Invasive Fungal Disease in Patients with Myeloid Malignancies: A Retrospective Cohort Study of a Diagnostic-Driven Care Pathway Withholding Mould-Active Prophylaxis
Abstract
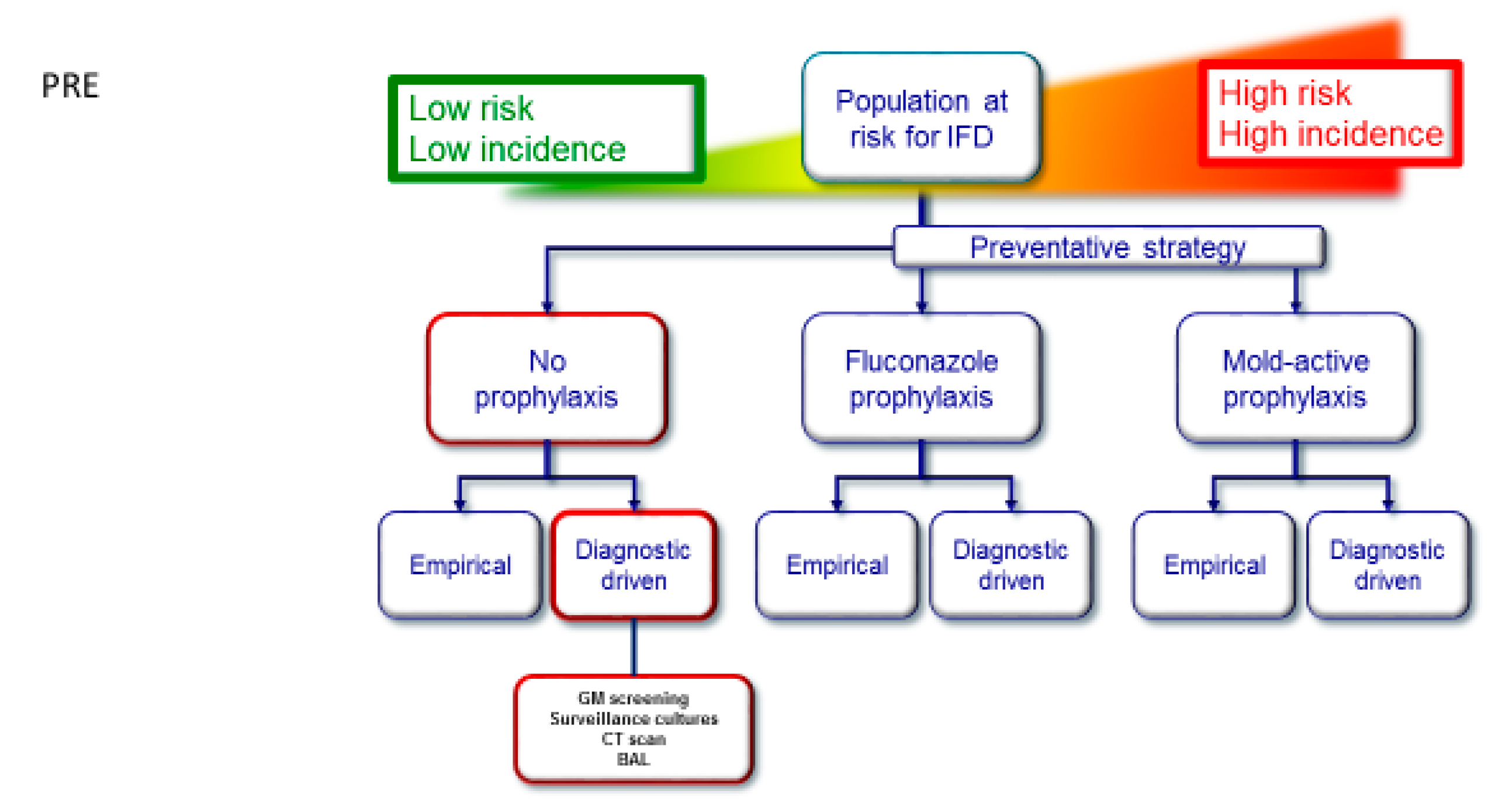
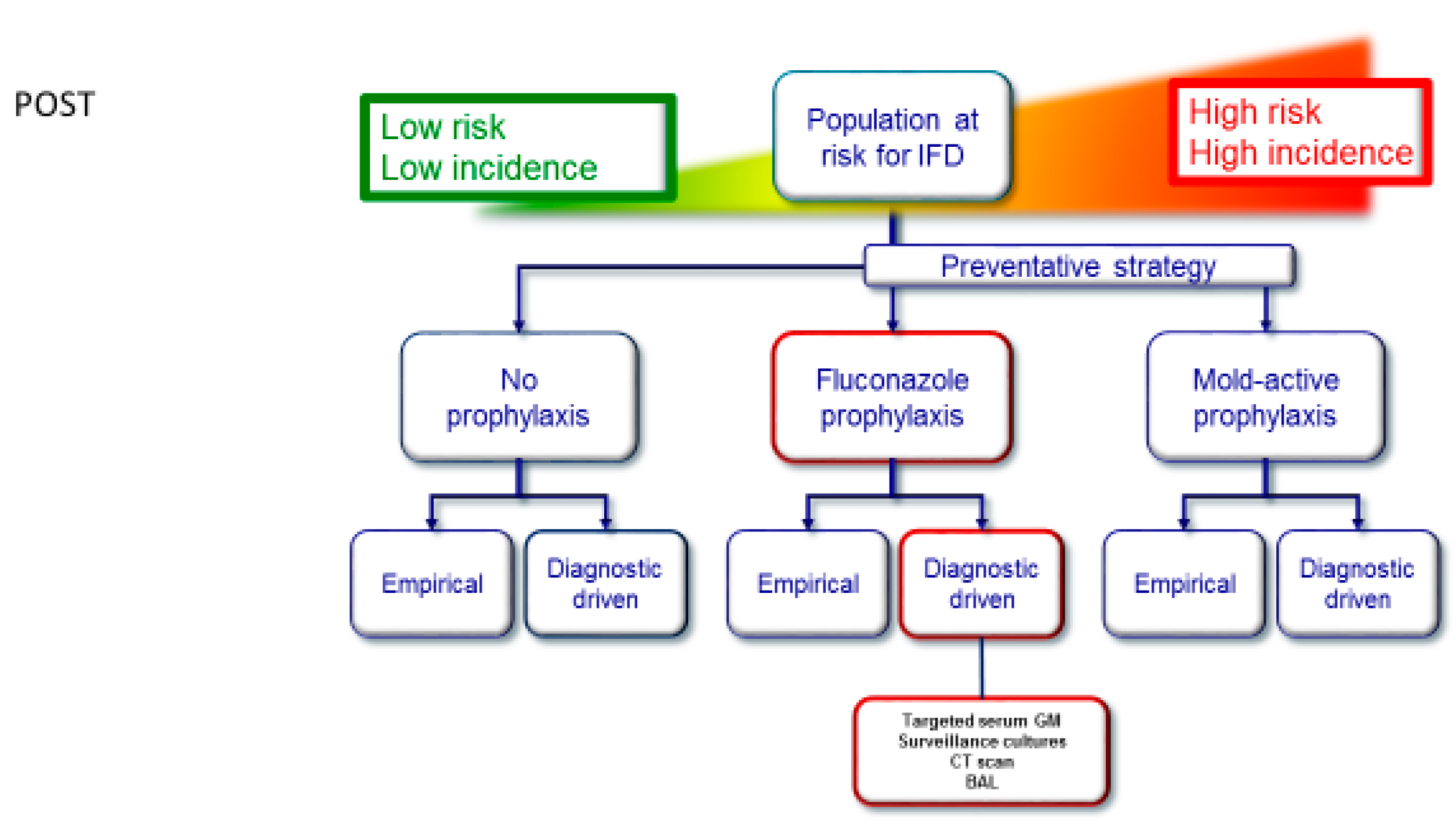
:1. Introduction
2. Methods
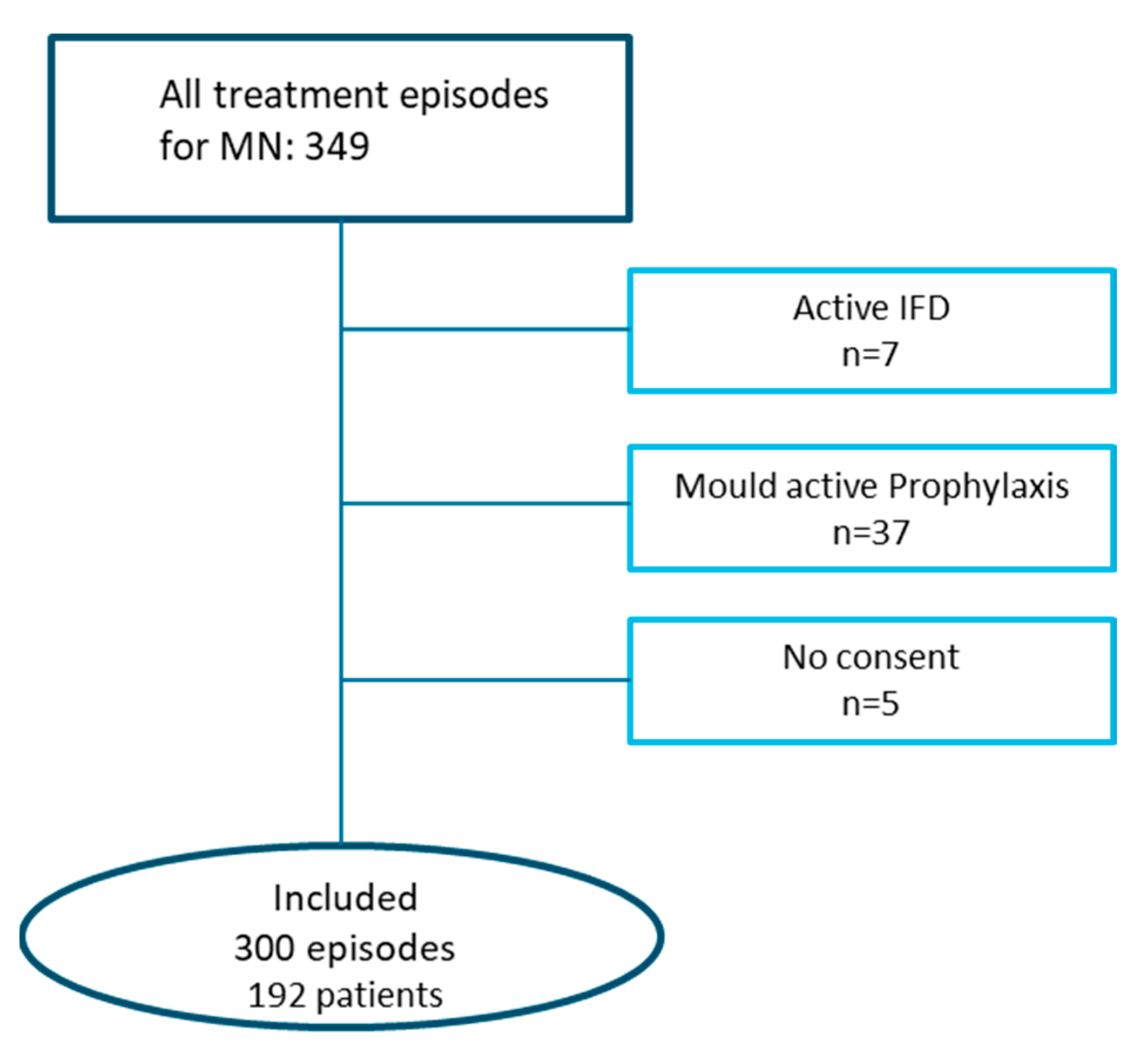
2.1. Patients and Treatment Protocol
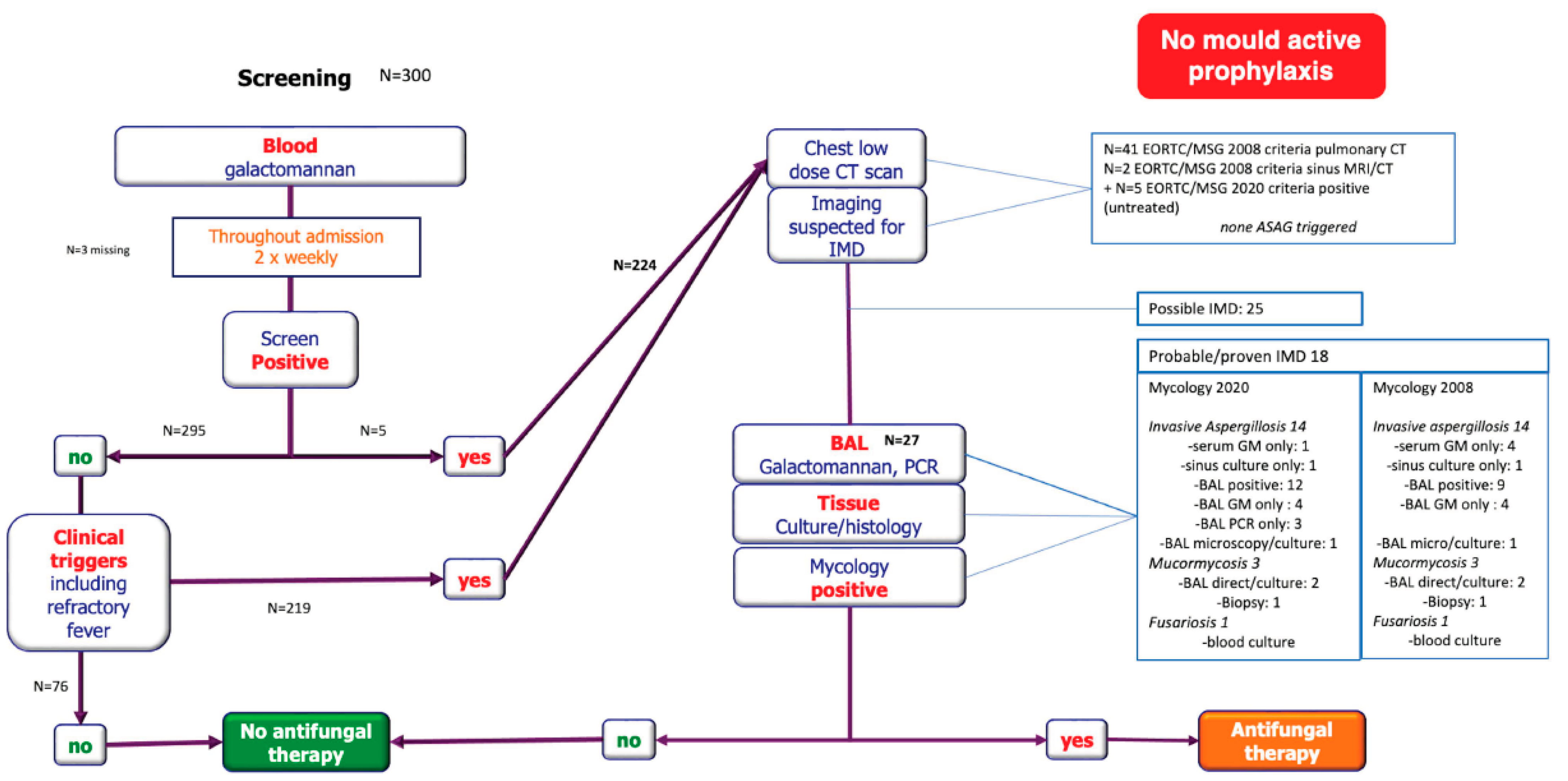
2.2. Diagnostic-Driven Care Pathway
2.3. Classification
2.4. Outcome
2.5. Statistical Analysis
3. Results
3.1. Patients and Treatment
3.2. Mould Infections
3.2.1. Classification
3.2.2. Radiology
3.2.3. Microbiology
3.3. Yeast Infections
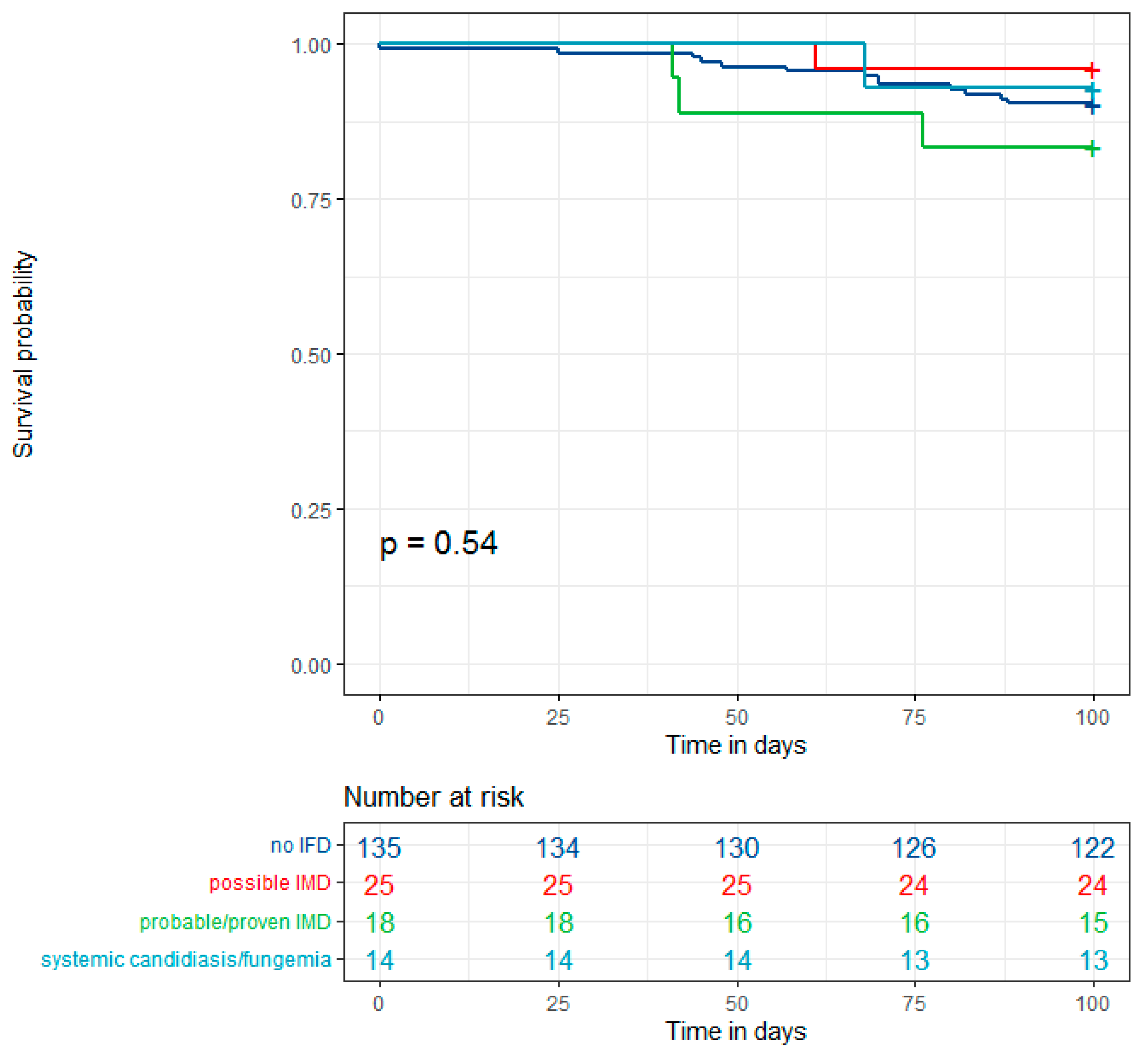
3.4. Outcome
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koehler, P.; Hamprecht, A.; Bader, O.; Bekeredjian-Ding, I.; Buchheidt, D.; Doelken, G.; Elias, J.; Haase, G.; Hahn-Ast, C.; Karthaus, M.; et al. Epidemiology of invasive aspergillosis and azole resistance in patients with acute leukaemia: The SEPIA Study. Int. J. Antimicrob. Agents 2017, 49, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Tavernier, E.; Hocquet, D.; Huynh, A.; Isnard, F.; Legrand, F.; Lhéritier, V.; Raffoux, E.; Dombret, H.; Ifrah, N.; et al. Epidemiology of invasive fungal infections during induction therapy in adults with acute lymphoblastic leukemia: A GRAALL-2005 study. Leuk. Lymphoma 2017, 58, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Raiola, A.M.; Piciocchi, A.; Algarotti, A.; Stanzani, M.; Cudillo, L.; Pecoraro, C.; Guidi, S.; Iori, A.P.; Montante, B.; et al. Incidence and Outcome of Invasive Fungal Diseases after Allogeneic Stem Cell Transplantation: A Prospective Study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol. Blood Marrow Transplant. 2014, 20, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, M.; Xu, J.-Y.; Zhou, R.-F.; Chen, B.; Wan, Y. Comparison of Antifungal Prophylaxis Drugs in Patients With Hematological Disease or Undergoing Hematopoietic Stem Cell Transplantation: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2020, 3, e2017652. [Google Scholar] [CrossRef]
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.A.; Cornely, O.A.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef]
- Brüggemann, R.J.; Verheggen, R.; Boerrigter, E.; Stanzani, M.; Verweij, P.E.; Blijlevens, N.M.A.; Lewis, R.E. Management of drug–drug interactions of targeted therapies for haematological malignancies and triazole antifungal drugs. Lancet Haematol. 2021, 9, e58–e72. [Google Scholar] [CrossRef]
- Agrawal, S.; Hope, W.; Sinkó, J.; Kibbler, C. Optimizing management of invasive mould diseases. J. Antimicrob. Chemother. 2011, 66, i45–i53. [Google Scholar] [CrossRef]
- Maertens, J.; Theunissen, K.; Verhoef, G.; Verschakelen, J.; Lagrou, K.; Verbeken, E.; Wilmer, A.; Verhaegen, J.; Boogaerts, M.; Eldere, J.V. Galactomannan and Computed Tomography–Based Preemptive Antifungal Therapy in Neutropenic Patients at High Risk for Invasive Fungal Infection: A Prospective Feasibility Study. Clin. Infect. Dis. 2005, 41, 1242–1250. [Google Scholar] [CrossRef]
- Morrissey, C.O.; Chen, S.C.A.; Sorrell, T.C.; Milliken, S.; Bardy, P.G.; Bradstock, K.F.; Szer, J.; Halliday, C.L.; Gilroy, N.M.; Moore, J.; et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: A randomised controlled trial. Lancet Infect. Dis. 2013, 13, 519–528. [Google Scholar] [CrossRef]
- Bergamasco, M.D.; Pereira, C.A.P.; Arrais-Rodrigues, C.; Ferreira, D.B.; Baiocchi, O.; Kerbauy, F.; Nucci, M.; Colombo, A.L. Epidemiology of Invasive Fungal Diseases in Patients with Hematologic Malignancies and Hematopoietic Cell Transplantation Recipients Managed with an Antifungal Diagnostic Driven Approach. J. Fungi 2021, 7, 588. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Cordonnier, C.; Cuenca-Estrella, M. A European prospective invasive mould disease audit. In Proceedings of the Twenty-Fourth European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, 10–13 May 2014. [Google Scholar]
- Lowenberg, B.; Pabst, T.; Maertens, J.; van Norden, Y.; Biemond, B.J.; Schouten, H.C.; Spertini, O.; Vellenga, E.; Graux, C.; Havelange, V.; et al. Therapeutic value of clofarabine in younger and middle-aged (18–65 years) adults with newly diagnosed AML. Blood 2017, 129, 1636–1645. [Google Scholar] [CrossRef]
- Ossenkoppele, G.J.; Breems, D.A.; Stuessi, G.; van Norden, Y.; Bargetzi, M.; Biemond, B.J.; von dem Borne, P.A.; Chalandon, Y.; Cloos, J.; Deeren, D.; et al. Lenalidomide added to standard intensive treatment for older patients with AML and high-risk MDS. Leukemia 2020, 34, 1751–1759. [Google Scholar] [CrossRef]
- Prentice, H.G. Towards a targeted, risk-based, antifungal strategy in neutropenic patients. Br. J. Haematol. 2001, 110, 273. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2019, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.R.; Ribaud, P.; Schlamm, H.T.; Herbrecht, R. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer 2008, 112, 2309–2312. [Google Scholar] [CrossRef]
- Orasch, C.; Weisser, M.; Mertz, D.; Conen, A.; Heim, D.; Christen, S.; Gratwohl, A.; Battegay, M.; Widmer, A.; Fluckiger, U. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2010, 45, 521–526. [Google Scholar] [CrossRef]
- Fisher, B.T.; Westling, T.; Boge, C.L.K.; Zaoutis, T.E.; Dvorak, C.C.; Nieder, M.; Zerr, D.M.; Wingard, J.R.; Villaluna, D.; Esbenshade, A.J.; et al. Prospective Evaluation of Galactomannan and (1→3) β-d-Glucan Assays as Diagnostic Tools for Invasive Fungal Disease in Children, Adolescents, and Young Adults With Acute Myeloid Leukemia Receiving Fungal Prophylaxis. J. Pediatric Infect. Dis. Soc. 2021, 10, 864–871. [Google Scholar] [CrossRef]
- Bitterman, R.; Hardak, E.; Raines, M.; Stern, A.; Zuckerman, T.; Ofran, Y.; Lavi, N.; Guralnik, L.; Frisch, A.; Nudelman, O.; et al. Baseline Chest Computed Tomography for Early Diagnosis of Invasive Pulmonary Aspergillosis in Hemato-oncological Patients: A Prospective Cohort Study. Clin. Infect. Dis. 2019, 69, 1805–1808. [Google Scholar] [CrossRef]
- Resendiz Sharpe, A.; Lagrou, K.; Meis, J.F.; Chowdhary, A.; Lockhart, S.R.; Verweij, P.E.; ISHAM/ECMM Aspergillus Resistance Surveillance Working Group. Triazole resistance surveillance in Aspergillus fumigatus. Med. Mycol. 2018, 56, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.; Abdolrasouli, A.; Dunne, K.; Sewell, T.R.; Zhang, Y.; Ballard, E.; Brackin, A.P.; van Rhijn, N.; Chown, H.; Tsitsopoulou, A.; et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 2022, 7, 663–674. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | 192 Patients |
|---|---|
| Patient age, median (IQR), y | 57 (46–63) |
| Male sex, no (%) | 110 (57%) |
Diagnoses, no. (%)
| 157 (82%) 18 (9%) 17 (9%) 2 2 2 6 5 |
Treatment courses, no.
| 196 193 3 104 |
Treatment year, no.
| 53 55 59 25 |
Candida−directed prophylaxis,no. (%)
| 107 (36%) 93 14 |
| Mould directed prophylaxis | 0 |
| Mould Infections | |
|---|---|
| Median time to diagnosis (days) | 20 (IQR 17–26) |
| Possible IMD | 16 |
Probable IMD
| 16 13 1 2 |
Proven IMD
| 2 1 1 |
Probable/proven IMD, no (%)
| 18 (9.3%) 9 (4.6%); 7 IA (3.6%) 9 (8.7%); 7 IA (6.7%) |
| Yeast infections | |
| Median time to diagnosis in days | 30 (IQR 16–43) |
Fungaemia
| 4 (3,1) 1 |
Disseminated hepatosplenic candidiasis
| 8 1 |
| Systemic Candida infection Patient (N = 192)
| 13 (6.8%) 13 (10%) 0 12 (6%) 1 (1%) |
| Patient nr | Age (Years) | Gender | Diagnosis | RI Cycle | Candidiasis | EORTC/MSG 2020 Category |
|---|---|---|---|---|---|---|
| 1 | 60 | F | AML | I | Hepatosplenic candidiasis | Possible |
| 2 | 66 | M | AML | I | Hepatosplenic candidiasis | Possible |
| 3 | 59 | M | aCML | I | Candidaemia | Proven |
| 4 | 50 | M | MDS-EB2 | I | Hepatosplenic candidiasis | Possible |
| 5 | 27 | F | AML | II | Candidaemia | Proven |
| 6 | 54 | M | AML | I | Hepatosplenic candidiasis | Possible |
| 7 | 64 | F | AML | I | Hepatosplenic candidiasis | Probable |
| 8 | 56 | M | AML | I | Hepatosplenic candidiasis | Possible |
| 9 | 40 | M | AML | I | Hepatosplenic candidiasis | Possible |
| 10 | 42 | F | AML | I | Hepatosplenic candidiasis | Possible |
| 11 | 60 | F | AML | I | Candidaemia | Proven |
| 12 | 60 | F | AML | I | Candidaemia | Proven |
| 13 | 44 | F | AML | I | Hepatosplenic candidiasis | Possible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Kort, E.A.; Buil, J.B.; Schalekamp, S.; Schaefer-Prokop, C.; Verweij, P.E.; Schaap, N.P.M.; Blijlevens, N.M.A.; Van der Velden, W.J.F.M. Invasive Fungal Disease in Patients with Myeloid Malignancies: A Retrospective Cohort Study of a Diagnostic-Driven Care Pathway Withholding Mould-Active Prophylaxis. J. Fungi 2022, 8, 925. https://doi.org/10.3390/jof8090925
De Kort EA, Buil JB, Schalekamp S, Schaefer-Prokop C, Verweij PE, Schaap NPM, Blijlevens NMA, Van der Velden WJFM. Invasive Fungal Disease in Patients with Myeloid Malignancies: A Retrospective Cohort Study of a Diagnostic-Driven Care Pathway Withholding Mould-Active Prophylaxis. Journal of Fungi. 2022; 8(9):925. https://doi.org/10.3390/jof8090925
Chicago/Turabian StyleDe Kort, Elizabeth A., Jochem B. Buil, Steven Schalekamp, Cornelia Schaefer-Prokop, Paul E. Verweij, Nicolaas P. M. Schaap, Nicole M. A. Blijlevens, and Walter J. F. M. Van der Velden. 2022. "Invasive Fungal Disease in Patients with Myeloid Malignancies: A Retrospective Cohort Study of a Diagnostic-Driven Care Pathway Withholding Mould-Active Prophylaxis" Journal of Fungi 8, no. 9: 925. https://doi.org/10.3390/jof8090925
APA StyleDe Kort, E. A., Buil, J. B., Schalekamp, S., Schaefer-Prokop, C., Verweij, P. E., Schaap, N. P. M., Blijlevens, N. M. A., & Van der Velden, W. J. F. M. (2022). Invasive Fungal Disease in Patients with Myeloid Malignancies: A Retrospective Cohort Study of a Diagnostic-Driven Care Pathway Withholding Mould-Active Prophylaxis. Journal of Fungi, 8(9), 925. https://doi.org/10.3390/jof8090925






