Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations
Abstract
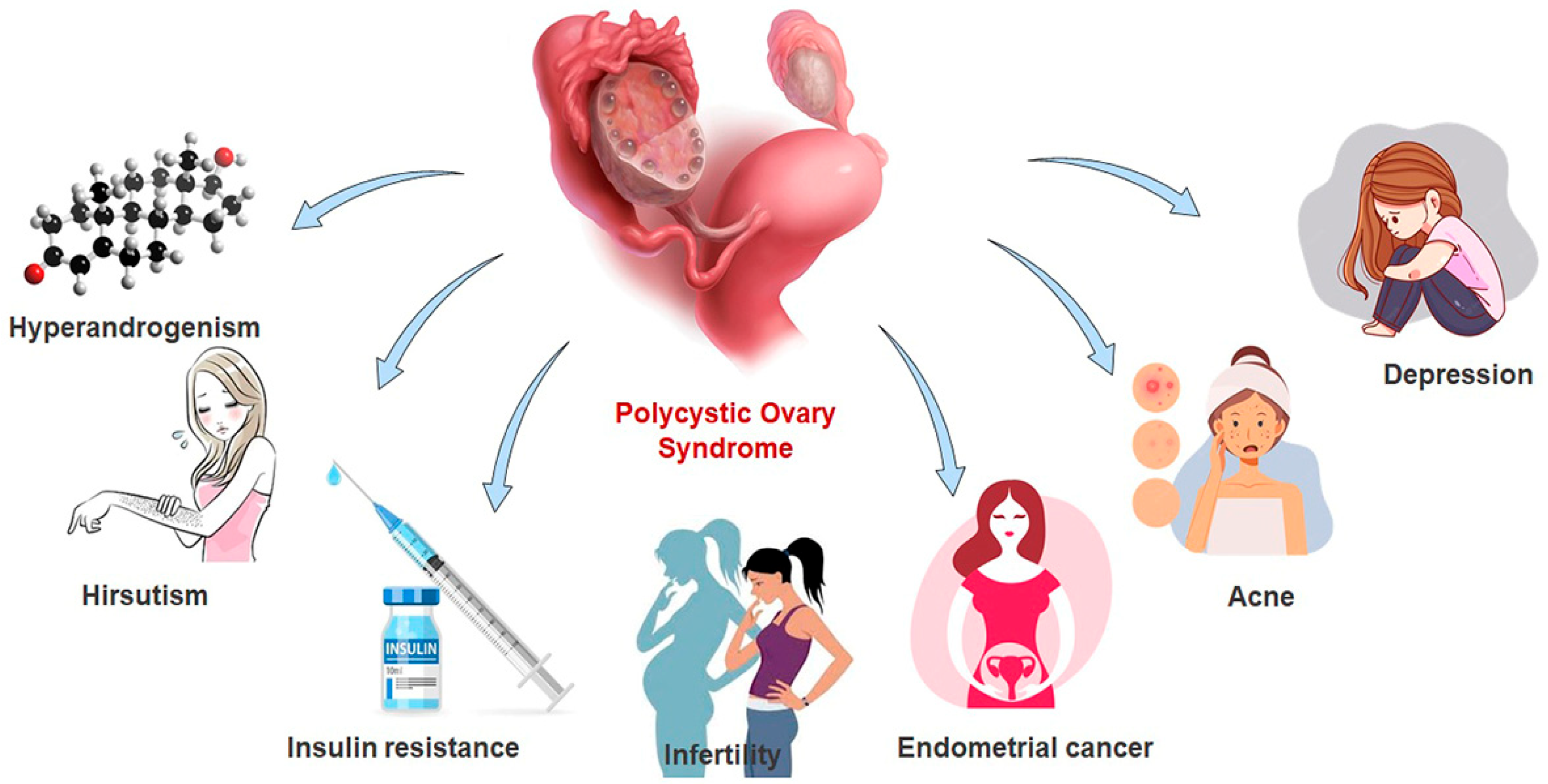
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Data Extraction
2.3. Clinical Diagnosis and Criteria
3. Results and Discussion
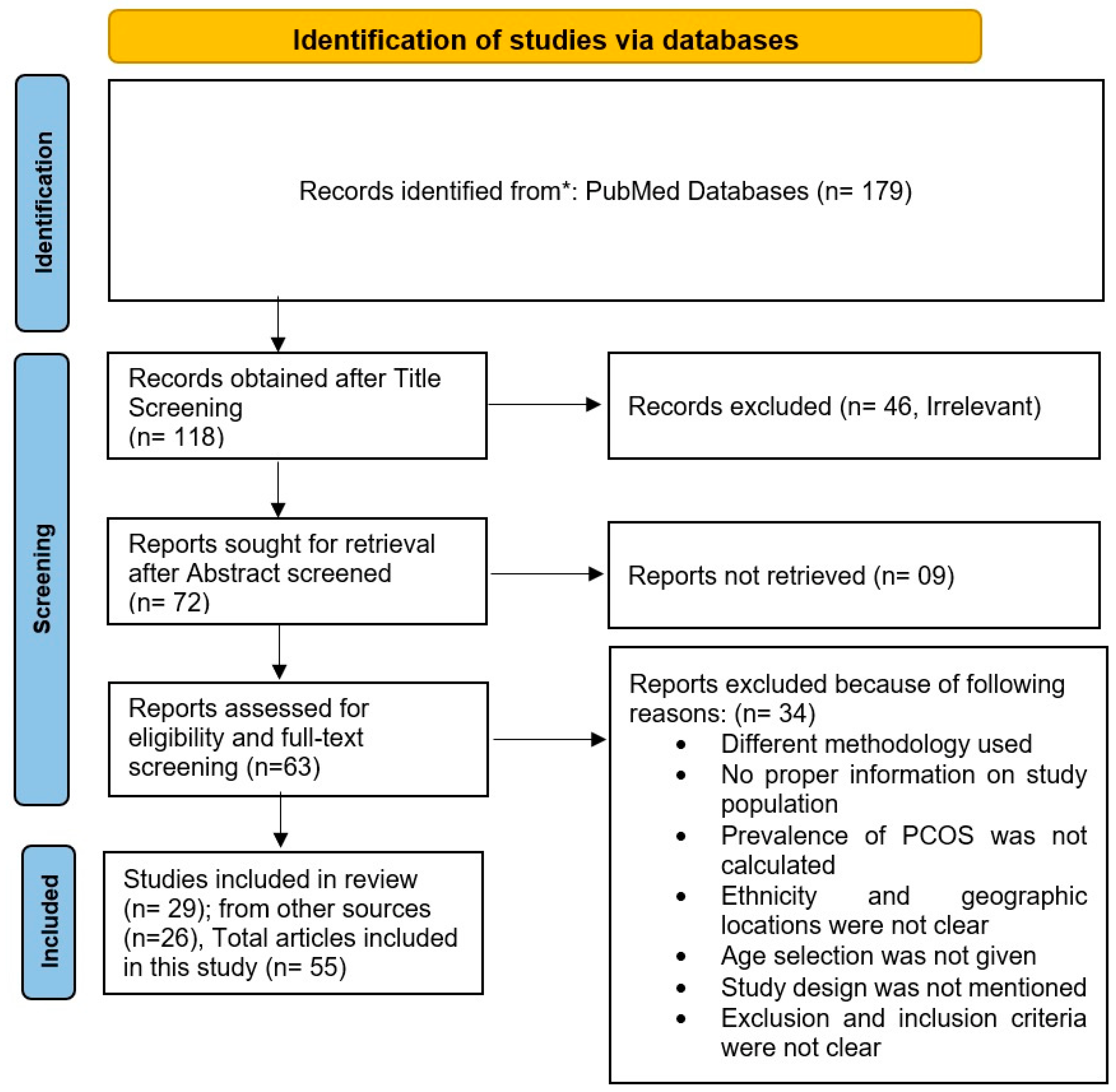
3.1. Search Results
3.2. Epidemiology in Different Ethnicities
3.3. Prevalence in Europe, America, and Australia
3.4. Prevalence in Iran
3.5. Prevalence in Asia
3.6. Prevalence in India
4. Management
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Azziz, R.; Adashi, E.Y. Stein and Leventhal: 80 years on. Am. J. Obstet. Gynecol. 2016, 214, 247.e1–247.e11. [Google Scholar] [CrossRef]
- Senaldi, L.; Gopi, R.P.; Milla, S.; Shah, B. Is ultrasound useful in the diagnosis of adolescents with polycystic ovary syndrome? J. Pediatr. Endocrinol. Metab. 2015, 28, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; E Oberfield, S.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, J.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Ganie, M.A.; Vasudevan, V.; Wani, I.A.; Baba, M.S.; Arif, T.; Rashid, A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J. Med. Res. 2019, 150, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Clayton, W.; Lipton, M.; Elford, J.; Rustin, M.; Sherr, L. A randomized controlled trial of laser treatment among hirsute women with polycystic ovary syndrome. Br. J. Dermatol. 2005, 152, 986–992. [Google Scholar] [CrossRef]
- Boomsma, C.; Eijkemans, M.; Hughes, E.; Visser, G.; Fauser, B.; Macklon, N. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Updat. 2006, 12, 673–683. [Google Scholar] [CrossRef]
- Harris, H.R.; Terry, K.L. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: A systematic review. Fertil. Res. Pr. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Lim, S.; Davies, M.; Norman, R.; Moran, L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2012, 18, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Kunselman, A.R.; Dodson, W.C.; Dunaif, A. Prevalence and Predictors of Risk for Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in Polycystic Ovary Syndrome: A Prospective, Controlled Study in 254 Affected Women. J. Clin. Endocrinol. Metab. 1999, 84, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Cífková, R.; Fanta, M.; Poledne, R.; Živný, J.; Skibová, J. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum. Reprod. 2000, 15, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Kim, H.O.; Cha, S.W.; Park, C.W.; Kim, J.Y.; Yang, K.M.; Song, I.O.; Koong, M.K.; Kang, I.S. Adverse pregnancy outcomes with assisted reproductive technology in non-obese women with polycystic ovary syndrome: A case-control study. Clin. Exp. Reprod. Med. 2011, 38, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Legro, R.S.; Kunselman, A.R.; Dunaif, A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am. J. Med. 2001, 111, 607–613. [Google Scholar] [CrossRef]
- Carmina, E.; Chu, M.C.; Longo, R.A.; Rini, G.B.; Lobo, R.A. Phenotypic Variation in Hyperandrogenic Women Influences the Findings of Abnormal Metabolic and Cardiovascular Risk Parameters. J. Clin. Endocrinol. Metab. 2005, 90, 2545–2549. [Google Scholar] [CrossRef]
- Romitti, M.; Fabris, V.C.; Ziegelmann, P.K.; Maia, A.L.; Spritzer, P.M. Association between PCOS and autoimmune thyroid disease: A systematic review and meta-analysis. Endocr. Connect. 2018, 7, 1158–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charalampakis, V.; Tahrani, A.A.; Helmy, A.; Gupta, J.K.; Singhal, R. Polycystic ovary syndrome and endometrial hyperplasia: An overview of the role of bariatric surgery in female fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 220–226. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Boyle, J.A.; Garad, R.M.; McAllister, V.; Downes, L.; Gibson-Helm, M.; Hart, R.J.; Rombauts, L.; Moran, L.; et al. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2018; Monash University: Melbourne, Australia, 2018. [Google Scholar] [CrossRef] [Green Version]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.; Futterweit, W.; Janssen, O.E.; Legro, R.; Norman, R.; Taylor, A.E.; et al. Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Azziz, R.; Marin, C.; Hoq, L.; Badamgarav, E.; Song, P. Health Care-Related Economic Burden of the Polycystic Ovary Syndrome during the Reproductive Life Span. J. Clin. Endocrinol. Metab. 2005, 90, 4650–4658. [Google Scholar] [CrossRef] [Green Version]
- Futterweit, W.; Dunaif, A.; Yeh, H.-C.; Kingsley, P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J. Am. Acad. Dermatol. 1988, 19, 831–836. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, M.P.; Bentzen, J.G.; Pinborg, A.; Loft, A.; Forman, J.L.; Thuesen, L.L.; Cohen, A.; Hougaard, D.M.; Andersen, A.N. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum. Reprod. 2014, 29, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Ovalle, F.; Azziz, R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil. Steril. 2002, 77, 1095–1105. [Google Scholar] [CrossRef]
- Sendur, S.N.; Yildiz, B.O. Influence of ethnicity on different aspects of polycystic ovary syndrome: A systematic review. Reprod. Biomed. Online 2020, 42, 799–818. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Knight, S.; White, V.; Claridge, C.; Davis, B.J.; Bell, R. Preliminary indication of a high prevalence of polycystic ovary syndrome in indigenous Australian women. Gynecol. Endocrinol. 2002, 16, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiao, J. Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids 2013, 78, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.M.; Wattick, R.A.; Kinkade, O.N.; Olfert, M.D. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef] [Green Version]
- Vutyavanich, T.; Khaniyao, V.; Wongtra-Ngan, S.; Sreshthaputra, O.; Sreshthaputra, R.; Piromlertamorn, W. Clinical, endocrine and ultrasonographic features of polycystic ovary syndrome in Thai women. J. Obstet. Gynaecol. Res. 2007, 33, 677–680. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, J.; Teede, H.J. Polycystic ovary syndrome—An update. Aust. Fam. Physician 2012, 41, 752–756. [Google Scholar]
- Yildiz, B.O. Diagnosis of hyperandrogenism: Clinical criteria. Best Pr. Res. Clin. Endocrinol. Metab. 2006, 20, 167–176. [Google Scholar] [CrossRef]
- Mitrašinović-Brulić, M.; Buljan, M.; Suljević, D. Association of LH/FSH ratio with menstrual cycle regularity and clinical features of patients with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2021, 26, 1–9. [Google Scholar] [CrossRef]
- Barbosa, G.; de Sá, L.B.P.C.; Rocha, D.R.T.W.; Arbex, A.K. Polycystic Ovary Syndrome (PCOS) and Fertility. Open J. Endocr. Metab. Dis. 2016, 06, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Polycystic Ovary Syndrome (PCOS) 2012. Bethes-da (MD): National Institutes of Health Library (US). 405p. Available online: http://prevention.nih.gov/workshops/2012/pcos/default.aspx (accessed on 12 November 2022).
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kenigsberg, L.E.; Agarwal, C.; Sin, S.; Shifteh, K.; Isasi, C.R.; Crespi, R.; Ivanova, J.; Coupey, S.M.; Heptulla, R.A.; Arens, R. Clinical utility of magnetic resonance imaging and ultrasonography for diagnosis of polycystic ovary syndrome in adolescent girls. Fertil. Steril. 2015, 104, 1302–1309.e4. [Google Scholar] [CrossRef] [Green Version]
- Moran, L.J.; Tassone, E.C.; Boyle, J.; Brennan, L.; Harrison, C.L.; Hirschberg, A.L.; Lim, S.; Marsh, K.; Misso, M.L.; Redman, L.; et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Lifestyle management. Obes. Rev. 2020, 21, e13046. [Google Scholar] [CrossRef]
- Kousta, E.; Tolis, G.; Franks, S. Polycystic ovary syndrome. Revised diagnostic criteria and long-term health consequences. Hormones 2005, 4, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Okoroh, E.M.; Hooper, W.C.; Atrash, H.K.; Yusuf, H.R.; Boulet, S.L. Prevalence of polycystic ovary syndrome among the privately insured, United States, 2003-2008. Am. J. Obstet. Gynecol. 2012, 207, 299.e1–299.e7. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2009, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Asunción, M.; Calvo, R.M.; Millá;n, J.L.S.; Sancho, J.; Avila, S.; Escobar-Morreale, H.F. A Prospective Study of the Prevalence of the Polycystic Ovary Syndrome in Unselected Caucasian Women from Spain1. J. Clin. Endocrinol. Metab. 2000, 85, 2434–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A Survey of the Polycystic Ovary Syndrome in the Greek Island of Lesbos: Hormonal and Metabolic Profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef] [PubMed]
- Sanchón, R.; Gambineri, A.; Alpañés, M.; García, M.M.; Pasquali, R.; Escobar-Morreale, H.F. Prevalence of functional disorders of androgen excess in unselected premenopausal women: A study in blood donors. Hum. Reprod. 2012, 27, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, L.G. Síndrome dos Ovários Policísticos: Uma Abordagem Epidemiológica. Master’s Thesis, Sistema Universi-tário de Bibliotecas UFBA, Salvador, Brazil, 2013. [Google Scholar]
- Moran, C.; Tena, G.; Moran, S.; Ruiz, P.; Reyna, R.; Duque, X. Prevalence of Polycystic Ovary Syndrome and Related Disorders in Mexican Women. Gynecol. Obstet. Investig. 2010, 69, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Kar, S.; Vanky, E.; Morin-Papunen, L.; Piltonen, T.; Puurunen, J.; Tapanainen, J.S.; Maciel, G.A.R.; Hayashida, S.A.Y.; Soares, J.M.; et al. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: A regional cross-sectional study. Am. J. Obstet. Gynecol. 2017, 217, 189.e1–189.e8. [Google Scholar] [CrossRef] [Green Version]
- Jalilian, A.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Khodaee, Z.; Akbari, M. Prevalence of polycystic ovary syndrome and its asso-ciated complications in Iranian women: A meta-analysis. Iran J. Reprod. Med. 2015, 13, 591–604. [Google Scholar]
- Dargham, S.R.; Ahmed, L.; Kilpatrick, E.S.; Atkin, S.L. The prevalence and metabolic characteristics of polycystic ovary syndrome in the Qatari population. PLoS ONE 2017, 12, e0181467. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Choi, Y.M. Phenotype and genotype of polycystic ovary syndrome in Asia: Ethnic differences. J. Obstet. Gynaecol. Res. 2019, 45, 2330–2337. [Google Scholar] [CrossRef]
- Ding, T.; Hardiman, P.J.; Petersen, I.; Wang, F.-F.; Qu, F.; Baio, G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: A systematic review and meta-analysis. Oncotarget 2017, 8, 96351–96358. [Google Scholar] [CrossRef] [Green Version]
- Kumarapeli, V.; Seneviratne, R.D.A.; Wijeyaratne, C.N.; Yapa, R.M.S.C.; Dodampahala, S.H. A Simple Screening Approach for Assessing Community Prevalence and Phenotype of Polycystic Ovary Syndrome in a Semiurban Population in Sri Lanka. Am. J. Epidemiol. 2008, 168, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Mani, H.; Davies, M.J.; Bodicoat, D.H.; Levy, M.J.; Gray, L.J.; Howlett, T.A.; Khunti, K. Clinical characteristics of polycystic ovary syndrome: Investigating differences in White and South Asian women. Clin. Endocrinol. 2015, 83, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Deswal, R.; Nanda, S.; Ghalaut, V.S.; Roy, P.S.; Dang, A.S. Cross-sectional study of the prevalence of polycystic ovary syndrome in rural and urban populations. Int. J. Gynecol. Obstet. 2019, 146, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, R.V.; Swetha, S.; Neerajaa, J.; Madhavica, J.V.; Janani, D.M.; Rekha, S.; Ramya, S.; Usha, B. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertil. Soc. J. 2017, 22, 313–316. [Google Scholar] [CrossRef]
- Rodin, D.A.; Bano, G.; Bland, J.M.; Taylor, K.; Nussey, S.S. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin. Endocrinol. 1998, 49, 91–99. [Google Scholar] [CrossRef]
- Sirmans, S.; Pate, K. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.Z.; Pang, L.H.; Li, M.J.; Fan, X.J.; Huang, R.D.; Chen, H.Y. Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2013, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, R.J.; Davies, M.; Lord, J.; Moran, L.J. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol. Metab. 2002, 13, 251–257. [Google Scholar] [CrossRef]
- Zain, M.M.; Jamaluddin, R.; Ibrahim, A.; Norman, R.J. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: A randomized controlled trial. Fertil. Steril. 2009, 91, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Kriedt, K.J.; Alchami, A.; Davies, M.C. PCOS: Diagnosis and management of related infertility. Obstet. Gynaecol. Reprod. Med. 2018, 29, 1–5. [Google Scholar] [CrossRef]
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Fossum, G.; Gitlin, S.; Gracia, C.; Hansen, K.; La Barbera, A.; et al. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): A guideline. Fertil. Steril. 2017, 108, 426–441. [Google Scholar] [CrossRef] [Green Version]
- Mejia, R.B.; Summers, K.M.; Kresowik, J.D.; Van Voorhis, B.J. A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil. Steril. 2019, 111, 571–578.e1. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.W.; Legro, R.S. Longterm management of Polycystic Ovarian Syndrome (PCOS). Mol. Cell. Endocrinol. 2012, 373, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farquhar, C.; Brown, J.; Marjoribanks, J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst. Rev. 2012, CD001122. [Google Scholar] [CrossRef] [PubMed]
- Dokras, A.; Stener-Victorin, E.; Yildiz, B.O.; Li, R.; Ottey, S.; Shah, D.; Epperson, N.; Teede, H. Androgen Excess- Polycystic Ovary Syndrome Society: Position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 2018, 109, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, A.; Kokanali, M.K.; Guzel, A.I.; Kara, A.; Topcu, H.O.; Cavkaytar, S. Polycystic Ovary Syndrome and Risk of Endometrial Cancer: A Mini-Review. Asian Pac. J. Cancer Prev. 2014, 15, 7011–7014. [Google Scholar] [CrossRef] [PubMed]

| Diagnostic Consensus | National Institutes of Health (NIH), 1990 [36] | Rotterdam, 2003 [37] | AE-PCOS Society, 2006 [20,23] | NHMRC-Evidence-Based Methodology Workshop Panel on PCOS, 2018 [19] |
|---|---|---|---|---|
| Symptomatic characteristics | i. Clinical or Biochemical hyperandrogenism, ii. Ovulation disorder (amenorrhea/oligomenorrhea) in the absence of non-classical adrenal hyperplasia | i. Androgen excess/hyperandrogenism ii. Oligo-ovulation or anovulation, Polycystic appearance of the ovaries on ultrasonography | i. Androgen excess ii. Ovarian dysfunction | i. Hyperandrogenism ii. Oligo-and/or anovulation iii. Polycystic appearance of the ovaries on ultrasonography |
| Criteria considered | Both criteria are considered | Considers any two of three criteria | Both criteria are required | i. Any two of three criteria are considered to identify sub-phenotypes ii. Polycystic ovaries on USG are required. |
| Parameters | Phenotype A | Phenotype B | Phenotype C | Phenotype D |
|---|---|---|---|---|
| HA | + | + | + | _ |
| OD | + | + | _ | + |
| PCOM | + | _ | + | + |
| Geographic Region | Ethnicity | Sample Size | Objectives | Findings | Consensus | Reference |
|---|---|---|---|---|---|---|
| Cross-Sectional Study | ||||||
| Spain | Caucasian | 154 women | Assessment of PCOS prevalence in an unbiased manner | PCOS prevalence rate of 6.5% | NIH Criteria | [43] |
| Mexico | Hispanic | 150 women | Assessment of PCOS prevalence | PCOS prevalence rate in Mexican women is similar to other populations but lower than reported in Mexican American women | NIH and Rotterdam criteria | [47] |
| Sri Lanka | South Asian | 3030 women | Assessment of PCOS prevalence and symptoms, large community-based | Oligo/amenorrhea and polycystic ovaries (91.4%) oligo/amenorrhea and hirsutism (48.3%) | Rotterdam criteria | [53] |
| Retrospective Study | ||||||
| Australia | Caucasian | 728 women | Assessment of PCOS prevalence with large cohort study | The Rotterdam and AES prevalence estimates were up to twice that obtained with the NIH criteria | NIH, Rotterdam, and the AES criteria | [42] |
| Meta-analysis | ||||||
| Iran | Iranian | 30 studies | Reviewed the prevalence of PCOS and its associated complications | PCOS prevalence rate in Iran is not high | NIH criteria, Rotterdam criteria & Ultrasound investigation | [49] |
| Different sources and regions | Caucasian, Middle Eastern, Chinese, and African American | 42 studies | Investigation four major contrasting ethnic groups | PCOS prevalence rate in Chinese women was lowest than in black woman | NIH, Rotterdam, and the AES criteria | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmin, A.; Roychoudhury, S.; Paul Choudhury, A.; Ahmed, A.B.F.; Dutta, S.; Mottola, F.; Verma, V.; Kalita, J.C.; Kumar, D.; Sengupta, P.; et al. Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations. Life 2022, 12, 1974. https://doi.org/10.3390/life12121974
Yasmin A, Roychoudhury S, Paul Choudhury A, Ahmed ABF, Dutta S, Mottola F, Verma V, Kalita JC, Kumar D, Sengupta P, et al. Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations. Life. 2022; 12(12):1974. https://doi.org/10.3390/life12121974
Chicago/Turabian StyleYasmin, Afrin, Shubhadeep Roychoudhury, Arun Paul Choudhury, A. B. Fuzayel Ahmed, Sulagna Dutta, Filomena Mottola, Vivek Verma, Jogen C. Kalita, Dhruv Kumar, Pallav Sengupta, and et al. 2022. "Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations" Life 12, no. 12: 1974. https://doi.org/10.3390/life12121974
APA StyleYasmin, A., Roychoudhury, S., Paul Choudhury, A., Ahmed, A. B. F., Dutta, S., Mottola, F., Verma, V., Kalita, J. C., Kumar, D., Sengupta, P., & Kolesarova, A. (2022). Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations. Life, 12(12), 1974. https://doi.org/10.3390/life12121974









