Effect of Benzotriazole on the Localized Corrosion of Copper Covered with Carbonaceous Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens and Solutions Preparation
2.2. X-ray Photoelectron Spectroscopy (XPS)
2.3. Electrochemical Tests
2.4. Surface Analysis after Potentiostatic Polarization Test
3. Results and Discussion
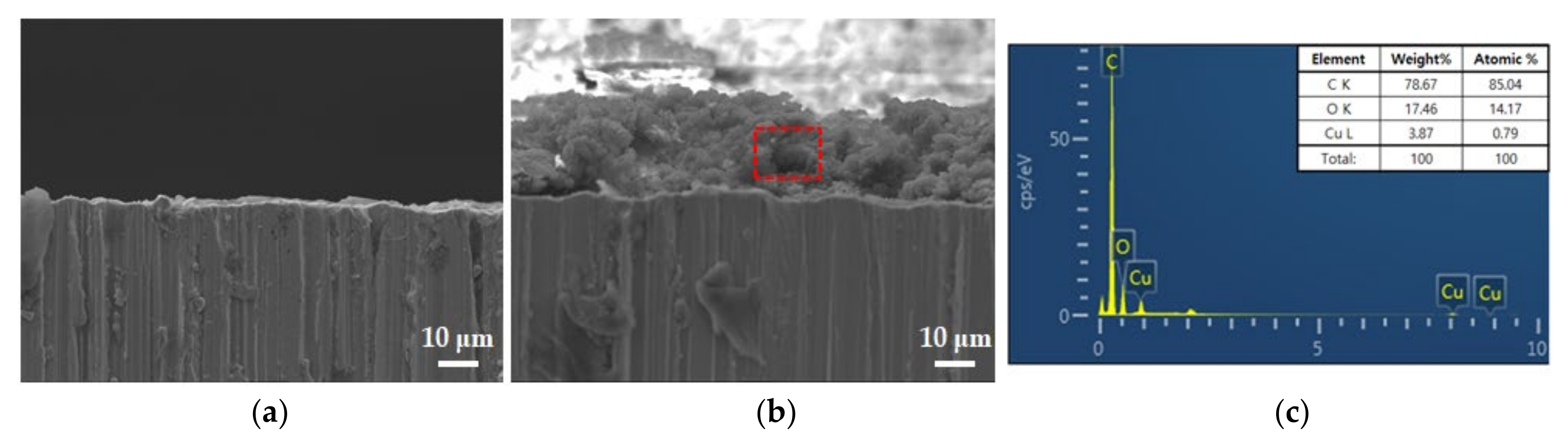
3.1. Carbonaceous Film Coating Analysis
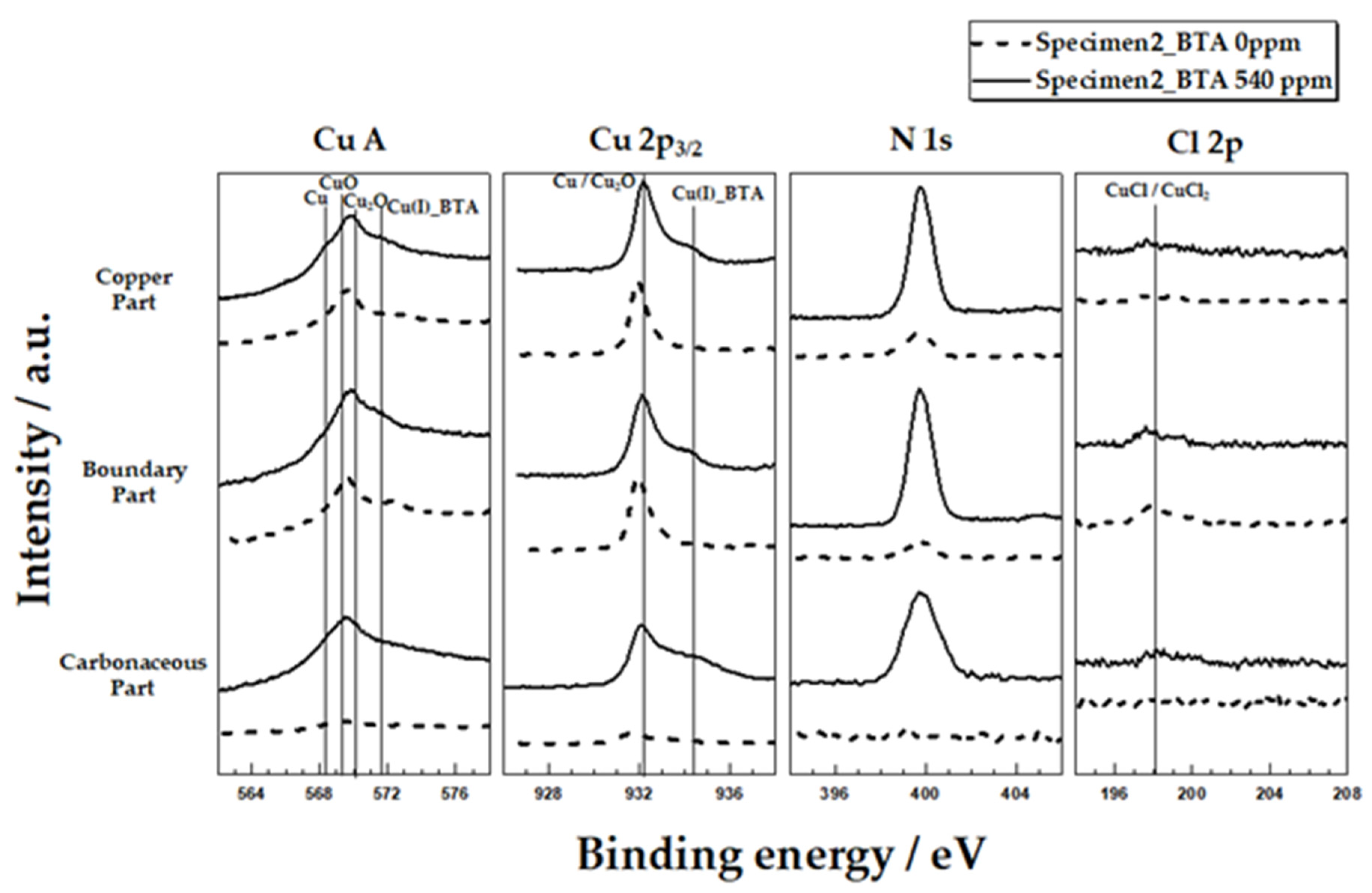
3.2. XPS Analysis
3.3. Electrochemical Analysis
3.3.1. Potentiodynamic Polarization Measurements
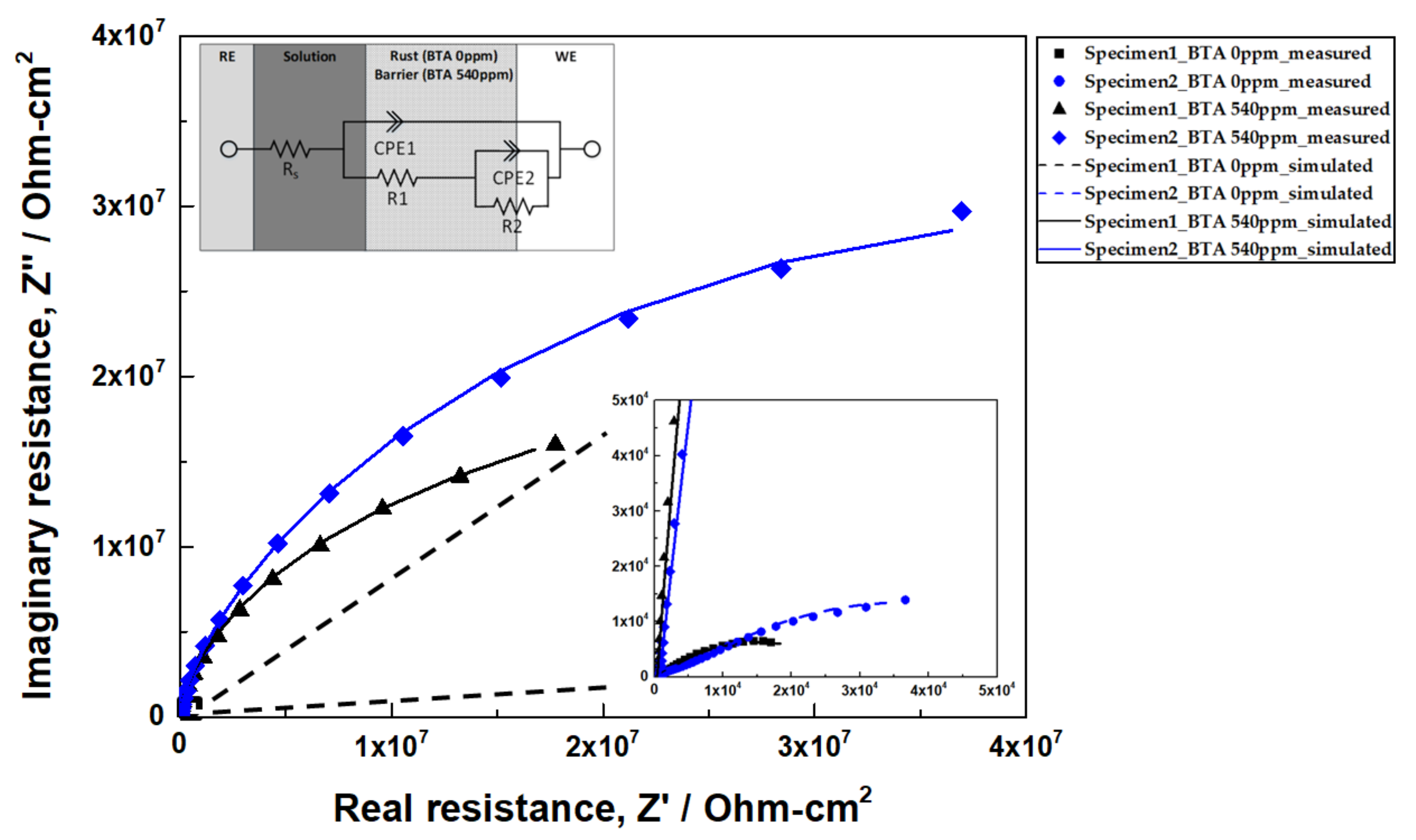
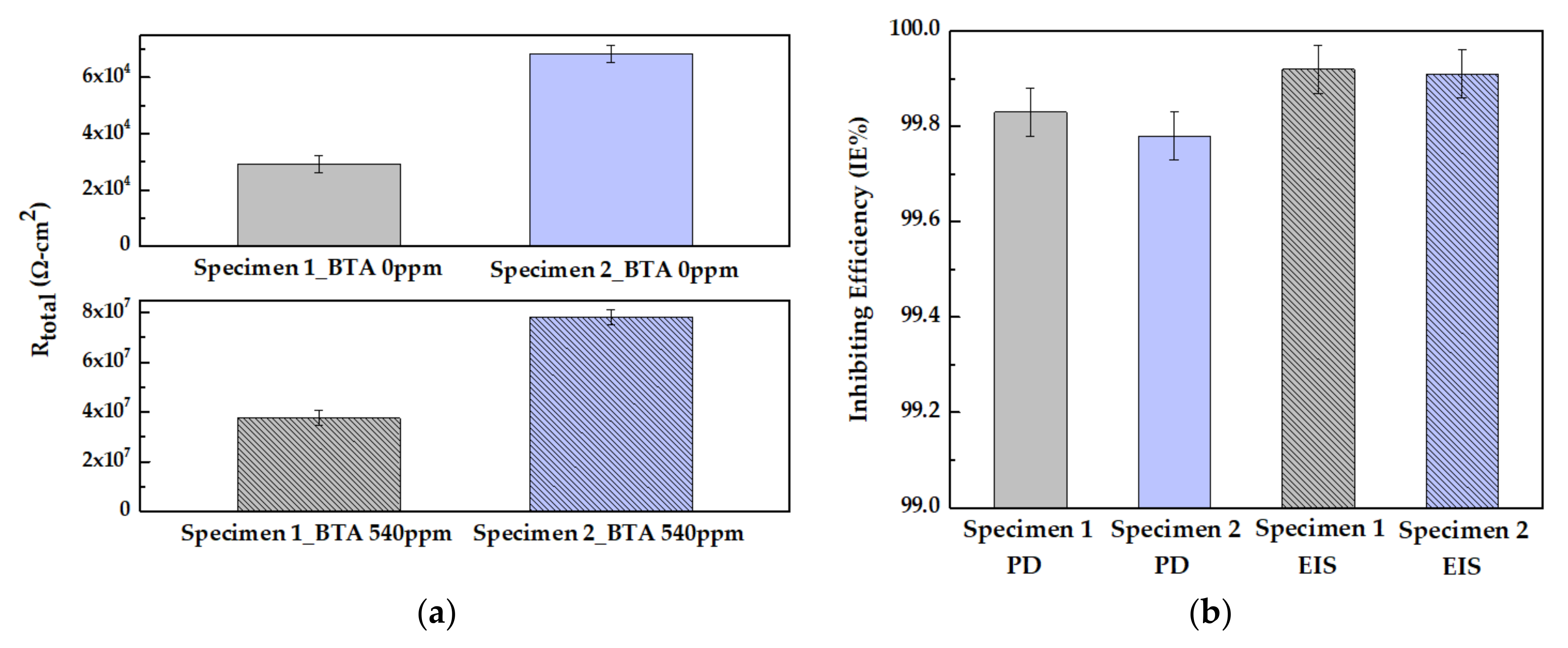
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
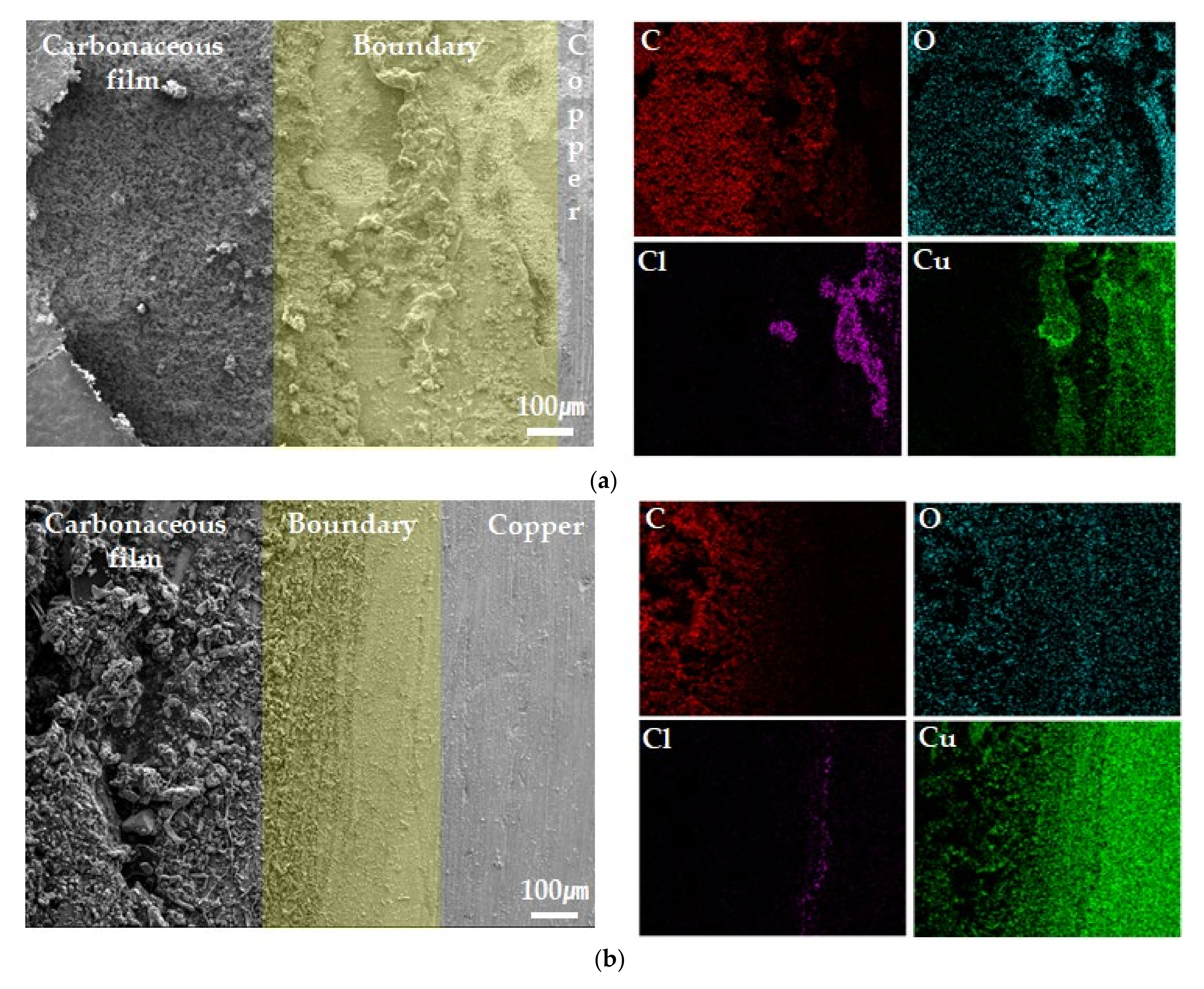
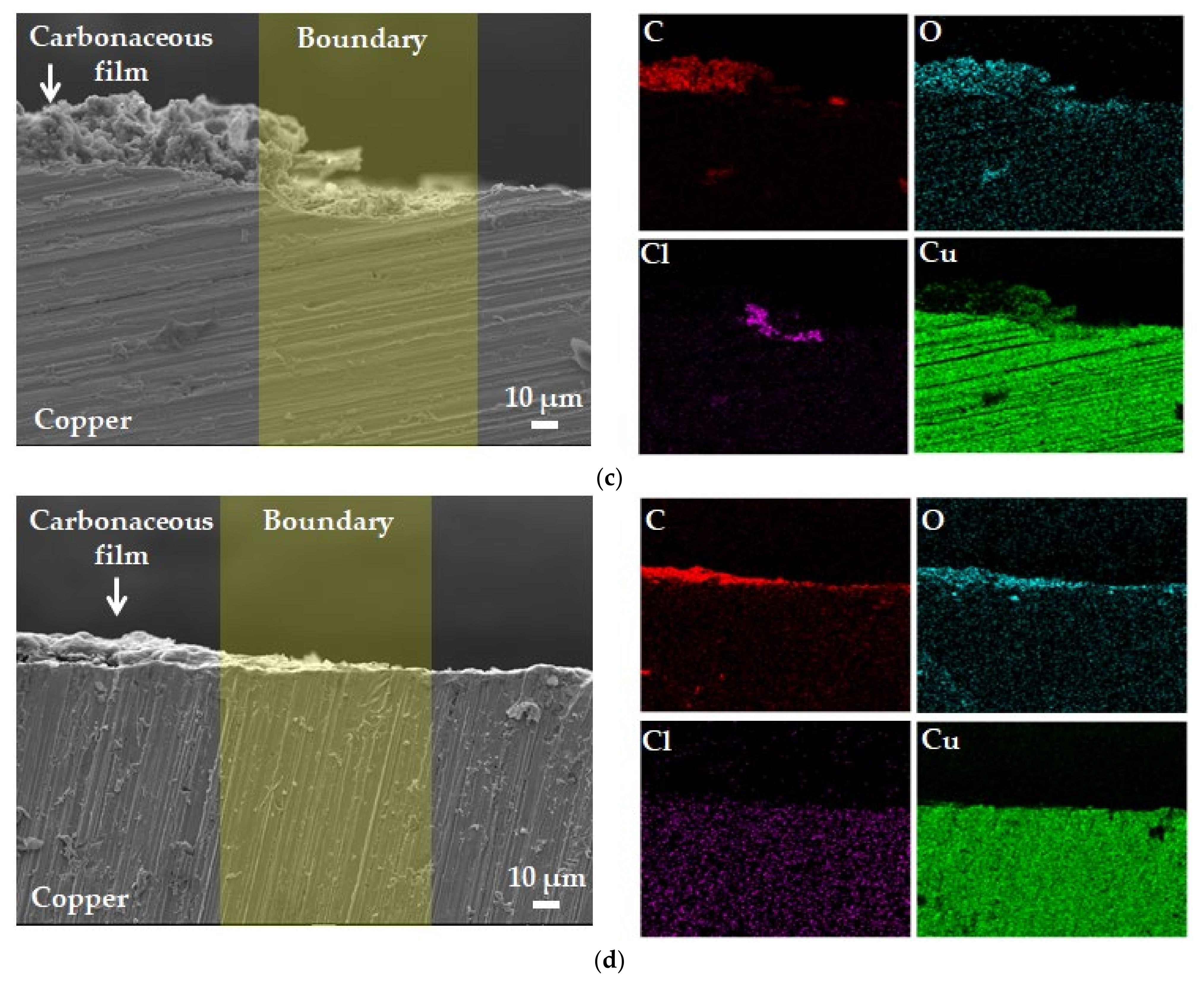
3.4. Surface Analysis after Potentiostatic Polarization Test
4. Conclusions
- In solutions containing BTA, Cu−BTA complex and N elements were detected in the copper part, carbonaceous film-copper boundary part, and carbonaceous film part through XPS analysis. In addition, BTA decreased icorr and increased R1 and n1. This is because BTA adsorbs well on the entire surface of copper with carbonaceous film to form a Cu−BTA protective layer;
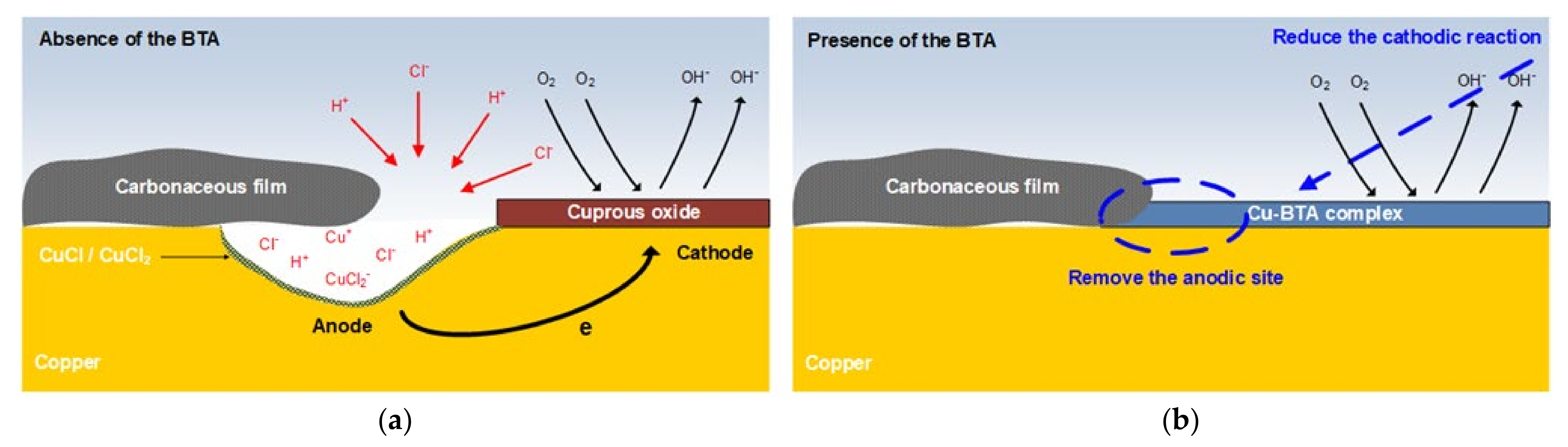
- XPS and SEM/EDS analyses indicated that localized corrosion and Cl concentration occurred in the carbonaceous film–copper boundary. It was inferred that this was due to crevice corrosion caused by the gap between the carbonaceous film and copper surface and the galvanic corrosion between the carbonaceous film and copper;
- BTA mitigates localized corrosion at the anodic site by forming the Cu−BTA complex in the carbonaceous film–copper boundary part. In addition, the formation of a Cu−BTA complex in the copper part inhibits the formation of an oxygen-concentration cell. Consequently, BTA suppresses localized corrosion caused by the carbonaceous film.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suh, S.H.; Suh, Y.; Yoon, H.G.; Oh, J.H.; Kim, Y.; Jung, K.; Kwon, H. Analysis of pitting corrosion failure of copper tubes in an apartment fire sprinkler system. Eng. Fail. Anal. 2016, 64, 111–125. [Google Scholar] [CrossRef]
- Cornwell, F.; Wildsmith, G.; Gilbert, P. Pitting corrosion in copper tubes in cold water service. Br. Corros. J. 1973, 8, 202–209. [Google Scholar] [CrossRef]
- Lytle, D.A.; Nadagouda, M.N. A comprehensive investigation of copper pitting corrosion in a drinking water distribution system. Corros. Sci. 2010, 52, 1927–1938. [Google Scholar] [CrossRef]
- El Warraky, A.; El Shayeb, H.; Sherif, E. Pitting corrosion of copper in chloride solutions. Anti Corros. Methods Mater. 2004, 51, 52–61. [Google Scholar] [CrossRef]
- Lytle, D.A.; Schock, M.R. Pitting corrosion of copper in waters with high pH and low alkalinity. J. Am. Water Work. Assoc. 2008, 100, 115–129. [Google Scholar] [CrossRef]
- Burleigh, T.D.; Gierke, C.G.; Fredj, N.; Boston, P.J. Copper tube pitting in Santa Fe municipal water caused by microbial induced corrosion. Materials 2014, 7, 4321–4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
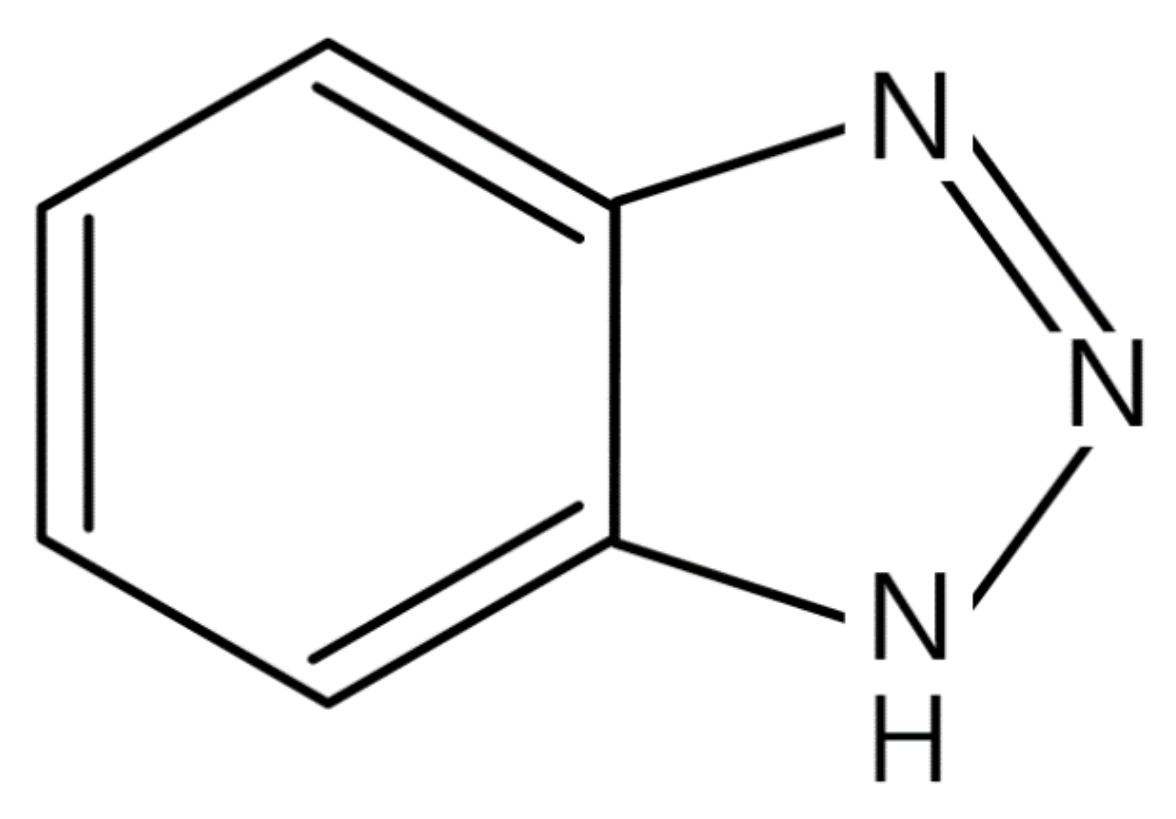
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1, 2, 3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-G. Electrochemical and quantum chemical studies of 1, 2, 3-benzotriazole as inhibitor for copper and steel in simulated tap water. Mater. Trans. 2017, 58, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Walker, R. Triazole, benzotriazole and naphthotriazole as corrosion inhibitors for copper. Corrosion 1975, 31, 97–100. [Google Scholar] [CrossRef]
- Hobbins, N.; Roberts, R. An ellipsometric study of thin films formed on copper by aqueous benzotriazole and benzimidazole. Surf. Technol. 1979, 9, 235–239. [Google Scholar] [CrossRef]
- Yu, P.; Liao, D.-M.; Luo, Y.-B.; Chen, Z.-G. Studies of benzotriazole and tolytriazole as inhibitors for copper corrosion in deionized water. Corrosion 2003, 59, 314–318. [Google Scholar] [CrossRef]
- Ross, T.; Berry, M. Benzotriazole as an inhibitor of the corrosion of Cu in flowing H* 12* sSO* 14* s. Corros. Sci. 1971, 11, 273–274. [Google Scholar] [CrossRef]
- Kosec, T.; Milošev, I.; Pihlar, B. Benzotriazole as an inhibitor of brass corrosion in chloride solution. Appl. Surf. Sci. 2007, 253, 8863–8873. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, Y.; Chen, C.; Chen, Y.; Liu, Y.; Yang, Z. New aspects of copper corrosion in a neutral NaCl solution in the presence of benzotriazole. Corrosion 2018, 74, 613–622. [Google Scholar] [CrossRef]
- Khan, P.F.; Shanthi, V.; Babu, R.K.; Muralidharan, S.; Barik, R.C. Effect of benzotriazole on corrosion inhibition of copper under flow conditions. J. Environ. Chem. Eng. 2015, 3, 10–19. [Google Scholar] [CrossRef]
- Oliphant, R. Causes of Copper Corrosion in Plumbing Systems; Foundation for Water Research: Buckinghamshire, UK, 2003. [Google Scholar]
- Iyasu, T.; Kuratani, M.; Ikeda, I.; Tanaka, N.; Yamada, Y.; Sakurada, O. A Study of Water Treatment Chemical Effects on Type I” Pitting Corrosion of Copper Tubes. Mater. Sci. Appl. 2020, 11, 494. [Google Scholar] [CrossRef]
- CEN. 1057: 2006 Copper and Copper Alloys—Seamleess, Round Copper Tubes for Water and Gas in Sanitary and Heating Applications; European Committee for Standardization: Brussels, Belgium, 2006. [Google Scholar]
- Lee, J.-B.; Jung, H. Investigation on Causes of Pitting Corrosion in Sprinkler Copper Tubes. Corros. Sci. Technol. 2014, 13, 6–14. [Google Scholar] [CrossRef] [Green Version]
- ASTM. B88: Standard Specification for Seamless Copper Water Tube; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Davis, J.R. Copper and Copper Alloys; ASM International: Ohio, OH, USA, 2001. [Google Scholar]
- Hong, M.-S.; Park, Y.; Kim, J.G.; Kim, K. Effect of incorporating MoS2 in organic coatings on the corrosion resistance of 316L stainless steel in a 3.5% NaCl solution. Coatings 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Bi, H.; Burstein, G.; Rodriguez, B.; Kawaley, G. Some aspects of the role of inhibitors in the corrosion of copper in tap water as observed by cyclic voltammetry. Corros. Sci. 2016, 102, 510–516. [Google Scholar] [CrossRef]
- Kosec, T.; Merl, D.K.; Milošev, I. Impedance and XPS study of benzotriazole films formed on copper, copper–zinc alloys and zinc in chloride solution. Corros. Sci. 2008, 50, 1987–1997. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Cui, C.; Zhang, Y.; Wang, L.; Wang, Z. The surface restructuring of copper oxides with mixed oxidation-states and their efficient CO oxidation properties. Mater. Lett. 2021, 289, 129378. [Google Scholar] [CrossRef]
- Lee, W.-J.; Lee, Y.-S.; Rha, S.-K.; Lee, Y.-J.; Lim, K.-Y.; Chung, Y.-D.; Whang, C.-N. Adhesion and interface chemical reactions of Cu/polyimide and Cu/TiN by XPS. Appl. Surf. Sci. 2003, 205, 128–136. [Google Scholar] [CrossRef]
- McIntyre, N.; Sunder, S.; Shoesmith, D.; Stanchell, F. Chemical information from XPS—applications to the analysis of electrode surfaces. J. Vac. Sci. Technol. 1981, 18, 714–721. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, J.G. Copper corrosion in potable water distribution systems: Influence of copper products on the corrosion behavior. Mater. Lett. 2004, 58, 2002–2006. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, T.; Lu, X. Chemical effects on the tribological behavior during copper chemical mechanical planarization. Mater. Chem. Phys. 2015, 153, 48–53. [Google Scholar] [CrossRef]
- Vasquez, R. CuO by XPS. Surf. Sci. Spectra 1998, 5, 262–266. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Zhang, G.; Qiu, Y.; Guo, X. Benzotriazole as a volatile corrosion inhibitor during the early stage of copper corrosion under adsorbed thin electrolyte layers. Corros. Sci. 2012, 65, 214–222. [Google Scholar] [CrossRef]
- Vasquez, R.P. CuCl2 by XPS. Surf. Sci. Spectra 1993, 2, 160–164. [Google Scholar] [CrossRef]
- Vasquez, R.P. CuCl by XPS. Surf. Sci. Spectra 1993, 2, 138–143. [Google Scholar] [CrossRef]
- Raman, R.S.; Banerjee, P.C.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Shi, W. Variable corrosion behavior of a thick amorphous carbon coating in NaCl solution. Rsc Adv. 2015, 5, 95750–95763. [Google Scholar] [CrossRef]
- Cho, B.-J.; Shima, S.; Hamada, S.; Park, J.-G. Investigation of Cu−BTA complex formation during Cu chemical mechanical planarization process. Appl. Surf. Sci. 2016, 384, 505–510. [Google Scholar] [CrossRef]
- Hong, M.-S.; Kim, S.-H.; Im, S.-Y.; Kim, J.-G. Effect of ascorbic acid on the pitting resistance of 316L stainless steel in synthetic tap water. Met. Mater. Int. 2016, 22, 621–629. [Google Scholar] [CrossRef]
- Liao, X.; Cao, F.; Zheng, L.; Liu, W.; Chen, A.; Zhang, J.; Cao, C. Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion; Macmillan: New York, NY, USA, 1992. [Google Scholar]
- Rosenfeld, I.; Marshakov, I. Mechanism of crevice corrosion. Corrosion 1964, 20, 115t–125t. [Google Scholar] [CrossRef]
- Modestov, A.; Zhou, G.-D.; Wu, Y.-P.; Notoya, T.; Schweinsberg, D. A study of the electrochemical formation of Cu (I)-BTA films on copper electrodes and the mechanism of copper corrosion inhibition in aqueous chloride/benzotriazole solutions. Corros. Sci. 1994, 36, 1931–1946. [Google Scholar] [CrossRef]


| Elements | Composition |
|---|---|
| Copper | 99.9 |
| Phosphorus | 0.015–0.04 |
| Carbon | 0004 |
| Silicon | 0.01 |
| Fe | 0.01 |
| Elements | Composition |
|---|---|
| Polyisobutylene (PIB) | 80–85 |
| Fatty ester | 15–20 |
| pH | Cl− | Mg2+ | Ca2+ | SO42− | BTA |
|---|---|---|---|---|---|
| 7.2 | 15.8 | 12.9 | 51.7 | 13.2 | 0, 540 |
| Specimen | CBTA (ppm) | Ecorr (mVSCE) | icorr (nA/cm2) | Eb (mVSCE) | IE% |
|---|---|---|---|---|---|
| Specimen 1 | 0 | −17.83 | 759.95 | - | - |
| 540 | 60.27 | 1.32 | 451.89 | 99.83 | |
| Specimen 2 | 0 | −2.83 | 351.51 | - | - |
| 540 | 56.87 | 0.76 | 544.14 | 99.78 |
| Specimen | CBTA (ppm) | Rs (Ω-cm2) | R1 (Ω-cm2) | CPE1 (F/cm2) | n1 | R2 (Ω-cm2) | CPE2 (F/cm2) | n2 | IE% |
|---|---|---|---|---|---|---|---|---|---|
| Specimen 1 | 0 | 2.49 × 102 | 1.86 × 103 | 2.22 × 10−5 | 0.66 | 2.72 × 104 | 9.00 × 10−5 | 0.51 | - |
| 540 | 4.35 × 102 | 8.14 × 106 | 1.51 × 10−6 | 0.96 | 2.94 × 107 | 4.59 × 10−7 | 0.66 | 99.92 | |
| Specimen 2 | 0 | 6.78 × 102 | 7.36 × 103 | 4.74 × 10−5 | 0.49 | 6.03 × 104 | 1.59 × 10−4 | 0.51 | - |
| 540 | 8.15 × 102 | 1.07 × 107 | 6.01 × 10−7 | 0.95 | 6.75 × 107 | 1.54 × 10−7 | 0.58 | 99.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Hong, M.-S.; Ko, S.-J.; Kim, J.-G. Effect of Benzotriazole on the Localized Corrosion of Copper Covered with Carbonaceous Residue. Materials 2021, 14, 2722. https://doi.org/10.3390/ma14112722
Lee Y-H, Hong M-S, Ko S-J, Kim J-G. Effect of Benzotriazole on the Localized Corrosion of Copper Covered with Carbonaceous Residue. Materials. 2021; 14(11):2722. https://doi.org/10.3390/ma14112722
Chicago/Turabian StyleLee, Yun-Ho, Min-Sung Hong, Sang-Jin Ko, and Jung-Gu Kim. 2021. "Effect of Benzotriazole on the Localized Corrosion of Copper Covered with Carbonaceous Residue" Materials 14, no. 11: 2722. https://doi.org/10.3390/ma14112722






