Kastoria and Mikri Prespa Lakes: The Impact of Anthropogenic Activities on the Differentiation in the Genotoxic and Toxic Profile of the Surface Water
Abstract
1. Introduction
2. Study Areas, Materials, and Methods
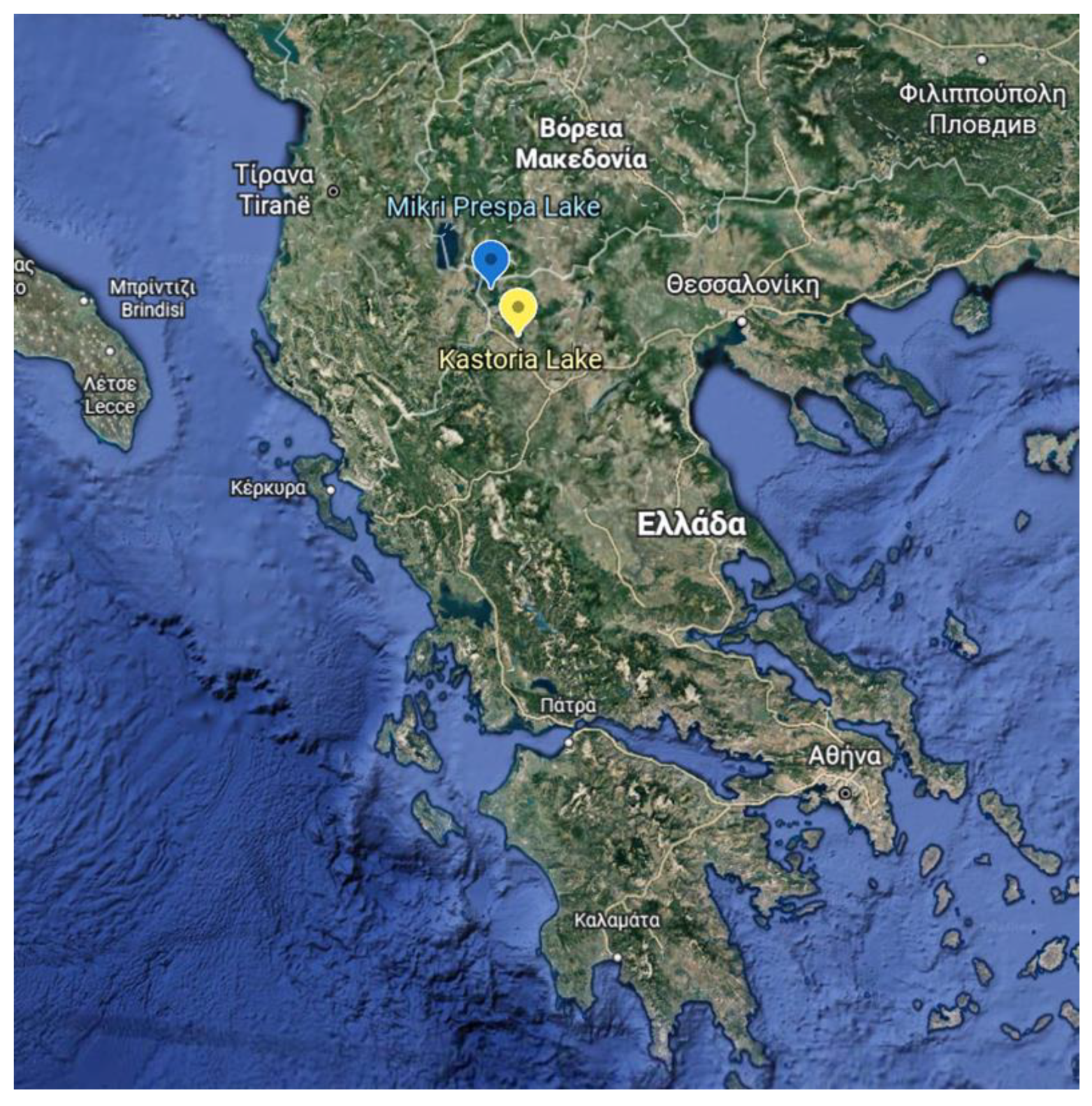
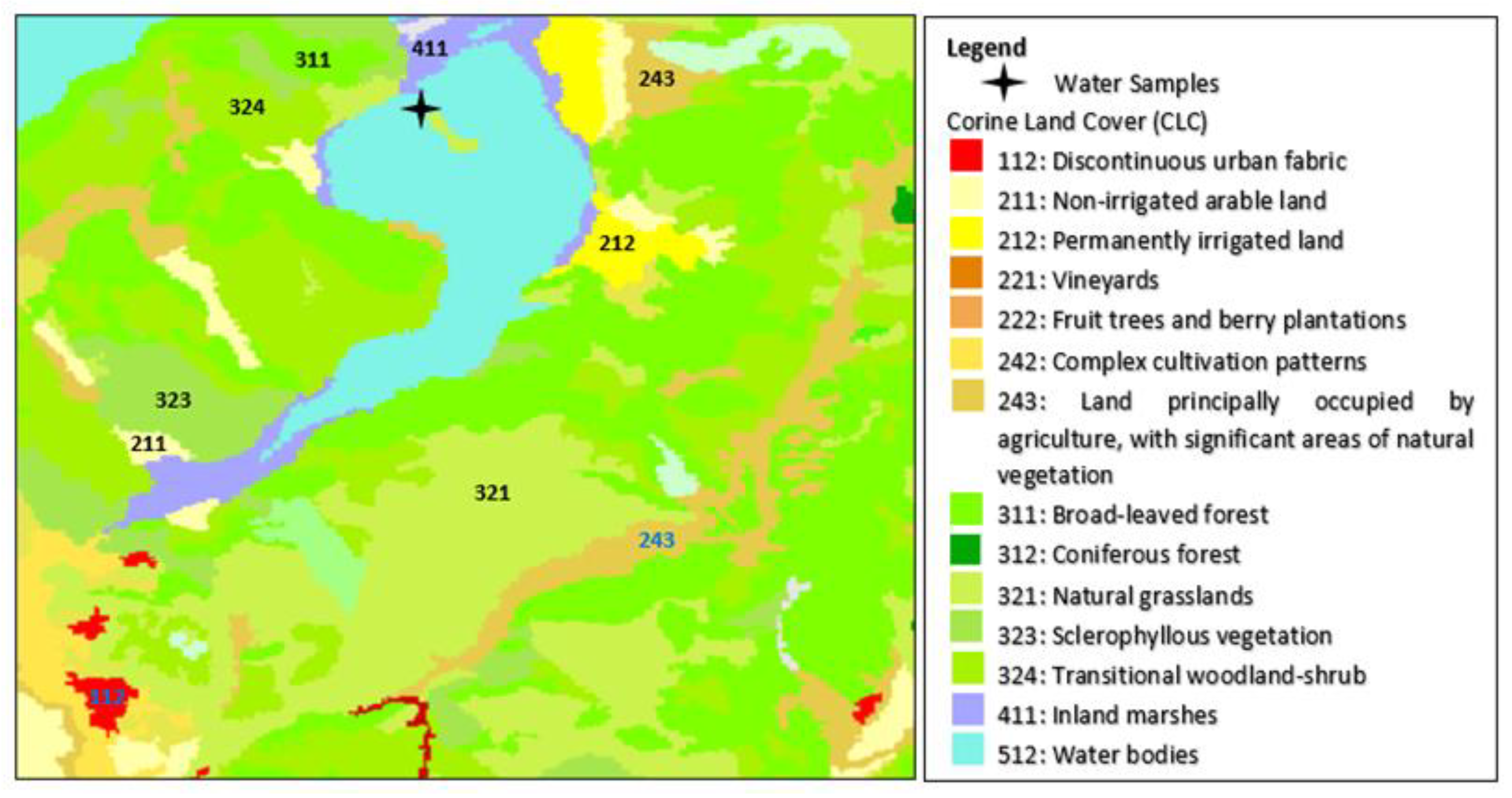
2.1. Study Areas
2.2. Water Samples Collection
2.3. Reagents
2.4. Physicochemical Analysis
2.5. ICP-MS/MS Analysis
2.6. Genotoxic and Cytotoxic Effects on Human Lymphocyte Cultures
2.6.1. Ethic Statement
2.6.2. Experimental Procedure
2.7. Aliivibrio Fischeri Toxicity Test (Microtox Assay)
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Analysis
3.2. Element Concentration via ICP-MS/MS
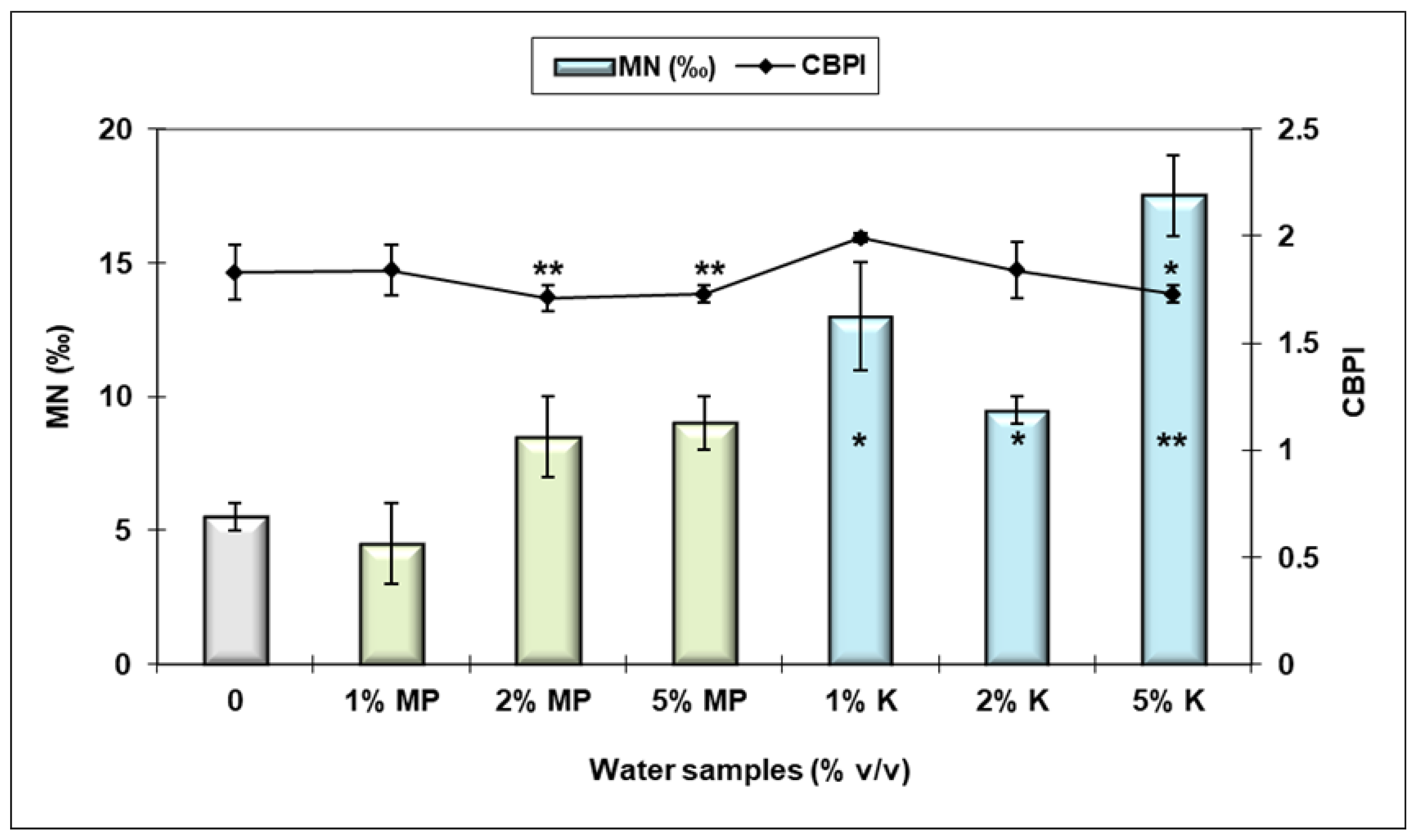
3.3. Genotoxic and Cytotoxic Effects Assessment
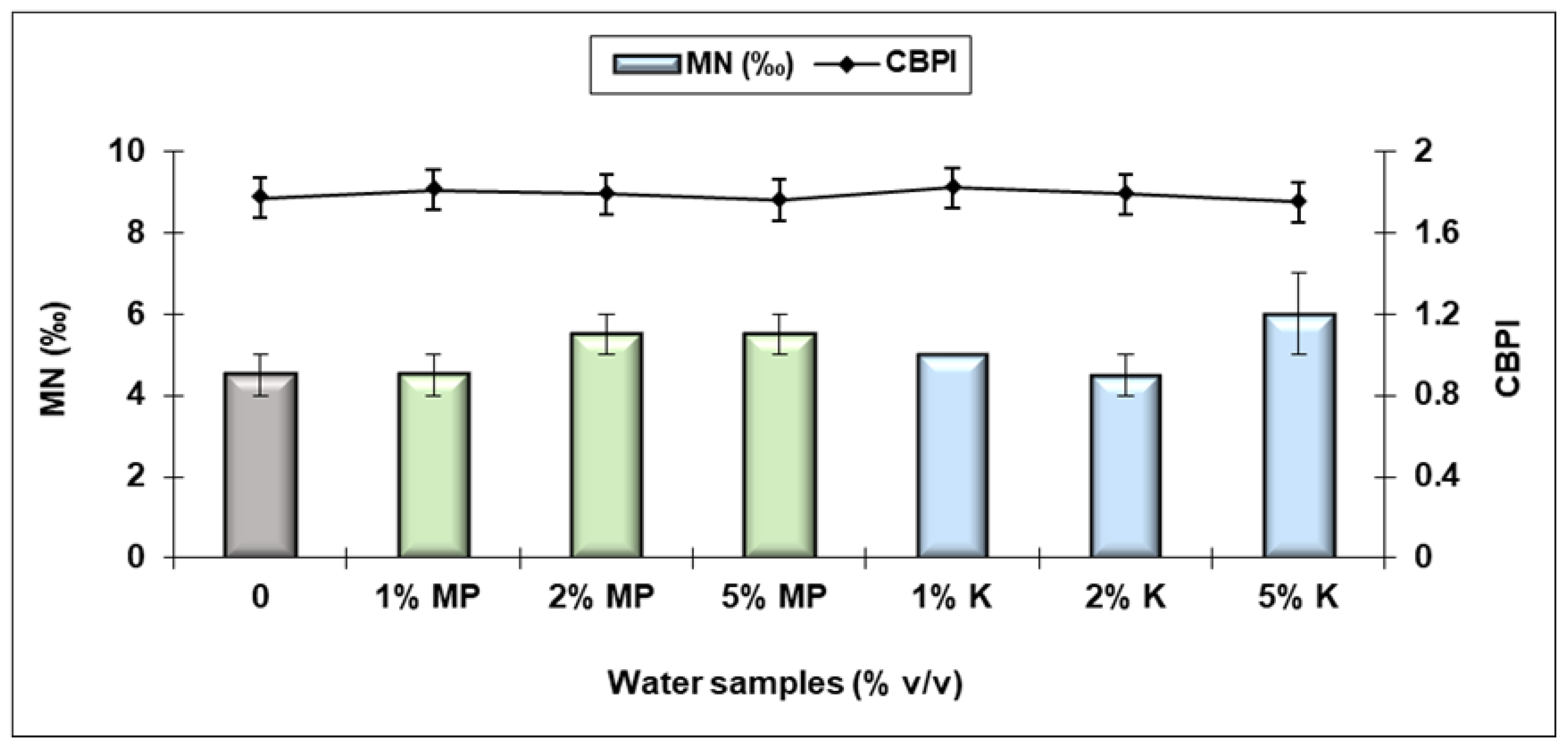
3.4. Ecological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-García, J.Y.; Ramírez-Gutiérrez, A.G.; Núñez-Ríos, J.E.; Cardoso-Castro, P.P.; Rojas, O.G. Systems thinking approach to sustainable performance in RAMSAR sites. Sustainability. 2019, 11, 6469. [Google Scholar] [CrossRef]
- Kosma, C.; Triantafyllidis, V.; Zotos, A.; Pittaras, A.; Kouneli, V.; Karydogianni, S.; Mavroeidis, A.; Kakabouki, I.; Beslemes, D.; Tigka, E.L.; et al. Assessing Spatial Variability of Soil Properties in Mediterranean Smallholder Farming Systems. Land 2022, 11, 557. [Google Scholar] [CrossRef]
- Romanelli, A.; Esquius, K.S.; Massone, H.E.; Escalante, A.H. GIS-based pollution hazard mapping and assessment framework of shallow lakes: Southeastern Pampean lakes (Argentina) as a case study. Environ. Monit. Assess. 2013, 185, 6943–6961. [Google Scholar] [CrossRef]
- Gezie, A.; Anteneh, W.; Dejen, E.; Mereta, S.T. Effects of human-induced environmental changes on benthic macroinvertebrate assemblages of wetlands in Lake Tana Watershed, Northwest Ethiopia. Environ. Monit. Assess. 2017, 189, 152. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Fulton, R.S. Ecology of harmful cyanobacteria. In Ecology of Harmful Algae. Ecological Studies; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 95–109. [Google Scholar]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water, 1st ed.; Chorus, I., Bartram, J., Eds.; E. & F.N. Spon: London, UK, 1999; pp. 41–110. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Vardaka, E.; Lanaras, T. Toxic cyanobacteria in Greek freshwaters, 1987-2000: Occurrence, toxicity, and impacts in the Mediterranean region. Acta Hydrochim. Hydrobiol. 2004, 32, 107–124. [Google Scholar] [CrossRef]
- Vardaka, E.; Moustaka-Gouni, M.; Cook, C.M.; Lanaras, T. Cyanobacterial blooms and water quality in Greek waterbodies. J. Appl. Phycol. 2005, 17, 391–401. [Google Scholar] [CrossRef]
- Crivelli, A.J.; Catsadorakis, G.; Lake, P. Northwestern Greece: A Unique Balkan Wetland; Springer Science and Business Media: Berlin/Heidelberg, Germany, 1997; Volume 351. [Google Scholar]
- Wagner, B.; Vogel, H.; Zanchetta, G.; Sulpizio, R. Environmental change within the Balkan region during the past ca. 50 ka recorded in the sediments from lakes Prespa and Ohrid. Biogeosciences 2010, 7, 3187–3198. [Google Scholar] [CrossRef]
- Hollis, G.E.; Stevenson, A.C. The physical basis of the Lake Mikri Prespa systems: Geology, climate, hydrology and water quality. In Lake Prespa, Northwestern Greece. Developments in Hydrobiology; Crivelli, A.J., Catsadorakis, G., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 1–19. [Google Scholar]
- Tryfon, E.; Moustaka-Gouni, M. Species composition and seasonal cycles of phytoplankton with special reference to the nanoplankton of Lake Mikri Prespa. Hydrobiologia 1997, 351, 61–75. [Google Scholar] [CrossRef]
- Strid, A.; Bergmeier, E.; Fotiadis, G. Flora and Vegetation of the Prespa National Park, Greece; Society for the Protection of Prespa: Agios Germanos, Greece, 2020. [Google Scholar]
- Karteris, M.A.; Pyrovetsi, M. Land Cover/Use Analysis of Prespa National Park, Greece. Environ. Conserv. 1986, 13, 319–324. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Tryfon, E. Phytoplankton species succession in a shallow Mediterranean lake (L. Kastoria, Greece): Steady-state dominance of Limnothrix redekei, Microcystis aeruginosa and Cylindrospermopsis raciborskii. Hydrobiologia 2007, 575, 129–140. [Google Scholar] [CrossRef]
- Frogley, M.R.; Griffiths, H.I.; Martens, K. Modern and fossil ostracods from ancient lakes. In The Ostracoda: Applications in Quaternary Research; Holmes, J.A., Chivas, A.R., Eds.; Wiley: Washington, DC, USA, 2002; pp. 167–184. [Google Scholar]
- Kagalou, I.; Psilovikos, A. Assessment of the typology and the trophic status of two Mediterranean lake ecosystems in Northwestern Greece. Water Resour. 2014, 41, 335–343. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Panitsa, M. Plant species richness and composition of a habitat island within Lake Kastoria and comparison with those of a true island within the protected Pamvotis lake (NW Greece). Biodivers. Data J. 2020, 8, e48704. [Google Scholar] [CrossRef] [PubMed]
- Touka, A.; Vareli, K.; Igglezou, M.; Monokrousos, N.; Alivertis, D.; Halley, J.; Hadjikakou, S.; Frillingos, S.; Sainis, I. Ancient European lakes: Reservoirs of hidden microbial diversity? The case of Lake Pamvotis (NW Greece). Open J. Ecol. 2018, 8, 537–578. [Google Scholar] [CrossRef][Green Version]
- Organization for Economic Cooperation and Development (OECD). Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Kirsch-Volders, M.; Fenech, M.; Bolognesi, C. Validity of the Lymphocyte Cytokinesis-Block Micronucleus Assay (L-CBMN) as biomarker for human exposure to chemicals with different modes of action: A synthesis of systematic reviews. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 47–52. [Google Scholar] [CrossRef]
- Charalampous, N.; Kindou, A.; Vlastos, D.; Tsarpali, V.; Antonopoulou, M.; Konstantinou, I.; Dailianis, S. A multidisciplinary assessment of river surface water quality in areas heavily influenced by human activities. Arch. Environ. Contam. Toxicol. 2015, 69, 208–222. [Google Scholar] [CrossRef]
- Yu, J.; Dong, H.W.; Shi, L.T.; Jiang, H.L.; Yu, J.W.; Zhao, Q.W.; Cai, S.C.; Han, D.; Tang, X.Y.; Liu, J.R. Re-Examination of the genotoxic activity of water taken from the Songhua River in P. R. China. Arch. Environ. Contam. Toxicol. 2013, 65, 78–88. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Hela, D.G.; Albanis, T.A. The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Part I. Review on occurrence and levels. Environ. Pollut. 2006, 141, 555–570. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater-2540 Solids. Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.030 (accessed on 15 September 2018).
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar]
- 17294-1:2004; Water Quality Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 1: General Guidelines. International Organization for Standardization (ISO): Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/32957.html (accessed on 15 September 2020).
- 17294-2:2016; Water Quality-Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization (ISO): Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/62962.html (accessed on 15 September 2020).
- Vlastos, D.; Skoutelis, C.G.; Theodoridis, I.T.; Stapleton, D.R.; Papadaki, M.I. Genotoxicity study of photolytically treated 2-chloropyridine aqueous solutions. J. Hazard. Mater. 2010, 177, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat. Res. 1997, 392, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Surrallés, J.; Xamena, N.; Creus, A.; Catalan, J.; Norppa, H.; Marcos, R. Induction of micronuclei by five pyrethroid insecticides in whole-blood and isolated human lymphocyte cultures. Mutat. Res. 1995, 341, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidis, V.; Hela, D.; Dimopoulos, P.; Albanis, T. Imidacloprid losses in surface runoff from plots cultivated with tobacco. Int. J. Environ. Anal. Chem. 2006, 86, 185–194. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Stamatis, N.; Triantafyllidis, V.; Hela, D.; Konstantinou, I. Occurrence and distribution of selected pharmaceutical compounds on sewage-impacted section of River Acheloos, Western Greece. Int. J. Environ. Anal. Chem. 2013, 93, 1602–1619. [Google Scholar] [CrossRef]
- Carmichael, W.W. Health effects of toxin producing cyanobacteria: ‘The cyanoHABs’. Hum. Ecol. Risk. Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Maliaka, V.; Verstijnen, Y.J.M.; Faassen, E.J.; Smolders, A.J.P.; Lürling, M. Effects of guanotrophication and warming on the abundance of green algae, cyanobacteria and microcystins in Lake Lesser Prespa, Greece. PLoS ONE 2020, 15, e0229148. [Google Scholar] [CrossRef]
- Christophoridis, C.; Zervou, S.K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Main, B.J.; Samardzic, K. Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity. Neurotox. Res. 2018, 33, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef] [PubMed]
- Ibelings, B.W.; Backer, L.C.; Kardinaal, W.E.A.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae. 2014, 40, 63–74. [Google Scholar] [CrossRef]
- Ellina, G.; Papaschinopoulos, G.; Papadopoulos, B. The use of fuzzy estimators for the construction of a prediction model concerning an environmental ecosystem. Sustainability 2019, 11, 5039. [Google Scholar] [CrossRef]


| Site | pH | 1 Cond | 2 TSS | 2 PO43− | 2 SO42− | 2 Cl− | 2 NO3− | 2 NO2− | 2 F− | 2 Br− | 2 NH4+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MP | S1 | 7.14 ± 0.20 | 55.82 ± 3.10 | 1.18 ± 0.45 | 0.00 ± 0.00 | 1.12 ± 0.21 | 5.42 ± 0.26 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| S2 | 7.22 ± 0.12 | 47.63 ± 2.05 | 1.24 ± 0.12 | 0.00 ± 0.00 | 0.64 ± 0.32 | 10.34 ± 0.27 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| K | S1 | 5.74 ± 0.12 | 193.27 ± 1.27 | 5.72± 0.18 | 15.34 ± 0.56 | 10.37 ± 0.39 | 31.67 ± 0.44 | 20.32 ± 0.37 | 12.67 ± 0.66 | 10.56 ± 0.72 | 0.25 ± 084 | 2.47 ± 0.26 |
| S2 | 7.01 ± 0.18 | 42.64 ± 2.01 | 1.05± 0.64 | 0.00 ± 0.00 | 2.24 ± 0.27 | 20.43 ± 0.38 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.03 |
| MP S1 | MP S2 | K S1 | K S2 | |
|---|---|---|---|---|
| Mg 1 | 14,981.69 | 9749.36 | 15,903.18 | 9922.45 |
| Al 1 | - | - | - | - |
| Si 1 | 4187.58 | 2380.99 | 4835.98 | 4199.23 |
| K 1 | 1598.09 | 1254.79 | 3495.23 | 1961.03 |
| Ca 1 | 10,696.50 | 5518.65 | 6405.31 | 6003.50 |
| P 2 | 6.47 | 6.47 | 7.11 | 6.35 |
| Cr 1 | - | - | - | - |
| Mn 1 | - | - | - | - |
| Fe 1 | - | - | - | - |
| Co 1 | - | - | - | - |
| Ni 1 | - | - | - | - |
| Cu 1 | - | 3.51 | - | 5.44 |
| Zn 1 | 3.62 | 8.90 | - | 7.58 |
| As 1 | - | - | - | - |
| Se 1 | - | - | - | - |
| Mo 1 | - | - | - | - |
| Ag 1 | - | - | - | - |
| Cd 1 | - | - | - | - |
| Sn 1 | - | - | - | - |
| Hg 1 | - | - | - | - |
| Tl 1 | - | - | - | - |
| Pb 1 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efthimiou, I.; Vlastos, D.; Triantafyllidis, V.; Antonopoulou, M. Kastoria and Mikri Prespa Lakes: The Impact of Anthropogenic Activities on the Differentiation in the Genotoxic and Toxic Profile of the Surface Water. Land 2023, 12, 119. https://doi.org/10.3390/land12010119
Efthimiou I, Vlastos D, Triantafyllidis V, Antonopoulou M. Kastoria and Mikri Prespa Lakes: The Impact of Anthropogenic Activities on the Differentiation in the Genotoxic and Toxic Profile of the Surface Water. Land. 2023; 12(1):119. https://doi.org/10.3390/land12010119
Chicago/Turabian StyleEfthimiou, Ioanna, Dimitris Vlastos, Vassilios Triantafyllidis, and Maria Antonopoulou. 2023. "Kastoria and Mikri Prespa Lakes: The Impact of Anthropogenic Activities on the Differentiation in the Genotoxic and Toxic Profile of the Surface Water" Land 12, no. 1: 119. https://doi.org/10.3390/land12010119
APA StyleEfthimiou, I., Vlastos, D., Triantafyllidis, V., & Antonopoulou, M. (2023). Kastoria and Mikri Prespa Lakes: The Impact of Anthropogenic Activities on the Differentiation in the Genotoxic and Toxic Profile of the Surface Water. Land, 12(1), 119. https://doi.org/10.3390/land12010119










