur, L.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Wyrwas, M.; Pawluczuk, N.; Lewandowski, W. Crystal Structure, Spectroscopic Characterization, Antioxidant and Cytotoxic Activity of New Mg(II) and Mn(II)/Na(I) Complexes of Isoferulic Acid. Materials 2021, 14, 3236. https://doi.org/10.3390/ma14123236
Kalinowska M, Gołębiewska E, Mazur L, Lewandowska H, Pruszyński M, Świderski G, Wyrwas M, Pawluczuk N, Lewandowski W. Crystal Structure, Spectroscopic Characterization, Antioxidant and Cytotoxic Activity of New Mg(II) and Mn(II)/Na(I) Complexes of Isoferulic Acid. Materials. 2021; 14(12):3236. https://doi.org/10.3390/ma14123236
Chicago/Turabian StyleKalinowska, Monika, Ewelina Gołębiewska, Liliana Mazur, Hanna Lewandowska, Marek Pruszyński, Grzegorz Świderski, Marta Wyrwas, Natalia Pawluczuk, and Włodzimierz Lewandowski. 2021. "Crystal Structure, Spectroscopic Characterization, Antioxidant and Cytotoxic Activity of New Mg(II) and Mn(II)/Na(I) Complexes of Isoferulic Acid" Materials 14, no. 12: 3236. https://doi.org/10.3390/ma14123236
APA StyleKalinowska, M., Gołębiewska, E., Mazur, L., Lewandowska, H., Pruszyński, M., Świderski, G., Wyrwas, M., Pawluczuk, N., & Lewandowski, W. (2021). Crystal Structure, Spectroscopic Characterization, Antioxidant and Cytotoxic Activity of New Mg(II) and Mn(II)/Na(I) Complexes of Isoferulic Acid. Materials, 14(12), 3236. https://doi.org/10.3390/ma14123236









