Novel 2,4,6-Trimethylbenzenesulfonyl Hydrazones with Antibacterial Activity: Synthesis and In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
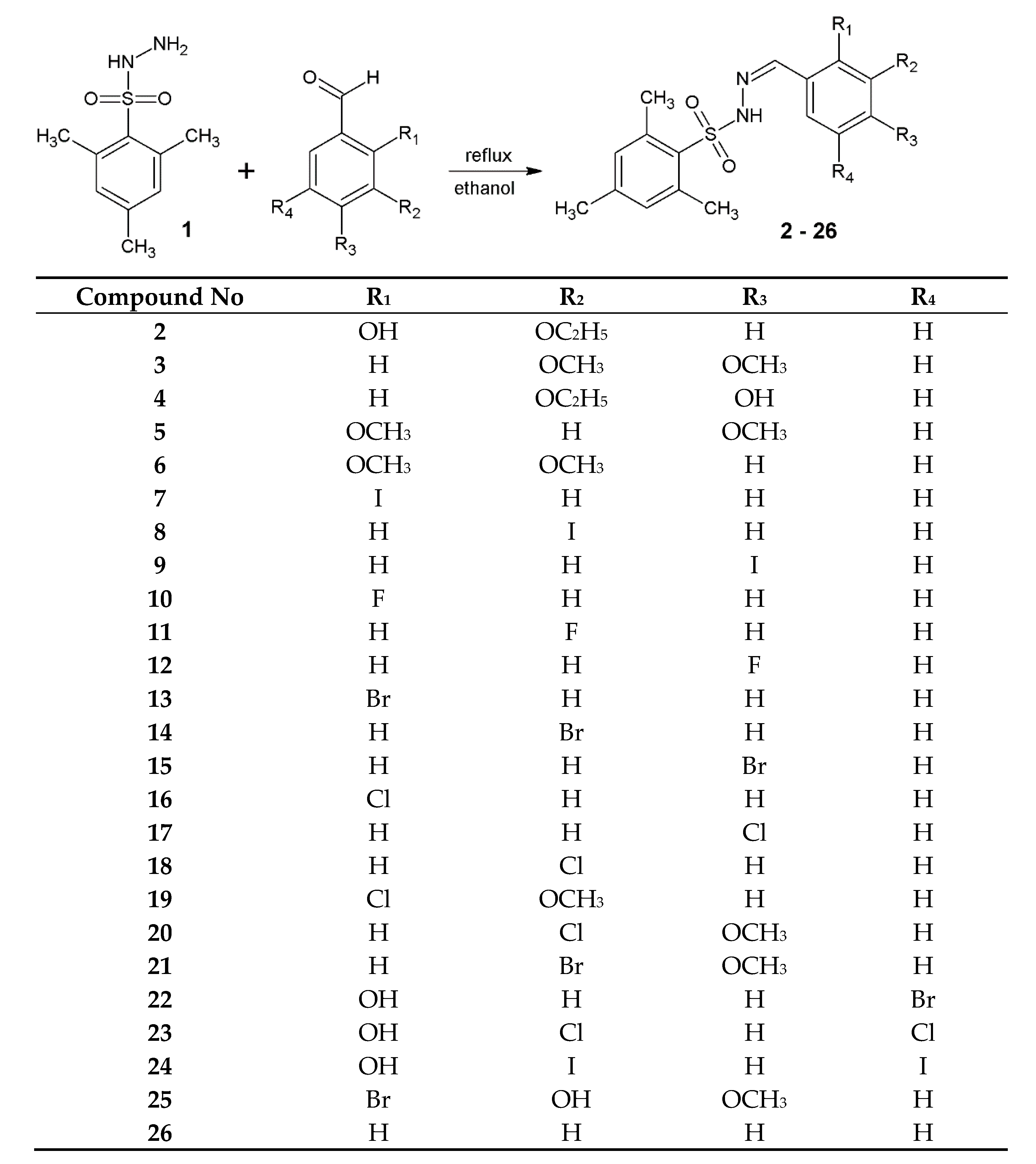
2.1.1. Preparation of 2,4,6-Trimethylbenzenesulfonyl Hydrazones (2–26)
2.1.2. Physicochemical Properties of 2,4,6-Trimethylbenzenesulfonyl Hydrazones (2–26)
2.2. Microbiology
In Vitro Antimicrobial Activity Assay
3. Results and Discussion
3.1. Chemistry
3.2. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moellering, R.C., Jr. Discovering new antimicrobial agents. Inter. J. Antimicrob. Agents 2011, 37, 2–9. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Morehead, M.S.; Scarbrough, C. Emergence of Global Antibiotic Resistance. Prim. Care Clin. Off. Pract. 2018, 45, 467–484. [Google Scholar] [CrossRef]
- Barluenga, J.; Moriel, P.; Valdés, C.; Aznar, F. N-Tosylohydrazones as Reagent for Cross-Coupling Reactions: A Route to Polysubtituted Olefins. Angew. Chem. Int. Ed. 2007, 46, 5587–5590. [Google Scholar] [CrossRef]
- Cunha, M.R.; Tavares, M.T.; Carvalho, C.F.; Silva, N.A.T.; Souza, A.D.F.; Pereira, G.J.V.; Ferreira, F.F.; Parise-Filho, R. Environmentaly Safe Condition for the Synthesis of Aryl and Alkyl Sulfonyl Hydrazones via One-Pot Reaction. ACS Sustain. Chem. Eng. 2016, 4, 1899–1905. [Google Scholar] [CrossRef]
- Yang, F.-L.; Tian, S.-K. Sulfonyl hydrazides as sulfonyl sources in organic synthesis. Tetrahedron Lett. 2017, 58, 487–504. [Google Scholar] [CrossRef]
- Arunprasath, D.; Bala, B.D.; Sekar, G. Luxury of N-Tosylhydrazones in Transition-Metal-Free Transformations. Adv. Synth. Catal. 2018, 361, 1172–1207. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Y.; Liu, Y.; Liu, Z.P. Synthesis of Sulfonylhydrazone- and Acylhydrazone-Substituted 8-Ethoxy-3-nitro-2H-chromones as Potent Antiproliferative and Apoptosis Inducing Agents. Arch. Pharm. 2014, 347, 576–588. [Google Scholar] [CrossRef]
- Xie, Z.; Song, Y.; Xu, L.; Guo, Y.; Zhang, M.; Li, L.; Chen, K.; Liu, X. Rapid Synthesis of N-Tosylhydrazones under Solvent-Free Conditions and Their Potential Application Against Human Triple-Negative Breast Cancer. Chem. Open 2018, 7, 977–983. [Google Scholar] [CrossRef]
- Wei, D.-C.; Pan, Y.; Wang, H.; Xu, W.-J.; Chen, C.; Zheng, J.-H.; Cai, D. Synthesis of substituted aromatic heterocyclic sulfonyl hydrazone compounds and in vitro anti-hepatoma activity: Preliminary results. Eur. Rev. Med. Pharm. Sci. 2018, 22, 4720–4729. [Google Scholar]
- Popiołek, Ł.; Gawrońska-Grzywacz, M.; Berecka-Rycerz, A.; Paruch, K.; Piątkowska-Chmiel, I.; Natorska-Chomicka, D.; Herbet, M.; Gumieniczek, A.; Dudka, J.; Wujec, M. New benzenesulphonohydrazide derivatives as potential antitumour agents. Oncol. Lett. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, J.-Q.; Luo, S.-H.; Mei, W.-J.; Lin, J.-Y.; Zhan, J.-Q.; Wang, Z.-Y. Synthesis of N-2(5H)-furanonyl sulfonyl hydrazone derivatives and their biological evaluation in vitro and in vivo activity against MCF-7 breast cancer cells. Bioorg. Chem. 2021, 107, 104518. [Google Scholar] [CrossRef] [PubMed]
- Siemann, S.; Evanoff, D.P.; Marrone, L.; Clarke, A.J.; Viswanatha, T.; Dmitrienko, G. N-Arylsulfonyl Hydrazones as Inhibitors of IMP-1 Metallo-β-Lactamase. Antimicrob. Agent Chemother. 2002, 46, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Ghiya, S.; Joshi, Y.C. Synthesis and antimicrobial evaluation of hydrazones derived from 4-methylbenzenesulfonohydrazide in aqueous medium. Med. Chem. Res. 2016, 25, 970–976. [Google Scholar] [CrossRef]
- Bhat, M.; Poojary, B.; Kumar, S.M.; Hussain, M.M.; Pai, N.; Revanasiddappa, B.C.; Byrappa, K. Structural, crystallographic, Hirshfeld surface, thermal and antimicrobial evaluation of new sulfonyl hydrazones. J. Mol. Struct. 2018, 1159, 55–66. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Zhou, T. Synthesis and Antibacterial Activity of Benzenesulfonylhydrazone Derivatives of Methyl Dehydroabietate. Russ. J. Gen. Chem. 2019, 89, 819–823. [Google Scholar] [CrossRef]
- Shaaban, M.M.; Ragab, H.M.; Akaji, K.; McGeary, R.P.; Bekhit, A.-E.A.; Hussein, W.M.; Kurz, J.L.; Elwakil, B.H.; Bekhit, S.A.; Ibrahim, T.M.; et al. Design, synthesis, biological evaluation and in silico studies of certain aryl sulfonyl hydrazones conjugated with 1,3-diaryl pyrazoles as potent metallo-β-lactamase inhibitors. Bioorg. Chem. 2020, 105, 104386. [Google Scholar] [CrossRef]
- Loncle, C.; Brunel, J.M.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef]
- Wang, H.; Ren, S.-X.; He, Z.-Y.; Wang, D.-L.; Yan, X.-N.; Feng, J.-T.; Zhang, X. Synthesis, Antifungal Activities and Qualitative Structure Activity Relationship of Carabrone Hydrazone Derivatives as Potential Antifungal Agents. Int. J. Mol. Sci. 2014, 15, 4257–4272. [Google Scholar] [CrossRef]
- Backes, G.L.; Neumann, D.M.; Jursic, B.S. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg. Med. Chem. 2014, 22, 4629–4636. [Google Scholar] [CrossRef]
- Gao, Z.; Lv, M.; Li, Q.; Xu, H. Synthesis of heterocycle-attached methylidenebenzenesulfonohydrazones as antifungal agents. Bioorg. Med. Chem. Lett. 2015, 25, 5092–5096. [Google Scholar] [CrossRef]
- Novakoski de Oliveira, K.; Costa, P.; Santin, J.R.; Mazzambani, L.; Bürger, C.; Mora, C.; Nunes, R.J.; de Souza, M.M. Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg. Med. Chem. 2011, 19, 4295–4306. [Google Scholar] [CrossRef]
- Abid, S.M.A.; Younus, H.A.; Al-Rashida, M.; Arshad, Z.; Maryum, T.; Gilani, M.A.; Alharthi, A.I.; Iqbal, J. Sulfonyl hydrazones derived from 3-formylchromone as non-selective inhibitors of MAO-A and MAO-B: Synthesis, molecular modeling and in-silico ADME evaluation. Bioorg. Chem. 2017, 75, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Aslan, H.G.; Özcan, S.; Karacan, N. Synthesis, characterization and antimicrobial activity of salicylaldehydebenzenesulfonylhydrazone (Hsalbsmh)and its Nickel(II), Palladium(II), Platinum(II), Copper(II), Cobalt(II) complexes. Inorg. Chem. Commun. 2011, 14, 1550–1553. [Google Scholar] [CrossRef]
- Özdemir, Ü.Ö.; Arslan, F.; Hamurcu, F. Synthesis, characterization, antibacterial activities and carbonic anhydrase enzyme inhibitor effects of new arylsulfonylhydrazone and their Ni(II), Co(II) complexes. Spectrochim. Acta A 2010, 75, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ü.Ö.; Akkaya, N.; Özbek, N. New nickel(II), palladium(II), platinum(II) complexes with aromatic methanesulfonylhydrazone based ligands. Synthesis, spectroscopic characterization and in vitro antibacterial evaluation. Inorg. Chim. Acta 2013, 400, 13–19. [Google Scholar] [CrossRef]
- Özbek, N.; Özdemir, Ü.Ö.; Altun, A.F.; Şahin, E. Sulfonamide-derived hydrazone compounds and their Pd(II) complexes: Synthesis, spectroscopic characterization, X-ray structure determination, in vitro antibacterial activity and computational studies. J. Mol. Struct. 2019, 1196, 707–719. [Google Scholar] [CrossRef]
- Çınarlı, M.; Çınarlı, E.; Ataol, Ç.Y.; İdil, O.; Kariptaş, E. Synthesis, structural characterization, Hirshfeld surface analysis, antimicrobial activity, and DNA cleavage studies of (Z)-4-methyl-N′-(phenyl(pyridin-2-yl)methylene)benzenesulfonohydrazide and its Co(II), Ni(II) and Zn(II) complexes. J. Mol. Struct. 2019, 1196, 760–770. [Google Scholar] [CrossRef]
- Özdemir, Ü.Ö.; Aktan, E.; Ilbiz, F.; Gündüzalp, A.B.; Özbek, N.; Sarı, M.; Çelik, Ö.; Saydam, S. Characterization, antibacterial, anticarbonic anhydrase II isoenzyme, anticancer, electrochemical and computational studies of sulfonic acid hydrazide derivative and its Cu(II) complex. Inorg. Chim. Acta 2014, 423, 194–203. [Google Scholar] [CrossRef]
- Almendras, I.; Huentupil, Y.; Novoa, N.; Roussel, P.; Melis, D.R.; Smith, G.S.; Arancibia, R. Trinuclear Ni(II), Pd(II) and Cu(II) complexes containing the 2-hydroxybenzaldehyde-ferrocenyl-sulfonylhydrazone ligand: Synthesis, structural characterization and antiplasmodial evaluation. Inorg. Chim. Acta 2019, 496, 119050. [Google Scholar] [CrossRef]
- Concha, C.; Quintana, C.; Klahn, A.H.; Artigas, V.; Fuentealba, M.; Biot, C.; Halloum, I.; Kremer, L.; Lopez, R.; Romanos, J.; et al. Organometallic tosyl hydrazones: Synthesis, characterization, crystal structures and in vitro evaluation of anti-Mycobecterium tuberculosis and antiproliferative activities. Polyhedron 2017, 131, 40–45. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A. Hydrazide-hydrazones of 3-methoxybenzoic acid and 4-tert-butylbenzoic acid with promising antibacterial activity against Bacillus spp. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. 1), 62–69. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Biernasiuk, A. Synthesis and investigation of antimicrobial activities of nitrofurazone analogues containing hydrazide-hydrazone moiety. Saudi Pharm. J. 2017, 25, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Rysz, B.; Biernasiuk, A.; Wujec, M. Synthesis of promising antimicrobial agents: Hydrazide-hydrazones of 5-nitrofuran-2-carboxylic acid. Chem. Biol. Drug Des. 2020, 95, 260–269. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A.; Malm, A. Synthesis and antimicrobial activity of new 1,3-thiazolidin-4-one derivatives obtained from carboxylic acid hydrazides. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 251–260. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Patrejko, P.; Gawrońska-Grzywacz, M.; Biernasiuk, A.; Berecka-Rycerz, A.; Natorska-Chomicka, D.; Piątkowska-Chmiel, I.; Gumieniczek, A.; Dudka, J.; Wujec, M. Synthesis and in vitro bioactivity study of new hydrazide-hydrazones of 5-bromo-2-iodobenzoic acid. Biomed. Pharm. 2020, 130, 110526. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST Discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]

| Species | MIC (MBC) [µg/mL] and {MBC/MIC} Ratio of the Studied Compounds and Positive Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 7 | 9 | 12 | CIP/VA* | NIT | CFX | APC | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 25923 | 250 (500) {2} | - | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | - | 0.48 (0.48) {1} | 15.62 (15.62) | 0.49 | nd |
| Staphylococcus aureus ATCC 43300 | 500 (500) {1} | - | 1000 (>1000) {>1} | - | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | - | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd | |
| Staphylococcus aureus ATCC 29213 | 500 (500) {1} | - | - | - | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | - | 0.48 (0.48) {1} | 15.62 (15.62) | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | 500 (500) {1} | - | 500 (>1000) {>2} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | - | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 1000 (>1000) {>1} | - | 500 (>1000) {>2} | - | - | - | - | - | 0.98 * (1.95) {2} | 7.81 (7.81) | nd | nd | |
| Micrococcus luteus ATCC 10240 | 500 (500) {1} | - | - | - | 1000 (>1000) {>1} | 250 (>1000) {>4} | - | 250 (>1000) {>4} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 500 (500) {1} | 500 (>1000) {>2} | 500 (>1000) {>2} | 250 (1000) {>4} | 125 (>1000) {>8} | 62.5 (>1000) {>16} | 250 (>1000) {>4} | 250 (1000) {4} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 500 (1000) {2} | 250 (>1000) {>4} | 1000 (>1000) {>1} | - | 1000 (>1000) {>1} | - | - | - | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
| Species | MIC (MBC) [µg/mL] and {MBC/MIC} Ratio of the Studied Compounds and Positive Controls | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 16 | 17 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | CIP/VA* | NIT | CFX | APC | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 25923 | - | - | - | - | - | - | 500 (>1000) {>2} | 500 (>1000) {>2} | 15.62 (15.62) {1} | 125 (>1000) {>8} | 0.48 (0.48) {1} | 15.62 (15.62) | 0.49 | nd |
| Staphylococcus aureus ATCC 43300 | - | - | - | - | - | - | 500 (>1000) {>2} | 500 (>1000) {>2} | 15.62 (15.62) {1} | 250 (>1000) {>4} | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd | |
| Staphylococcus aureus ATCC 29213 | - | 500 (>1000) {>2} | - | - | - | - | 500 (>1000) {>2} | 250 (>1000) {>4} | 15.62 (62.5) {4} | 125 (>1000) {>8} | 0.48 (0.48) {1} | 15.62 (15.62) | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | - | 250 (>1000) {>4} | 1000 (>1000) {>1} | 500 (>1000) {>2} | - | - | 125 (500) {4} | 250 (>1000) {>4} | 15.62 (15.62) {1} | 125 (1000) {8} | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | - | - | - | - | - | - | - | 125 (>1000) {>8} | 15.62 (62.5) {4} | 250 (>1000) {>4} | 0.98 * (1.95) {2} | 7.81 (7.81) | nd | nd | |
| Micrococcus luteus ATCC 10240 | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 7.81 (15.62) {2} | 7.81 (15.62) {2} | 125 (250) {2} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 1000 (>1000) {>1} | 125 (1000) {8} | 250 (1000) {4} | 250 (1000) {4} | 1000 (>1000) {>1} | 500 (>1000) {>2} | 31.25 (500) {16} | 7.81 (15.62) {2} | 7.81 (7.81) {1} | 62.5 (125) {2} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 1000 (>1000) {>1} | 1000 (>1000) {>1} | - | - | - | 500 (>1000) {>2} | 250 (1000) {4} | 15.62 (15.62) {1} | 7.81 (15.62) {2} | 125 (>1000) {>8} | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiołek, Ł.; Szeremeta, S.; Biernasiuk, A.; Wujec, M. Novel 2,4,6-Trimethylbenzenesulfonyl Hydrazones with Antibacterial Activity: Synthesis and In Vitro Study. Materials 2021, 14, 2723. https://doi.org/10.3390/ma14112723
Popiołek Ł, Szeremeta S, Biernasiuk A, Wujec M. Novel 2,4,6-Trimethylbenzenesulfonyl Hydrazones with Antibacterial Activity: Synthesis and In Vitro Study. Materials. 2021; 14(11):2723. https://doi.org/10.3390/ma14112723
Chicago/Turabian StylePopiołek, Łukasz, Sylwia Szeremeta, Anna Biernasiuk, and Monika Wujec. 2021. "Novel 2,4,6-Trimethylbenzenesulfonyl Hydrazones with Antibacterial Activity: Synthesis and In Vitro Study" Materials 14, no. 11: 2723. https://doi.org/10.3390/ma14112723
APA StylePopiołek, Ł., Szeremeta, S., Biernasiuk, A., & Wujec, M. (2021). Novel 2,4,6-Trimethylbenzenesulfonyl Hydrazones with Antibacterial Activity: Synthesis and In Vitro Study. Materials, 14(11), 2723. https://doi.org/10.3390/ma14112723







