Candida glabrata Empyema Thoracis—A Post-COVID-19 Complication
Abstract
:1. Introduction
2. Case Presentation
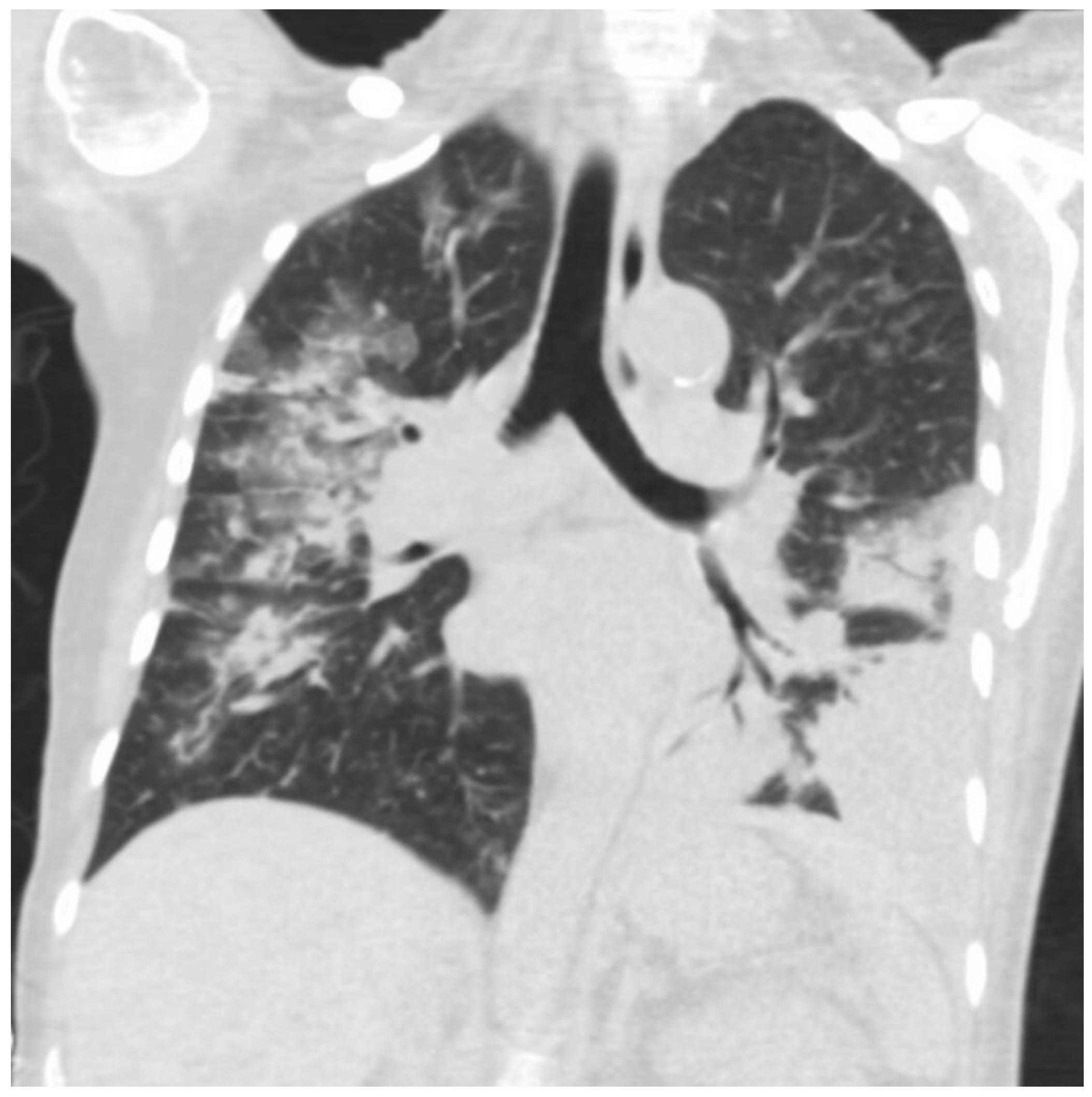
3. Investigations
4. Differential Diagnosis
5. Treatment
6. Outcome and Follow-Up
7. Discussion
8. Conclusions
- Candida empyema thoracis is an invasive candidiasis that frequently occurs in the absence of candidemia;
- Fungal empyema thoracis commonly presents as a polymicrobial infection, predominantly with a concomitant bacterial infection;
- COVID-19 is a newly identified risk factor for fungal empyema;
- The management of Candida empyemas involves prompt pleural drainage and systemic antifungals;
- Optimum treatment for non-bloodstream candidiasis may vary in important ways from the management of candidemia, and there are pharmacologic reasons for predicting that azoles are superior to echinocandins in the management of Candida empyemas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions? J. Fungi 2020, 6, 211. Available online: https://pubmed.ncbi.nlm.nih.gov/33050019/ (accessed on 14 April 2022). [CrossRef] [PubMed]
- Senger, S.S.; Thompson, G.R., 3rd; Samanta, P.; Ahrens, J.; Clancy, C.J.; Nguyen, M.H. Candida empyema thoracis at two academic medical centers: New insights into treatment and outcomes. Open Forum Infect. Dis. 2021, 8, ofaa656. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Singh, S.; Rudramurthy, S.M.; Ghosh, A.K.; Jayashree, M.; Narayana, Y.; Ray, P.; Chakrabarti, A. Candidaemia in a tertiary care centre of developing country: Monitoring possible change in spectrum of agents and antifungal susceptibility. Indian J. Med. Microbiol. 2020, 38, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. Available online: https://www.thelancet.com/article/S1473-3099(20)30086-4/fulltext (accessed on 14 April 2022). [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Executive summary: Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016, 62, 409–417. [Google Scholar] [CrossRef]
- Keighley, C.; Cooley, L.; Morris, A.J.; Ritchie, D.; Clark, J.E.; Boan, P.; Worth, J.L.; Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive candidiasis in haematology, oncology and intensive care settings, 2021. Intern. Med. J. 2021, 51, 89–117. [Google Scholar] [CrossRef]
- Nigo, M.; Vial, M.R.; Munita, J.M.; Jiang, Y.; Tarrand, J.; Jimenez, C.A.; Kontoyiannis, D.P. Fungal empyema thoracis in cancer patients. J. Infect. 2016, 72, 615–621. [Google Scholar] [CrossRef]
- Lin, K.H.; Liu, Y.M.; Lin, P.C.; Ho, C.M.; Chou, C.H.; Wang, J.H.; Chi, C.-Y.; Ho, M.-W.; Wang, J.-H. Report of a 63-case series of Candida empyema thoracis: 9-year experience of two medical centers in central Taiwan. J. Microbiol. Immunol. Infect. 2014, 47, 36–41. [Google Scholar] [CrossRef]
- Ko, S.C.; Chen, K.Y.; Hsueh, P.R.; Luh, K.T.; Yang, P.C. Fungal empyema thoracis: An emerging clinical entity. Chest 2000, 117, 1672–1678. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, B.; Ditullio, M.; Wilson, E.; Henning, S.A.; Penzak, S.R.; Danner, R.L.; Pennic, G.; Rinaldi, M.G.; Zelazny, A.M.; Gea-Banacloche, J.; et al. Pharmacokinetics of anidulafungin in pleural fluid during the treatment of a patient with Candida empyema. Antimicrob. Agents Chemother. 2011, 55, 2478–2480. [Google Scholar] [CrossRef] [PubMed]
- Rieder-Nelissen, C.M.; Hasse, J.; Yeates, R.A.; Sarnow, E. Fluconazole concentrations in pulmonary tissue and pericardial fluid. Infection 1997, 25, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Mulet Bayona, J.V.; Salvador García, C.; Tormo Palop, N.; Valentín Martín, A.; González Padrón, C.; Colomina Rodríguez, J.; Peman, J.; Gimeno Cardona, C. Novel chromogenic medium CHROMagarTM Candida Plus for detection of Candida auris and other Candida species from surveillance and environmental samples: A multicenter study. J. Fungi 2022, 8, 281. [Google Scholar] [CrossRef]
- Hospenthal, D.R.; Beckius, M.L.; Floyd, K.L.; Horvath, L.L.; Murray, C.K. Presumptive identification of Candida species other than C. albicans, C. krusei, and C. tropicalis with the chromogenic medium CHROMagar Candida. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 1. [Google Scholar] [CrossRef]
- Posteraro, B.; Ruggeri, A.; De Carolis, E.; Torelli, R.; Vella, A.; De Maio, F.; Ricciardi, W.; Postteraro, P.; Sanguinetti, M. Comparative Evaluation of BD Phoenix and Vitek 2 Systems for Species Identification of Common and Uncommon Pathogenic Yeasts. J. Clin. Microbiol. 2013, 51, 3841–5384. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Efremov, L.; Leoncini, E.; Amore, R.; Posteraro, P.; Ricciardi, W.; Sanguinetti, M. Are the Conventional Commercial Yeast Identification Methods Still Helpful in the Era of New Clinical Microbiology Diagnostics? A Meta-Analysis of Their Accuracy. J. Clin. Microbiol. 2015, 53, 2439–2450. Available online: https://jcm.asm.org/content/53/8/2439 (accessed on 14 April 2022). [CrossRef] [PubMed]
- Samantaray, S.; Singh, R. Evaluation of MALDI-TOF MS for identification of species in the Candida parapsilosis complex from candidiasis cases. J. Appl. Lab. Med. 2022, 7, 889–900. [Google Scholar] [CrossRef]
- Xie, T.-A.; Liu, Y.-L.; Liang, C.; Huang, Y.-Y.; Li, J.-W.; Li, Z.-W.; Fan, S.-J.; Chen, J.-T.; Xia, Y.; Li, X.-Y.; et al. Accuracy of matrix-assisted LASER desorption ionization-time of flight mass spectrometry for identification of Candida. Biosci. Rep. 2019, 39, BSR20190859. [Google Scholar] [CrossRef]
- Vervaeke, S.; Vandamme, K.; Boone, E.; De Laere, E.; Swinne, D.; Surmont, I. A case of Candida lambica fungemia misidentified as Candida krusei in an intravenous drug abuser. Med. Mycol. 2008, 46, 853–856. [Google Scholar] [CrossRef]
- Trowbridge, J.; Ludmer, L.M.; Riddle, V.D.; Levy, C.S.; Barth, W.F. Candida lambica polyarthritis in a patient with chronic alcoholism. J. Rheumatol. 1999, 26, 1846–1848. [Google Scholar]
- Krüger, W.H.; Kröger, N.; Rüssmann, B.; Renges, H.; Kabisch, H.; Zander, A.R. Treatment of mycotic infections after haemopoietic progenitor cell transplantation with liposomal amphotericin-B. Bone Marrow Transplant. 1998, 22, S10–S13. [Google Scholar] [PubMed]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidar, C.; Falces-Romero, I.; Machado, M.; de la Vila, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March-August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F. Invasive aspergillosis in coronavirus disease 2019: A practical approach for clinicians. Curr. Opin. Infect. Dis. 2022, 35, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chandley, P.; Subba, P.; Rohatgi, S. COVID-19-Associated Mucormycosis: A Matter of Concern Amid the SARS-CoV-2 Pandemic. Vaccines 2022, 10, 1266. [Google Scholar] [CrossRef]
- Vaseghi, N.; Sharifisooraki, J.; Khodadadi, H.; Nami, S.; Safari, F.; Ahangarkani, F.; Meis, J.M.; Badali, H.; Morovati, H. Global prevalence and subgroup analyses of coronavirus disease (COVID-19) associated Candida auris infections (CACa): A systematic review and meta-analysis. Mycoses 2022, 65, 683–703. [Google Scholar] [CrossRef]
- Erami, M.; Raiesi, O.; Momen-Heravi, M.; Getso, M.I.; Fakhrehi, M.; Mehri, N.; Yarahmadi, M.; Amiri, S.; Raissi, V.; Hashemi, S.J. Clinical Impact of Candida Respiratory Tract Colonization and Acute Lung Infections in Critically Ill Patients with COVID-19 Pneumonia. Microb. Pathog. 2022, 166, 105520. Available online: https://pubmed.ncbi.nlm.nih.gov/35405278/ (accessed on 14 April 2022). [CrossRef]
- Sharma, A.; Mehta, N.; Pirrotta, S.; Sheldon, D.; Juarez, R. A tale of two species: First reported case of an empyema secondary to co-infection with SARS-CoV-2 and candida albicans. Chest 2020, 158, A717. [Google Scholar] [CrossRef]
- Qasem, N.M.; Barakat, M.; Elamin, N.; Bashir, M.; Hassan, I.F. Candida albicans empyema in COVID-19 infected patient: The first reported case in Qatar. J. Emerg. Med. Trauma Acute Care 2022, 2022, 10. [Google Scholar]
- Glendening, J.; Koroscil, M. A Report of Fungal Empyema Following Recovery of Severe SARS-CoV-2 Infection. Chest 2020, 158, A566. Available online: https://journal.chestnet.org/article/S0012-3692(20)32718-5/fulltext (accessed on 14 April 2022). [CrossRef]

| DRUG | MIC |
|---|---|
| Voriconazole | 0.12 ug/mL |
| Anidulafungin | 0.06 ug/mL |
| Caspofungin | 0.06 ug/mL |
| Fluconazole | 4 ug/mL |
| Itraconazole | 0.5 ug/mL |
| Isavuconazole | 0.12 ug/mL |
| Posaconazole | 0.5 ug/mL |
| Micafungin | 0.015 ug/mL |
| Amphotericin B (E-test) | 0.19 ug/mL |
| 5-Fluorocytosine (E-test) | 0.016 ug/mL |
| Study | Age, Gender | Comorbid Conditions | Fungus Isolated | COVID-19 Management | Empyema Treatment | Concomitant Bacterial Infection | Outcome |
|---|---|---|---|---|---|---|---|
| Sharma et al. [27] | 55, male | Hypertension | C. albicans | Not mentioned | Tube thoracostomy, micafungin | MRSA in respiratory culture | Unclear: still admitted at the time of publication |
| Qasem et al. [28] | 52, male | None | C. albicans | ECMO | Chest tube, decortication | - | Expired |
| Glendening et al. [29] | 73, male | Congestive heart failure | C. albicans | Hydroxychloroquine intubated | Chest tube, fluconazole | Moraxella bacteremia | Discharged |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swaminathan, N.; Anderson, K.; Nosanchuk, J.D.; Akiyama, M.J. Candida glabrata Empyema Thoracis—A Post-COVID-19 Complication. J. Fungi 2022, 8, 923. https://doi.org/10.3390/jof8090923
Swaminathan N, Anderson K, Nosanchuk JD, Akiyama MJ. Candida glabrata Empyema Thoracis—A Post-COVID-19 Complication. Journal of Fungi. 2022; 8(9):923. https://doi.org/10.3390/jof8090923
Chicago/Turabian StyleSwaminathan, Neeraja, Katherine Anderson, Joshua D. Nosanchuk, and Matthew J. Akiyama. 2022. "Candida glabrata Empyema Thoracis—A Post-COVID-19 Complication" Journal of Fungi 8, no. 9: 923. https://doi.org/10.3390/jof8090923





