Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Agent
2.2. Animals and Treatment
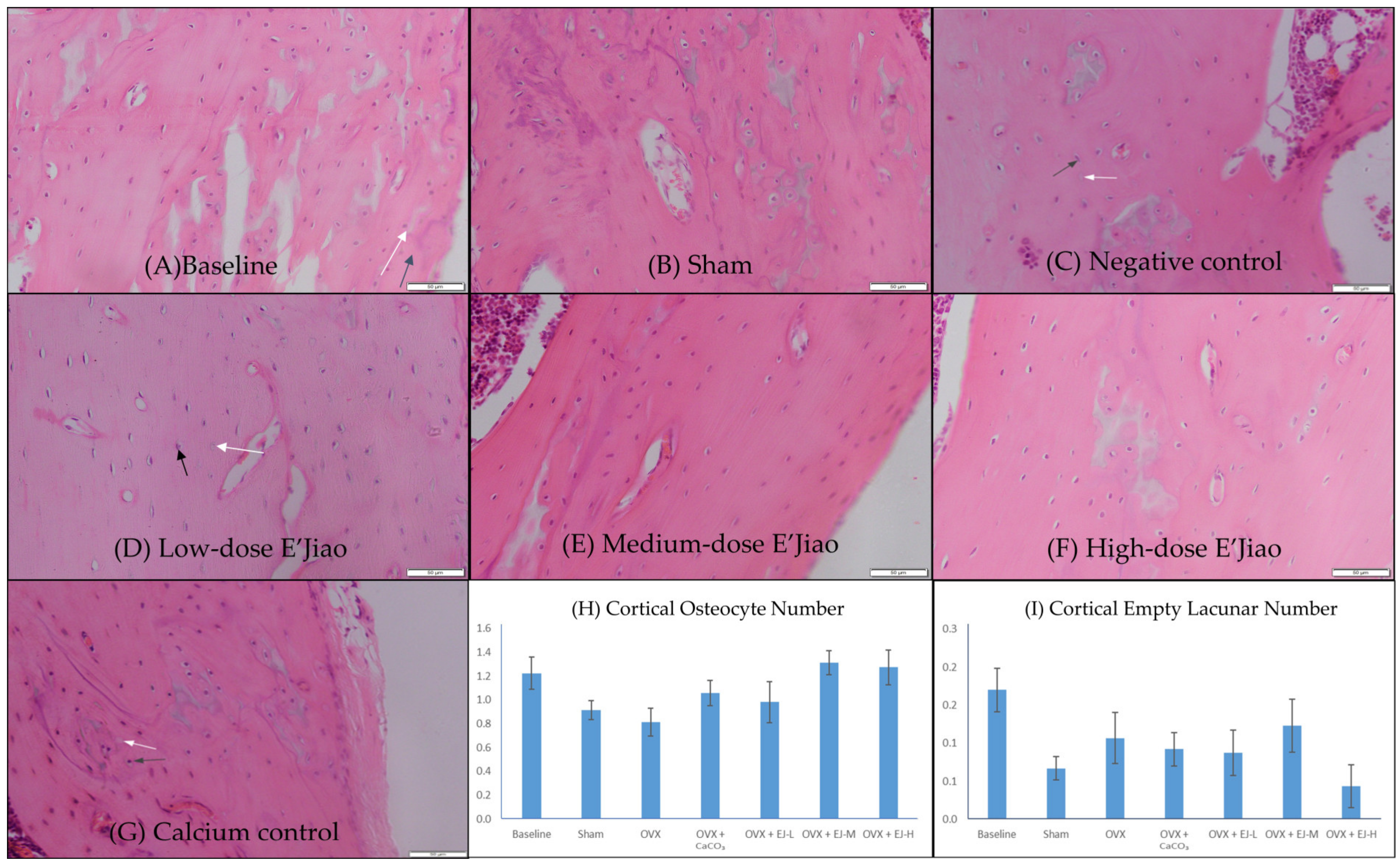
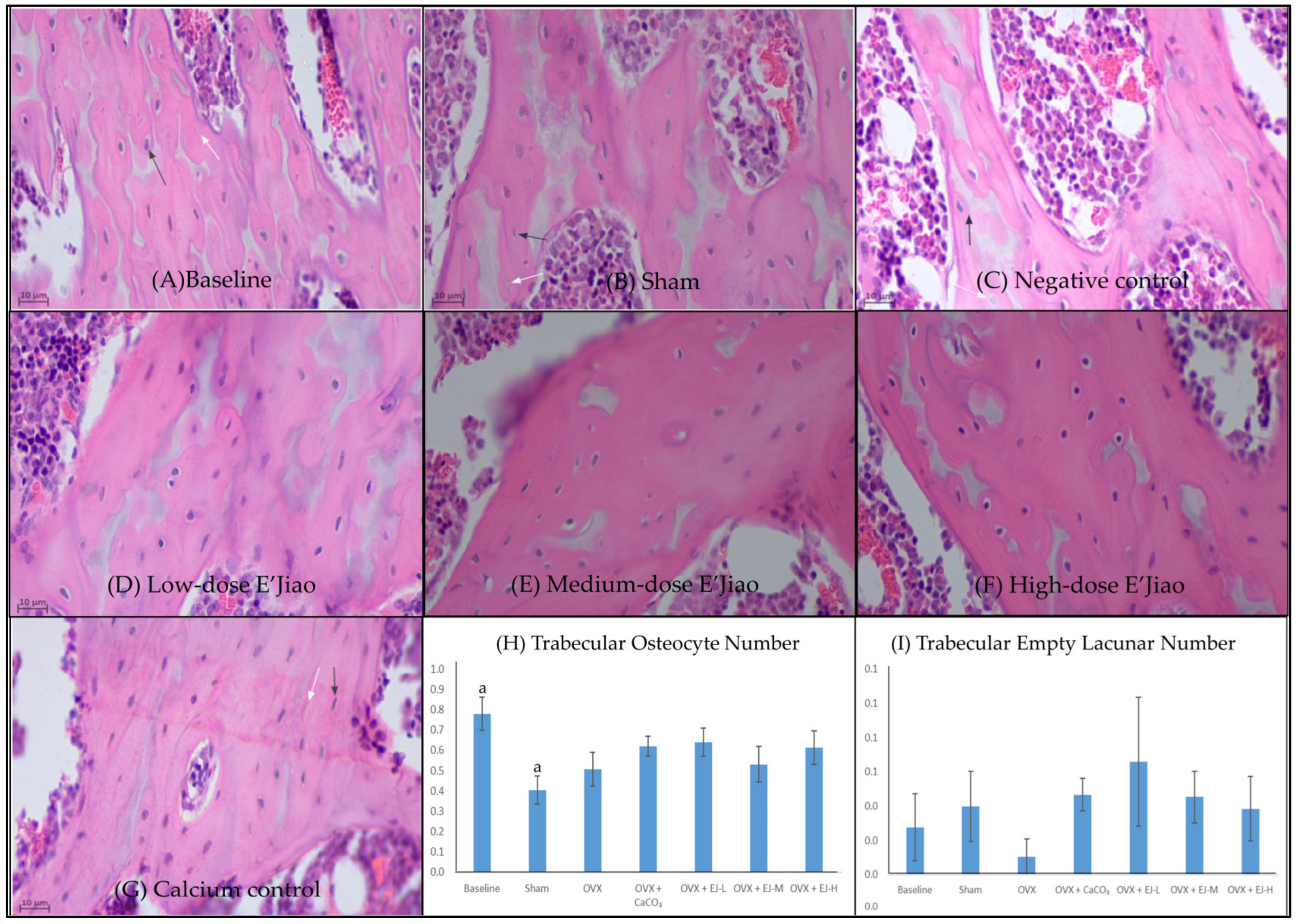
2.3. Measurement of Osteocyte and Empty Lacunar Number
2.4. FTIR Spectral Acquisition and Band Area Analysis
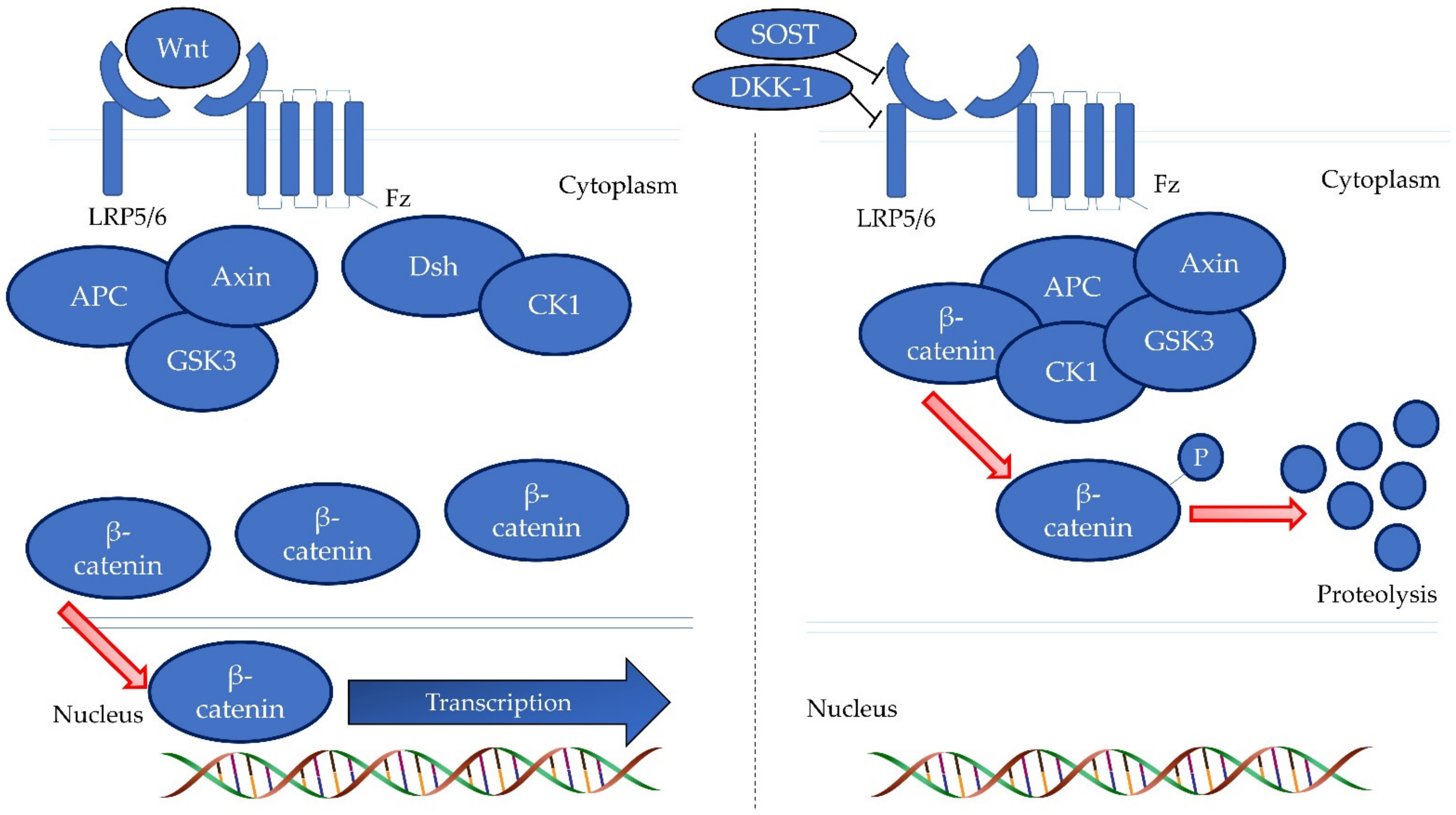
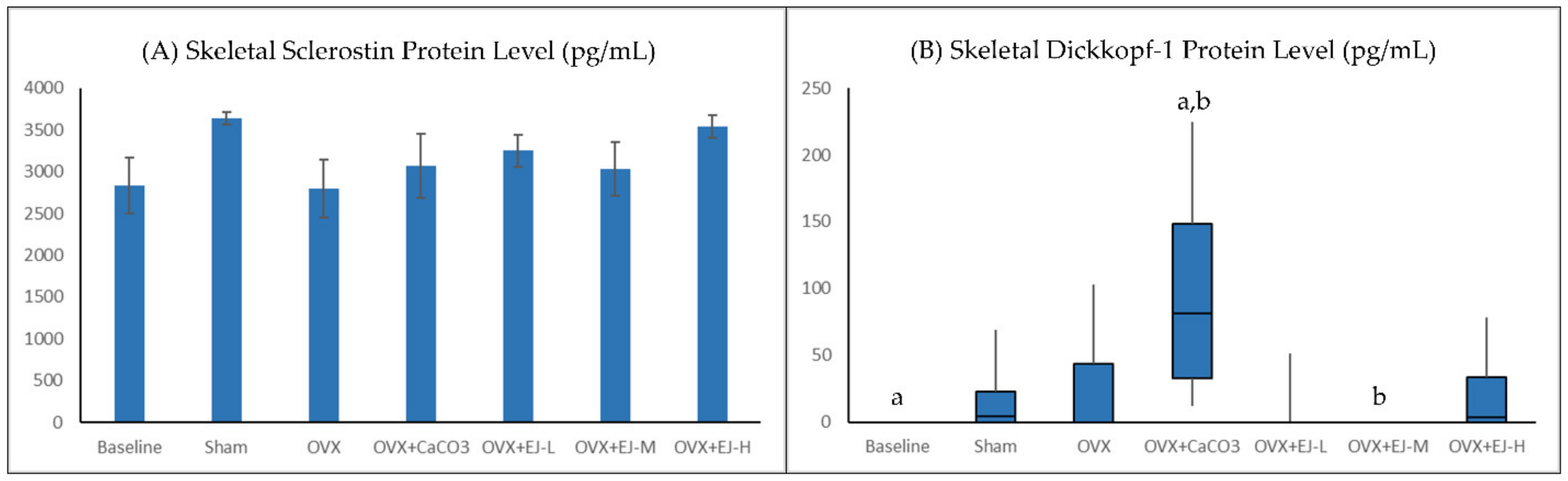
2.5. Measurement of WNT Inhibitors
2.6. Ethical Consideration
2.7. Statistical Analysis
3. Results
3.1. Osteocyte and Empty Lacunar Number
3.2. FTIR Analysis
3.3. Bone Biochemical Markers
4. Discussion
4.1. The Effects of Ovariectomy and E’Jiao on Osteocyte Parameters
4.2. The Effects of Ovariectomy and E’Jiao on Mineral/Matrix Ratio
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef]
- Alswat, K.A. Gender Disparities in Osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.h.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y. The Relationship between Follicle-stimulating Hormone and Bone Health: Alternative Explanation for Bone Loss beyond Oestrogen? Int. J. Med. Sci. 2018, 15, 1373–1383. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Adv. Musculoskelet Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef]
- Vig, S.; Fernandes, M.H. Bone Cell Exosomes and Emerging Strategies in Bone Engineering. Biomedicines 2022, 10, 767. [Google Scholar] [CrossRef]
- Chou, H.C.; Lin, S.Y.; Chou, L.Y.; Ho, M.L.; Chuang, S.C.; Cheng, T.L.; Kang, L.; Lin, Y.S.; Wang, Y.H.; Wei, C.W.; et al. Ablation of Discoidin Domain Receptor 1 Provokes an Osteopenic Phenotype by Regulating Osteoblast/Osteocyte Autophagy and Apoptosis. Biomedicines 2022, 10, 2173. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Weng, Y.; Ishihara, Y.; Odagaki, N.; Ei Hsu Hlaing, E.; Izawa, T.; Okamura, H.; Kamioka, H. Loading history changes the morphology and compressive force-induced expression of receptor activator of nuclear factor kappa B ligand/osteoprotegerin in MLO-Y4 osteocytes. PeerJ 2020, 8, e10244. [Google Scholar] [CrossRef]
- Guo, L.; Chen, K.; Yuan, J.; Huang, P.; Xu, X.; Li, C.; Qian, N.; Qi, J.; Shao, Z.; Deng, L.; et al. Estrogen inhibits osteoclasts formation and bone resorption via microRNA-27a targeting PPARgamma and APC. J. Cell Physiol. 2018, 234, 581–594. [Google Scholar] [CrossRef]
- Saad, F.A. Novel insights into the complex architecture of osteoporosis molecular genetics. Ann. N. Y. Acad. Sci. 2020, 1462, 37–52. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The regulation of bone metabolism and disorders by Wnt signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ekeuku, S.O.; Pang, K.-L. Sclerostin in the development of osteoarthritis: A mini review. Malays. J. Pathol. 2022, 44, 1–18. [Google Scholar]
- Ramli, F.F.; Chin, K.Y. A review of the potential application of osteocyte-related biomarkers, fibroblast growth factor-23, sclerostin, and Dickkopf-1 in predicting osteoporosis and fractures. Diagnostics 2020, 10, 145. [Google Scholar] [CrossRef]
- Baron, R.; Gori, F. Targeting WNT signaling in the treatment of osteoporosis. Curr. Opin. Pharmacol. 2018, 40, 134–141. [Google Scholar] [CrossRef]
- Fontalis, A.; Kenanidis, E.; Kotronias, R.A.; Papachristou, A.; Anagnostis, P.; Potoupnis, M.; Tsiridis, E. Current and emerging osteoporosis pharmacotherapy for women: State of the art therapies for preventing bone loss. Expert Opin. Pharm. 2019, 20, 1123–1134. [Google Scholar] [CrossRef]
- Palacios, S. Medical treatment of osteoporosis. Climacteric 2022, 25, 43–49. [Google Scholar] [CrossRef]
- Sheu, S.C.; Huang, J.Y.; Lien, Y.Y.; Lee, M.S. Specific, sensitive and rapid authentication of donkey-hide gelatine (Colla corii asini) in processed food using an isothermal nucleic acid amplification assay. J. Food Sci. Technol. 2020, 57, 2877–2883. [Google Scholar] [CrossRef]
- Wu, W.-J.; Li, L.-F.; Fung, H.-Y.; Cheng, H.-Y.; Kong, H.-Y.; Wong, T.-L.; Zhang, Q.-W.; Liu, M.; Bao, W.-R.; Huo, C.-Y.; et al. Qualitative and Quantitative Analysis of Ejiao-Related Animal Gelatins through Peptide Markers Using LC-QTOF-MS/MS and Scheduled Multiple Reaction Monitoring (MRM) by LC-QQQ-MS/MS. Molecules 2022, 27, 4643. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Che, X.; Ye, L.; Zhao, N.; Guo, D.; Peng, Y.; Lin, Y.; Liu, X. A Synergetic Strategy for Brand Characterization of Colla Corii Asini (Ejiao) by LIBS and NIR Combined with Partial Least Squares Discriminant Analysis. Molecules 2023, 28, 1778. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Hong, Z.; Gong, S.; Liu, W.; Zhou, X.; Sun, Y.; Qian, J.; Qu, H. Transcriptome profiling analysis reveals the potential mechanisms of three bioactive ingredients of Fufang E’jiao Jiang during chemotherapy-induced myelosuppression in mice. Front. Pharmacol. 2018, 9, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, T.; Gong, S.; Hong, Z.; Zhou, X.; Liu, H.; Qu, H.; Qian, J. RNA-sequencing based bone marrow cell transcriptome analysis reveals the potential mechanisms of E’jiao against blood-deficiency in mice. Biomed. Pharm. 2019, 118, 109291. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Lee, T.H.; Lee, H.P.; Li, T.M.; Lee, I.T.; Shieh, P.C.; Tang, C.H. Kuei-Lu-Er-Xian-Jiao extract enhances BMP-2 production in osteoblasts. Biomedicine 2017, 7, 2. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Chin, K.-Y.; Qian, J.; Zhang, Y.; Qu, H.; Mohd Ramli, E.S.; Wong, S.K.; Mohd Noor, M.M.; Ima-Nirwana, S. Suppression of high bone remodelling by E’Jiao in ovariectomised rats. Biomed. Pharm. 2022, 152, 113265. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared assessment of bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef]
- Cao, F.; Chen, Y.; Chang, C.; Ru, W.; Chen, J.; Zheng, Y.; Qian, L.; He, X.; Wang, D.; Zhu, J. Nutrient Compositions and Antioxidant Capacity of E’jiao Gao. J. Food Sci. Nut. 2019, 5, 43. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Effect of tocotrienol from Bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des. Dev. Ther. 2018, 12, 555–564. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. Effects of tocotrienol from Bixa orellana (annatto) on bone histomorphometry in a male osteoporosis model induced by buserelin. Biomed. Pharm. 2018, 103, 453–462. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Self-emulsified annatto tocotrienol improves bone histomorphometric parameters in a rat model of oestrogen deficiency through suppression of skeletal sclerostin level and RANKL/OPG ratio. Int. J. Med. Sci. 2021, 18, 3665–3673. [Google Scholar] [CrossRef]
- Imbert, L.; Gourion-Arsiquaud, S.; Villarreal-Ramirez, E.; Spevak, L.; Taleb, H.; van der Meulen, M.C.H.; Mendelsohn, R.; Boskey, A.L. Dynamic structure and composition of bone investigated by nanoscale infrared spectroscopy. PLoS ONE 2018, 13, e0202833. [Google Scholar] [CrossRef]
- Menges, F. SpectraGryph, Version 1.2.16.1. Available online: http://www.effemm2.de/spectragryph/ (accessed on 27 November 2022).
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Effects of tocotrienol on bone peptides in a rat model of osteoporosis induced by metabolic syndrome: The possible communication between bone cells. Int. J. Environ. Res. Public Health 2019, 16, 3313. [Google Scholar] [CrossRef]
- Emerton, K.B.; Hu, B.; Woo, A.A.; Sinofsky, A.; Hernandez, C.; Majeska, R.J.; Jepsen, K.J.; Schaffler, M.B. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone 2010, 46, 577–583. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Yang, D.; Bouxsein, M.L.; Saito, H.; Galvin, R.J.S.; Kuhstoss, S.A.; Thomas, C.C.; Schipani, E.; Baron, R.; et al. Suppression of Wnt Signaling by Dkk1 Attenuates PTH-Mediated Stromal Cell Response and New Bone Formation. Cell Metab. 2010, 11, 161–171. [Google Scholar] [CrossRef]
- Zammel, N.; Oudadesse, H.; Allagui, I.; Lefeuvre, B.; Rebai, T.; Badraoui, R. Evaluation of lumbar vertebrae mineral composition in rat model of severe osteopenia: A Fourier Transform Infrared Spectroscopy (FTIR) analysis. Vib. Spectrosc. 2021, 115, 103279. [Google Scholar] [CrossRef]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Mark-Lee, W.F.; Chong, Y.Y.; Qian, J.; Zhang, Y.; et al. Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats. Life 2023, 13, 570. https://doi.org/10.3390/life13020570
Chin K-Y, Ng BN, Rostam MKI, Muhammad Fadzil NFD, Raman V, Mohamed Yunus F, Mark-Lee WF, Chong YY, Qian J, Zhang Y, et al. Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats. Life. 2023; 13(2):570. https://doi.org/10.3390/life13020570
Chicago/Turabian StyleChin, Kok-Yong, Ben Nett Ng, Muhd Khairik Imran Rostam, Nur Farah Dhaniyah Muhammad Fadzil, Vaishnavi Raman, Farzana Mohamed Yunus, Wun Fui Mark-Lee, Yan Yi Chong, Jing Qian, Yan Zhang, and et al. 2023. "Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats" Life 13, no. 2: 570. https://doi.org/10.3390/life13020570
APA StyleChin, K.-Y., Ng, B. N., Rostam, M. K. I., Muhammad Fadzil, N. F. D., Raman, V., Mohamed Yunus, F., Mark-Lee, W. F., Chong, Y. Y., Qian, J., Zhang, Y., Qu, H., Syed Hashim, S. A., & Ekeuku, S. O. (2023). Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats. Life, 13(2), 570. https://doi.org/10.3390/life13020570







